Primary Gastric Melanoma with Rhabdoid Features: A Case Report
Article information
Malignant melanomas display a wide variety of morphologic patterns.1 Melanomas with rhabdoid appearance are rare and appear mostly as a recurrent or metastatic form, and only atypically arise as a primary lesion. Just eight cases of primary rhabdoid melanomas can be found in the literature since 1994.2-7 Malignant melanomas frequently metastasize to the gastrointestinal tract; they can be located in the stomach and are generally metastases of cutaneous melanomas. Primary gastric melanomas are extremely rare, and, to the best of our knowledge, a primary melanoma with rhabdoid features has not been reported thus far.
Here, we report a case of primary gastric melanoma with rhabdoid features along with its immunohistochemical panels and ultrastructural findings.
CASE REPORT
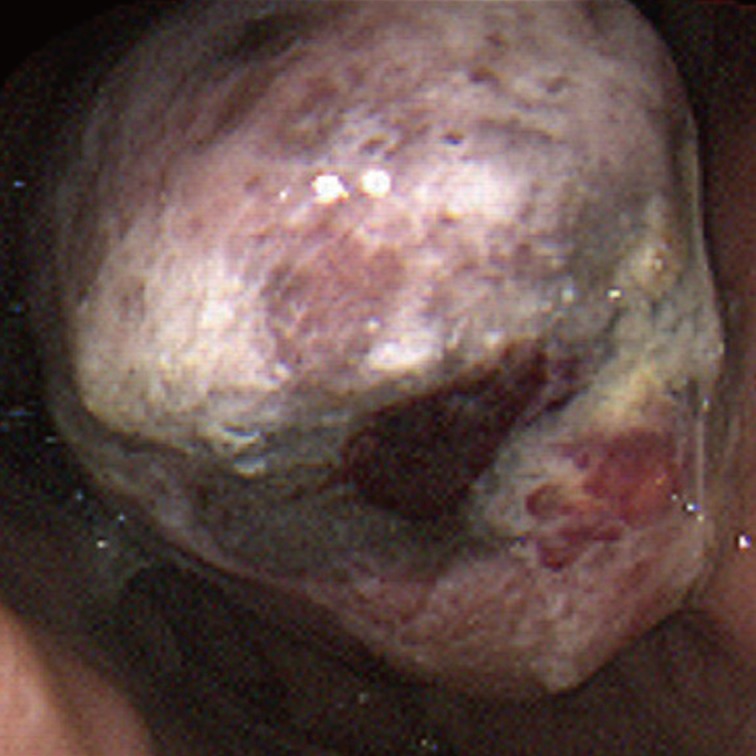
A 59-year-old male presented with a gastric mass that was found incidentally on gastroesophagoscopy. The only relevant history was a recent cerebral hemorrhage that occurred three months prior. Endoscopically, the mass was polypoid in appearance at the posterior wall of the fundus (Fig. 1). Stomach computed tomography showed an enhancing mass measuring 4.0 cm in diameter with central necrotic change at the cardia, and one enlarged regional lymph node, measuring 1.0 cm in diameter, posterior to the cardia. Under the impression of gastric carcinoma, total gastrectomy was performed. Preoperative endoscopic biopsy was not undertaken. A fungating grayish-tan colored mass was found at the posterior wall of the cardia, involving the esophagogastric junction. The cut surface of the polypoid mass revealed a homogeneous grayish-tan in appearance with focal hemorrhagic changes and focal mucosal ulcer. The mass invaded mucosa, submucosa, and inner proper muscle. It extended to the esophagogastric junction. Histologically, a solid growth pattern and pseudoalveolar clefts were found (Fig. 2A). The mass was infiltrated by round, small-to-large-sized cells with plump eosinophilic cytoplasm and peripherally compressed nuclei, creating an occasional rhabdoid appearance. The tumor cells had an irregular shape with angulated nuclei, irregular nuclear contours, and fine chromatin. Occasional marked pleomorphic cells were found. Mitotic figures were counted up to 15/10 high power fields. Metastatic foci were demonstrated in one of 20 regional lymph nodes. The observed normal-appearing gastric and esophageal mucosa showed no abnormalities. Under the impression of malignant melanoma, gastrointestinal stromal tumor, poorly differentiated rhabdomyosarcoma, myeloid sarcoma, lymphoma, or plasmacytoma, immunohistochemistry and electron microscopic examination was performed. The tumor cells, both rhabdoid and nonrhabdoid, were diffusely positive for vimentin (prediluted, V9, Dako, Glostrup, Denmark) and S-100 protein (prediluted, polyclonal, Dako) (Fig. 2B). They showed focal nuclear positivity for Sox 10 (prediluted, goat polyclonal, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) (Fig. 2C). They were negative for human melanoma black-45 antigen (HMB-45 antigen, prediluted, Dako), Melan-A (prediluted, A103, Dako), CD117 (prediluted, c-kit, Dako), CD34 (prediluted, QBEnd10, Dako), calretinin (1:100, calret 1, Dako), pancytokeratin (prediluted, AE1/AE3, Dako), epithelial membrane antigen (prediluted, E29, Dako), myoD-1 (1:50, 5.8A, Dako), myogenin (1:50, MyG007, Biocare Medical, Concord, CA, USA), smooth muscle actin (prediluted, IA4, Dako), desmin (prediluted, D33, Dako), synaptophysin (prediluted, SY38, Dako), chromogranin (prediluted, DAK-A3, Dako), CD56 (1:100, 123C2, Dako), CD68 (1:50, PG-M1, Dako), leukocyte common antigen (prediluted, Dako), CD79a (1:50, JCB117, Dako), CD138 (prediluted, MI/5, Dako), and CD99 (prediluted, 12E7, Dako). Staining with Fontana-Masson technique highlighted dark, black-colored cytoplasmic granular pigments within the tumor cells just beneath the ulcer (Fig. 2D), although some of those melanin pigments were identified on hematoxylin and eosin staining. The cells showed negative results for Prussian blue, periodic acid-Schiff, myeloperoxidase, toluidine blue, lysozyme, and terminal deoxynucleotidyl transferase tests. Ultrastructurally, closely-apposed oval-shaped tumor cells displayed a moderate amount of cytoplasm containing mitochondria and secondary lysosomes. Extensive repeated ultrastructural evaluation revealed a few stage-3 melanosomes (Fig. 3A). Some tumor cells had focal paranuclear accumulation of intermediate filaments with entrapped organelles (Fig. 3B). No demonstrable cell junctions were observed. All of these findings were consistent with malignant melanoma with rhabdoid features. The patient was re-evaluated. Upon careful physical examination of his entire body surface, including oral and anal mucosa, no lesions were found, and fundoscopic examination of the eye was also normal. He was diagnosed as a presumptive primary malignant melanoma with a rhabdoid phenotype, although no demonstrable intraepithelial melanocytosis or in situ lesion was observed. Three months later, the patient developed low back pain; spine magnetic resonance imaging with enhancement study showed a compression fracture at L3 and a small bone marrow lesion at S5, suggesting a metastatic spinal tumor at L3 and S5, which was confirmed to be meta static melanoma by percutaneous needle biopsy. Vertebroplasty was performed, and during the four months following the operation, palliative chemoradiotherapy with dacarbazine was administered.
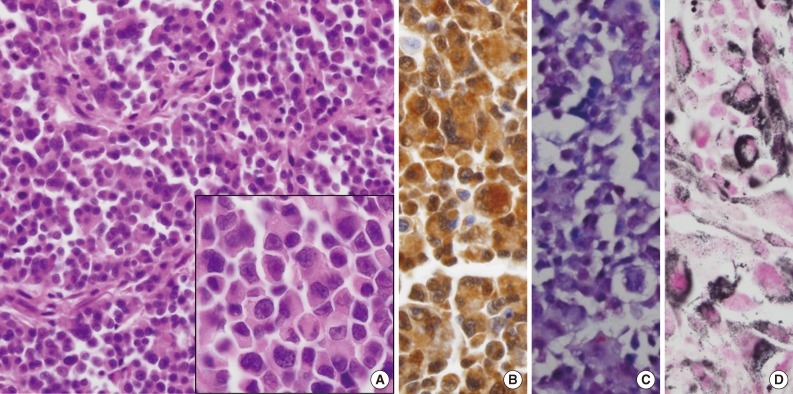
Microscopic findings. (A) The tumor is composed of round, small-to-large-sized cells with abundant eosinophilic cytoplasm. Inset indicates magnification of rhabdoid cells. The tumor cells show diffuse positivity for S-100 protein (B) and nuclear positivity for Sox 10 (C). (D) Melanin pigments are prominent on Fontana-Masson stain.
DISCUSSION
From an embryologic viewpoint, melanocytes of neural crest origin migrate to the skin, uvea, oral mucosa, leptomeninges, or anal mucosa.8 The pathogenesis of primary gastric melanoma is derived from noncutaneous melanocytes, which are not normal constituents of the gastric mucosa. Two possible theories on the origin of these tumor cells have been suggested: one theory involves ectopic migration of melanocyte precursors from their neural crest origin, and the other is that tumor cells are derived from amine precursor uptake and decarboxylation cells.8 The current tumor had neither an in situ lesion nor a precursor mucosal lesion. Rare cases of gastrointestinal melanosis, with or without melanosis cutis, have been reported. For example, esophageal melanocytosis is characterized by nontumoral intraepithelial melanocytes with increased melanin pigmentation.9 Therefore, it is possible that primary gastric melanoma may infrequently occur from these rare precursor lesions. Of recorded primary malignant rhabdoid melanomas, only two cases had primary visceral occurrence, and the remaining six cases occurred in the skin.2-7 Due to its rarity and unfamiliar and aberrant immunohistochemical features, diagnosis of primary, not metastatic or recurrent, melanoma with rhabdoid features is challenging. The most relevant differential diagnoses include gastrointestinal stromal tumor, poorly differentiated rhabdomyosarcoma, and hematologic malignancies including malignant lymphoma, myeloid sarcoma, and plasmacytoma. Negative immunoreactions for CD117, myogenin/desmin, leukocyte common antigen, myeloperoxidase, and CD138 can exclude these entities. Malignant melanoma with rhabdoid features, either primary, metastatic, or recurrent, aberrantly expresses melanoma markers (i.e., negativity for specific melanocytic markers such as HMB-45 antigen, Melan-A, and MART-1, but positivity only for S-100 protein and vimentin).2-7 In our case, rhabdoid cells were negative for HMB-45 antigen and Melan-A, but positive for S-100 protein and Sox 10, which participates in the late stage of neural crest cell formation with maintaining as multipotent stem cells destined for melanocytes and Schwann cells. Staining with Fontana-Masson technique highlighted melanin pigments within the tumor cells that had a small amount of equivocal pigmentation under hematoxylin and eosin staining. When melanotic granules in melanoma are totally absent or sparse, as in the present case, rhabdoid melanoma may be distinguished from undifferentiated sarcoma and poorly differentiated adenocarcinoma. Frequent loss of conventional melanoma-markers in rhabdoid melanoma may be ascribed to the clonal selection of a subpopulation in the progression of a tumor, or to the dedifferentiation of neoplastic tissue by tumor growth and/or chemotherapy regimens.10 In particular, the loss of S-100 protein in rhabdoid melanoma defines the most dedifferentiated form. Electron microscopic findings of rhabdoid melanoma have been rarely described;4,10 a rhabdoid appearance is attributed to the ultrastructural presence of paranuclear whorls of intermediate filaments containing entrapped organelles, with or without a few melanosomes. As in the current case, a thorough search for melanosomes is a useful diagnostic tool for identifying rhabdoid melanoma, especially when the tumor displays a total loss of specific melanocyte markers and negativity for S-100 protein.2,3,10
Malignant tumors demonstrating rhabdoid features are generally regarded as phenotypic variants. Most previously reported melanomas with rhabdoid features appeared as a recurrent or metastatic form.1 In particular, we emphasize the immunohistochemical variability of rhabdoid melanoma. Although primary gastric melanoma with a rhabdoid appearance has not been previously reported in the stomach, pathologists, surgeons, and gastroenterologists should be aware of the features of this rare disease, in order to provide an accurate diagnosis and an appropriate course of treatment.
Notes
No potential conflict of interest relevant to this article was reported.