A Case of Metastatic Angiosarcoma Diagnosed by Liquid-Based Preparation: Peculiar Cytoplasmic Changes
Article information
Abstract
Angiosarcoma with predominantly epithelioid features is a rare soft tissue neoplasm and the interpretation of its cytopathologic findings may be difficult. We report a case of metastatic angiosarcoma with predominantly epithelioid features diagnosed by liquid-based cytology. The cytopathologic findings in this case differed from those of the conventional preparation and we found a clean background, no hyperchromatic nuclei and several cytoplasmic changes, including intracytoplasmic vacuoles with peculiar shapes, juxtanuclear condensation and perinuclear clearing. Identification of these changes using liquid-based cytology supplemented with immunochemistry may be helpful in reaching a correct cytopathologic diagnosis.
Liquid-based preparation (LBP) has been widely used in gynecologic cytology because it aids in accurate diagnosis and reduces the required examination time compared to the conventional preparation (CP). However, LBP may alter cytopathologic findings, such as the cellularity, background, architecture, and cellular morphology, and these changes result in incorrect diagnoses.1
As of now, LBP is increasingly used for nongynecologic cytology, including aspiration cytology. The reported cytopathologic findings of angiosarcoma, particularly those with epithelioid features by CP are variable nuclear atypia, intracellular red blood cells (RBCs), bloody background and vasoformative features such as intracytoplasmic lumen, microacinar arrangement or well-formed vessels.2,3 However, there have only been a few reports on LBP,4 and the cytopathologic findings by LBP are required for the correct diagnosis. In this article, we report a case of metastatic angiosarcoma with predominantly epithelioid features and its cytopathologic findings by LBP, and discuss the differences compared to CP and cytologic differential diagnosis.
CASE REPORT
A 73-year-old man visited an outside hospital with right chest pain lasting 2 weeks. A right pleural effusion was identified by chest plain film. Pleural fluid cytology was performed and the cytopathologic diagnosis was metastatic adenocarcinoma. The positron emission tomography-computed tomography revealed multifocal uptakes in soft tissue of the right thigh, multiple bones and lymph nodes at mediastinal, right inguinal and left supraclavicular area. He was provisionally diagnosed as having pulmonary adenocarcinoma with multiple metastases and was referred to our hospital.
An excisional biopsy of the right inguinal node and needle biopsy and cytology of the mediastinal lymph nodes guided by endobronchial ultrasound (EBUS) were performed. The histopathologic and cytopathologic diagnosis was metastatic angiosarcoma of the epithelioid type. The patient was started on adjuvant chemotherapy with taxol-cisplatin. During chemotherapy, the patient complained of back pain, and bone metastases at the right scapula and at the lumbar vertebra were diagnosed by bone scan and magnetic resonance imaging (MRI). The patient received 10 cycles of adjuvant radiotherapy for the spine metastasis. MRI scan of the thigh showed a persistent subcutaneous mass with ill-defined circumscription and heterogeneous contrast enhancement that was considered to be a soft tissue angiosarcoma without additional pathologic assessment. Chest computed tomography (CT) revealed a small spiculate nodule in the right apical lung, reduced right pleural effusion, and some enlarged lymph nodes in the right supraclavicular, upper paratracheal and hilar areas. Abdominal CT revealed multiple lesions, including masses in the spleen and both hepatic lobes, multiple enlarged lymph nodes at the inguinal, aortocaval and para-aortic areas, and ascites considered to be cancer peritonei. Neither thickening nor mass lesions in the gastrointestinal tract were identified, and no endoscopic evaluation was performed owing to the patient's refusal of the procedure. Several radiologic findings were suspicious for disease progression. The patient was alive at the time of writing, five months after pathologic and cytopathologic diagnosis, but he stopped adjuvant chemotherapy due to poor overall health.
The histopathologic and cytopathologic findings
An inguinal lymph node (4.5×4.5×3.3 cm) was excised and its cut surface revealed a solid mass, nearly replacing the entire lymph node. The lesion was grayish to whitish tan with hemorrhage. Microscopically, it was a high-grade malignant tumor with rudimentary vascular channel formation (Fig. 1A). The major tumor cells were large and epithelioid, and arranged in a solid sheet and nest (Fig. 1B). The nuclei were round, oval, indented or multilobulated with clumped chromatin and one to three prominent nucleoli. The features of the cytoplasm also varied from little to plump in amount, and from amphophilic to eosinophilic in color. The other minor tumor cell components were spindles with small and hyperchromatic nuclei and a small amount of eosinophilic cytoplasm. Vascular channel formation was identified in both components, although it was more predominant in the latter component, and focal in the former component. Mitotic figures were numerous throughout the tumor. Immunohistochemically, tumor cells were positive for vimentin and CD31 (Fig. 1C), albeit with weak expression, and negative for other immunostaining, including CD34, factor VIII-related antigen, epithelial membrane antigen (EMA), cytokeratin AE1/AE3, cytokeratin 7, HBME1, and calretinin. The final histopathologic diagnosis of the lymph node was metastatic epithelioid angiosarcoma.
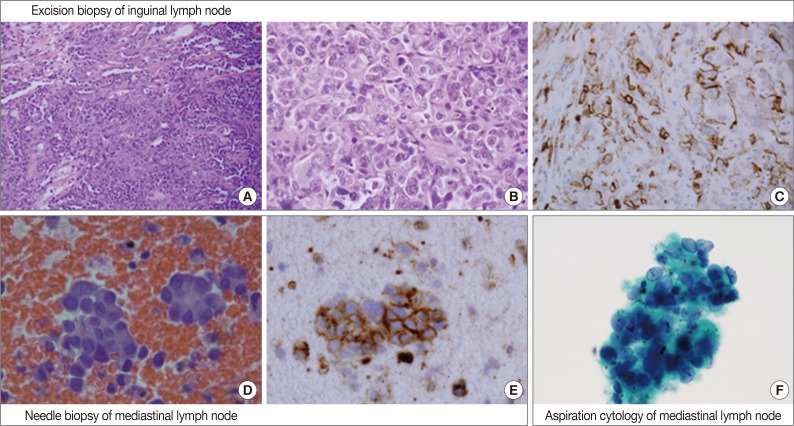
Overall histopathologic and cytopathologic findings in excision of inguinal lymph node, needle biopsy of mediastinal lymph node, and aspiration cytology by liquid-based preparation (LBP) of mediastinal lymph nodes. (A) Epithelioid tumor cells are arranged in a solid sheet and nest with focal vascular channel formation. (B) The major tumor cells are large and epithelioid with round nuclei, prominent nucleoli and sparse to plump cytoplasm. (C) Tumor cells are positive for CD31. (D) A few loose clusters or microacini with a central lumen are identified. (E) Tumor cells are positive for CD31. (F) Vague microacini formation is also identified in aspiration cytology by LBP (Papanicolaou stain).
By EBUS-guided needle biopsy, the mediastinal lymph nodes were diagnosed as malignant, compatible with metastatic angiosarcoma. Tumor cells were arranged in individual cells, cords, loose clusters, or microacini with central lumens in a blood-rich background, which has been frequently found in other mediastinal lymph node biopsies guided by EBUS (Fig. 1D). Tumor cells were epithelioid with round, irregularly indented or lobulated nuclei with prominent nucleoli and a scant amount of cytoplasm. Some tumor cells were plasmacytoid with intracytoplasmic amphophilic vacuoles (Fig. 2A), perinuclear clearing (Fig. 2E), or eosinophilic perinuclear condensation (Fig. 2I). However, evident intracytoplasmic hemosiderin pigment or erythrophagocytosis was not identified. Immunohistochemically, tumor cells were positive for CD31 and vimentin (Fig. 1E).
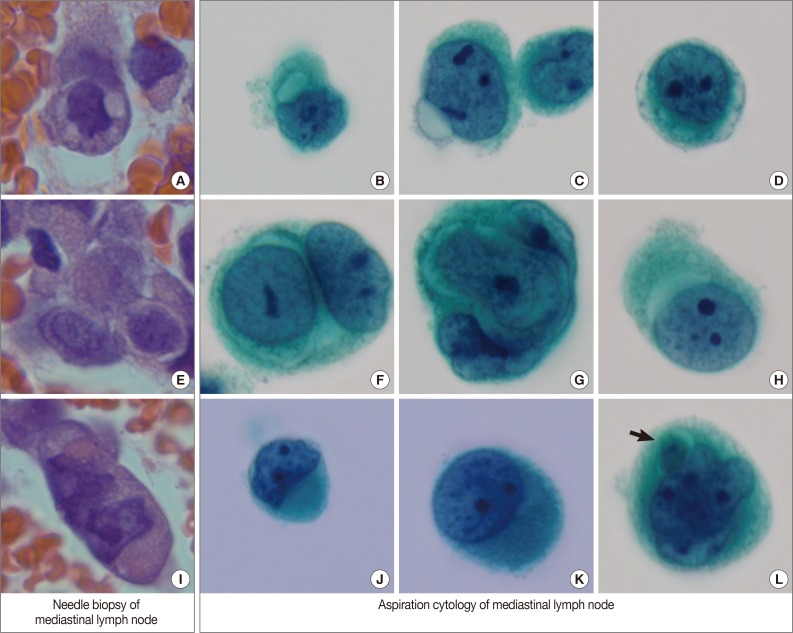
Variable cytoplasmic features in needle biopsy of mediastinal lymph nodes and aspiration cytology by liquid-based preparation of mediastinal lymph nodes. Needle biopsy demonstrates intracytoplasmic vacuoles (A), perinuclear clearing (E), and juxtanuclear condensation (I). These histopathologic findings correspond to the cytopathologic findings in aspiration cytology. Intracytoplasmic vacuoles exhibit variable shapes: prominent intracytoplasmic vacuoles (B), a protruding vacuole beyond the cytoplasmic border (C), and several small vacuoles encircling the nuclei to form a band-like appearance (D). Perinuclear clearing is identified circumferentially (F, G) or sectionally (H). Condensed juxtanuclear cytoplasm with nuclear indentation makes a rhabdoid appearance (J, K). Intracytoplasmic degenerative red blood cells (L, arrow) are present (B-D, F-H, J-L, Papanicolaou stain).
Cytologic examination of the mediastinal lymph nodes was also performed by EBUS-guided aspiration and it was prepared using liquid-based cytology (Thin Prep, Cytyc Corporation, Boxborough, MA, USA) by Papanicolaou stain. The cellularity was relatively low and tumor cells were individually scattered with a few small loosely cohesive clusters on a clean background. A few tumor cells were arranged in vague microacini, linear cords or tight small clusters (Fig. 1F). The nuclear features ranged from a bland nucleus with round borders and fine chromatin to a pleomorphic nucleus with irregular borders and clumped chromatin. The nucleoli were often seen in multiples and appeared small, but distinct. Tumor cells occasionally exhibited binucleation or multinucleation (Fig. 3A) and few Reed-Sternberg cell-like cells with markedly irregular nuclei and prominent nucleoli were identified (Fig. 3B). None of the tumor cells, even pleomorphic cells, exhibited nuclear hyperchromasia. There were no mitotic figures. The cytoplasm was scant to moderate in amount, and finely granular to densely glassy in texture. Many tumor cells had fine to prominent vacuoles, which were located inside the cytoplasm or protruded beyond the cellular outline (Fig. 2B, C). Several intracytoplasmic vacuoles along the nuclei seemed to be fused, resulting in a band-like appearance (Fig. 2D). Some tumor cells exhibited perinuclear clearing circumferentially or in section (Fig. 2F-H). In the cytoplasm of a few tumor cells, there were condensed small blobs (Fig. 2J, K) or yellowish green, round bodies, which were considered degenerative RBCs (Fig. 2L), but no definite intracytoplasmic hemosiderin deposits were identified. Some tumor cells had elongated cytoplasm and eccentrically located nuclei, creating a plasmacytoid appearance. A few tumor cells had a relatively long, elongated cytoplasmic projection, like a tail. In particular, the intracytoplasmic vacuoles and juxtanuclear condensations were frequent in plasmacytoid cells, where an occasional rhabdoid appearance was made by juxtanuclear condensation and eccentric nuclei. Immunocytochemically, tumor cells were positive for vimentin and negative for cytokeratin 7, pan-cytokeratin, EMA, and leukocyte common antigen. The cytopathologic diagnosis was malignancy compatible with metastatic angiosarcoma.
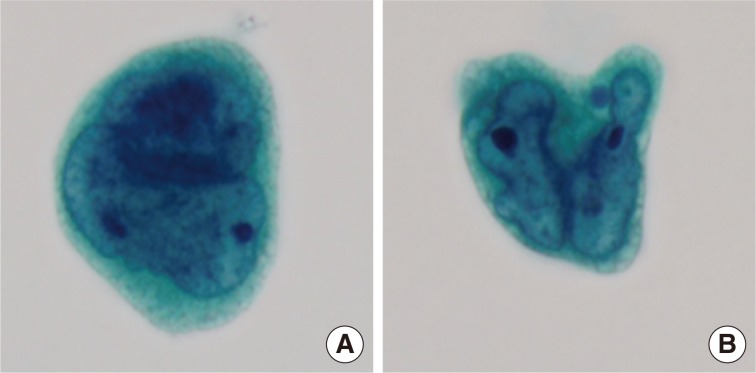
Variable nuclear features in aspiration cytology by liquid-based preparation (LBP) of mediastinal lymph nodes. (A) Tumor cells show binucleated, large nuclei with irregular borders. Many small vacuoles are present in the cytoplasm. (B) Tumor cells show markedly irregular nuclei with prominent nucleoli, resembling Reed-Sternberg cells (A, B, Papanicolaou stain).
DISCUSSION
Angiosarcoma has diverse histologic growth patterns, consisting of papillary, spindle, and epithelioid type cells. When angiosarcoma is predominantly or exclusively composed of large, rounded, so-called epithelioid cells, it can be classified as an epithelioid angiosarcoma variant. According to Pohar-Marinsek and Lamovec2 epithelioid angiosarcoma can be classified into the following three subgroups by CP: pleomorphic, carcinoma-like, and benign in appearance. The first pleomorphic subgroup has large tumor cells with large, hyperchromatic nuclei, coarse chromatin, and small to prominent nucleoli. This group of tumors exhibits moderate anisonucleosis. They may have plasmacytoid features such as eccentric nuclei and abundant cytoplasm. The second carcinoma-like subgroup has moderately large tumor cells, which have less marked nuclear pleomorphism and a smaller amount of cytoplasm than the pleomorphic group. The third benign-appearing subgroup has small and monomorphic tumor cells with bland nuclei and scant cytoplasm. In our case, the tumor cells were variable with bland to pleomorphic nuclei and anisonucleosis, indicating more of a mixed pleomorphic and carcinoma-like subtype.
Angiosarcoma has characteristic features resulting from the tendency to recapitulate normal endothelium. The cytopathologic features related to this tendency include a hemorrhagic background, erythrophagocytosis, and hemosiderin pigmentation.3 Although the background of our case was clean without hemorrhage, it is uncertain whether the clean background is a property related to tumor subtypes or the result of clearance of the RBCs during the course of LBP.1 Some investigators have reported that there have been no cases of epithelioid angiosarcoma with a hemorrhagic background by CP,5 while others have reported that epithelioid angiosarcoma can also have a hemorrhagic background.3 Erythrophagocytosis may be rare in cytology smears, but it has been reported that erythrophagocytosis is more frequent in the epithelioid subtype than the classic subtype (80% vs. 33%, respectively).3,5 The LBPs in our case exhibited rare intracytoplasmic yellowish-green bodies, which were suggestive of phagocytotic RBCs with degeneration. However, this feature was a very focal finding, and therefore diligent search was required. Although the vasoformative features of angiosarcoma in cytology is characterized by variable arrangements such as microacinar lumen formation, pseudorosette formation, intracytoplasmic lumens/vacuoles, and a signet-ring cell-like feature, almost all angiosarcomas by CP tend to present with many dissociated cells with only a few vasoformative features, or even without them in some cases.2,3,4 In our case by LBP, a few microacinar lumenal formations and occasional intracytoplasmic vacuoles were also identified. The vacuoles tended to be fine to small, protruding beyond the cellular outline and fused along the nuclei in a band-like pattern. We think the two latter shapes of vacuoles are different from those of previously described vacuoles in angiosarcomas.
In addition to the vasoformative features and peculiar intracytoplasmic vacuoles, there were some characteristic cytopathologic features in our case. The first, juxtanuclear cytoplasmic density, was described only by the Diff-Quik stain in a previous report,6 but it was identified on the Papanicolaou stain in our case. This was confirmed to be intermediate filaments forming intracytoplasmic aggregates or perinuclear whorls by electron microscope examination.7 These filaments were also found to push the nucleus to the side to form a rhabdoid appearance. Because this rhabdoid feature can be identified in other diseases, it may only be helpful for considering the possibility of angiosarcoma with epithelioid features. The second characteristic feature, juxtanuclear clearing, was originally described in epithelioid sarcoma,8 but has not been described in angiosarcoma with epithelioid features until now. With the review of synchronous needle biopsy of a mediastinal lymph node, we found a few tumor cells having perinuclear light cytoplasms, even though they were less evident than those in LBP. Further work will be needed to determine the diagnostic significance. Another diagnostic clue, bi- and multinucleated cells, was also identified.4 Some cells were found to have binucleation with mirror image and prominent nucleoli, giving them a Reed-Sternberg cell appearance.
If spindle cells with or without epithelioid cells are presented with the above cytopathologic features, the cytopathologists may suggest the tissues is a stromal tumor with vascular differentiation. However, if the epithelioid cells are predominant, its differential diagnosis may be difficult because of its rare incidence and overlapping morphologic features with other diseases. We also had to differentiate our case from metastatic adenocarcinoma, which is the most common incorrect diagnosis made in cases of angiosarcoma with predominantly epithelioid features due to similar cytologic characteristics, such as epithelioid appearance, acinar arrangement and intracytoplasmic vacuoles. Acinar arrangement is more frequent in adenocarcinoma than in angiosarcoma, and in adenocarcinoma it exhibits a scalloped outline with sharp borders,9 which was not identified in our case of angiosarcoma with epithelioid features. Also, it may be helpful to keep in mind that the necrotic background and large vacuoles commonly seen in adenocarcinoma, contrasts the clean or hemorrhagic background and fine vacuoles seen in angiosarcoma, particularly in single cell predominant cases.4 If a primary focus for metastatic adenocarcinoma is not clinically detected, cytopathologists have to consider the possibility of angiosarcoma with predominantly epithelioid features and characterize the vacuoles and background, even in cases where there is an absence of a hemorrhagic background by LBP. In addition to the elimination of these helpful diagnostic findings in LBP, an indistinct "vasoformative feature" may mimic other differentiation patterns. Several other findings in our case, such as vacuoles with peculiar shapes, juxtanuclear condensation and perinuclear clearing may be helpful in cases that are difficult to diagnose.
When cytopathologists can use immunostaining to complement their diagnosis, this can provide valuable information for diagnosis. With respect to immunostaining, LBP has an advantage over CP. Among the immunostaining targets for endothelial differentiation (i.e., CD31, CD34, factor VIII, and UEA1), CD31 has a high specificity and sensitivity and is considered the best choice according to some reports.3 However, because a small fraction of angiosarcomas may not express these markers, the usage of a panel of vascular markers is preferable. Furthermore, angiosarcomas can express many epithelial markers including cytokeratin AE1/AE3, cytokeratin CAM5.2, cytokeratin 7, B72.3, and EMA.3,10 This unexplained expression may confound the differential diagnosis and therefore, the immunochemical interpretation should be made with caution, and meticulous morphological evaluation with clinicopathologic correlation is required in these instances.
Besides adenocarcinoma, angiosarcoma with predominantly epithelioid features may be misdiagnosed as variable epithelioid diseases, including renal cell carcinoma (RCC), melanoma, malignant lymphoma and epithelioid benign and malignant mesenchymal tumors.4,5,6
Conventional RCC is usually hypercellular with hemorrhagic and necrotic background. Tumor cells are arranged as individual cells, loosely cohesive clusters or have a pseudopapillary pattern resulting from tumor cells anchored to capillaries, of which, the latter may be a diagnostic clue. RCC exhibits bland to pleomorphic nuclei, occasional intranuclear inclusions and abundant vacuolated cytoplasm with indistinct cytoplasmic borders. Compared with vacuoles in angiosarcoma, the vacuoles in RCC are more numerous and smaller, resulting in a frothy appearance.11 Extracellular and intracellular hemosiderin deposits in RCC have been reported and therefore cytopathologists should be careful not to interpret the presence of hemosiderin deposits as vascular differentiation of a tumor.12 Immunostaining for RCC markers including vimentin, EMA, CD10, RCC, PAX2, and PAX8 may be helpful.13,14
Melanoma is often arranged as predominantly dispersed individual cells, and exhibits an epithelioid feature with eccentric nuclei and moderate to abundant cytoplasm. Compared with angiosarcoma, melanoma tends to have a necrotic background, significant nuclear pleomorphism and more mitotic activity. Melanin pigment, as a diagnostic characteristic, is highly detected (up to 80%) in LBP,15 and it is finer than hemosiderin pigment of angiosarcoma, which is coarsely granular and refractive.9 By the May-Grüwald-Giemsa method, melanin pigment appears grey, in contrast to the brown color of hemosiderin pigment.5 Another ancillary test, such as the Fontana-Masson, Perls, human melanoma black 45 or Melan A stains, may be helpful.
Malignant lymphoma, particularly a high-grade lymphoma such as large B-cell lymphoma and anaplastic large cell lymphoma, is also a potential differential diagnosis.14 Unlike the general cytologic findings of lymphoma, malignant lymphoma may form a cohesive cluster or exhibit pleomorphic nuclei, abundant cytoplasm and "signet-ring"-like appearance, which mimics carcinoma with EMA expression. For an accurate differential diagnosis, combined immunostaining with a lymphoid marker, such as CD45, CD20, CD3, CD30, or ALK1, and a vascular marker, may be helpful.16,17
Epithelioid sarcoma is usually hypercellular and arranged as predominantly dispersed individual cells with rare disorganized cell clusters. Tumor cells are epithelioid with eccentric nuclei with prominent nucleoli and dense cytoplasm. The cytoplasm exhibits occasional intracytoplasmic vacuoles and a paranuclear dense zone.8 Brisk mitotic activity and necrosis are frequent.18 It has a characteristic clinical manifestation and predominantly occurs in young adults, with the most common location in the distal part of the upper extremities. Immunostaining for CD31 and CD34 may be positive in epithelioid sarcoma, and therefore, combined immunostaining of factor VIII, vimentin, cytokeratin, and EMA is also helpful in determining the diagnosis.14
Epithelioid hemangioendothelioma is arranged as predominantly dispersed individual cells with occasional cell clusters. It exhibits vasoformative features like intracytoplasmic vacuoles, erythrophagocytosis, hemorrhagic background and positive reaction to vascular markers, similar to angiosarcoma with predominantly epithelioid features. Although the cytologic features of epithelioid hemangioendothelioma may overlap with those of angiosarcoma with predominantly epithelioid features, the predominance of individually dispersed cells, lower cellularity, intranuclear cytoplasmic inclusions, insignificant nuclear atypia, and lower mitotic activity are considered the main differences in comparison to angiosarcoma with predominantly epithelioid features.18
In conclusion, the LBP of angiosarcoma with predominantly epithelioid features may exhibit vasoformative features and erythrophagocytosis. However, the other diagnostic clues described in CP, such as a hemorrhagic background and nuclear hyperchromasia, were not found. Knowledge of the cytopathologic findings in angiosarcoma with predominantly epithelioid features and identification of vacuoles with peculiar shapes, juxtanuclear condensation and perinuclear clearing may be helpful in reaching the correct diagnosis by LBP. Nevertheless, when it is difficult to differentiate epithelioid lesions morphologically, immunostaining of LBP specimens may provide another important clue for differential diagnosis by cytopathologists.
Notes
No potential conflict of interest relevant to this article was reported.