Alveolar Soft Part Sarcoma of the Uterine Cervix: A Case Report and Review of the Literature
Article information
Abstract
Alveolar soft part sarcoma (ASPS) of the uterine cervix is a rare malignancy, and 21 cases have been reported the literature from every language (including our case). Herein, we describe a 17-yearold female patient who presented with active vaginal bleeding. Pelvic examination revealed a 1.6 ×1.0×0.5-cm-sized soft mass protruding from the uterine cervix. The final pathological diagnosis was ASPS of the uterine cervix. Immunohistochemically, tumor cells were strongly nuclear positive for transcription factor E3. The patient remained disease free for 24 months without adjuvant therapy. The prognosis of ASPS in the cervix is considerably better than that of ASPS in soft tissues due to early clinical detection, small size, and resectability. ASPS should be considered in the differential diagnosis of an unusual epithelioid neoplasm showing organoid appearance with mild cytologic atypia and no/rare mitotic figures, particularly in young women. Pathologists should be aware of those unusual locations where ASPS may originate.
Alveolar soft part sarcoma (ASPS) is a rare mesenchymal malignancy accounting for 0.5–1% of all soft part sarcomas[1]. It affects mainly adolescents and young adults in the second and third decade of life and there is a slight female predominance. In adults, ASPS occurs most commonly in the deep soft tissues of the thigh or buttock while the head and neck regions are often involved in children and infants[1].
ASPS also occurs in the female reproductive tract, where fewer than 1% of all cervical sarcomas can be classified as ASPS[2,3]. ASPS of the uterine cervix is an extremely rare malignancy, and 21 cases have been reported in the literature from every language (including our case)[2-11]. To the best of our knowledge, this is the third case of a patient with such presentation in Korea[4,9]. Herein, we report a case of ASPS that originated from the uterine cervix, along with morphological and immunohistochemical features, clinical aspects, and the detection of nuclear immunoreactivity for transcription factor E3 (TFE3). Additionally, we provide a review of the literature.
CASE REPORT
We describe a 17-year-old female patient, gravida 0, who presented with active vaginal bleeding for three days with severe anemia after coitus. Her past medical and familial histories were unremarkable. Pelvic examination revealed a protruding 1.6× 1.0×0.5-cm-sized, soft mass in the lateral wall of the uterine cervix. Computed tomography examination revealed a mass lesion, 1.6 cm in diameter, mainly located in the vagina originating in the uterine cervix (Fig. 1) and no evidence of distant metastasis. The initial clinical diagnosis was a cervical myoma. Cervical cytology was negative and the results of routine laboratory studies were unremarkable, except for a decreased hemoglobin and hematocrit level. The hemoglobin level was 5.1 g/dL (normal range, 12 to 16 g/dL), and the hematocrit level was 16.4% (normal range, 37% to 47%). Tumor markers cancer antigen (CA) 125, CA19-9, and carcinoembryonic antigen were all within normal limits. A transvaginal cervical myomectomy was carried out. During the procedure, the cervical mass was fragile and hemorrhage was encountered. The surgically-resected tumor was well-circumscribed and located beneath the surface mucosa. The tumor cells were relatively uniform in appearance and exhibited a solid nested architectural pattern (Fig. 2A). The nests of cells were separated by thicker fibrous septa and stromal hyalinization. The tumor was composed of large polygonal cells with distinct cell borders and abundant eosinophilic cytoplasm. The round to oval nuclei often contained single prominent nucleoli (Fig. 2B). The chromatin was uniformly arranged and the nuclear pleomorphism was mild to moderate. Neither mitotic figures nor necrosis were observed. The nucleus/cytoplasm (N/C) ratio was not high due to abundant eosinophilic cytoplasm. No vascular invasion was present. Periodic acid–Schiff (PAS) and PAS with diastase staining showed few granules and no rod-shaped crystals (Fig. 3A, B). Immunohistochemically, the tumor cells stained diffusely nuclear positive for TFE3 (Fig. 3C), positive for MyoD1 (Fig. 3D), and focal positive for smooth muscle actin (SMA), neuron specific enolase (NSE), and myoglobin. The Ki-67 proliferating index was 3%. In addition, ASPS was negative for epithelial markers (cytokeratin [CK], high-molecular weight cytokeratin [HMWCK], CK8/18), neuroendocrine markers (CD56, chromogranin A, synaptophysin), melanocytic markers (human melanoma black 45 [HMB45], S-100), myogenic markers (desmin, myogenin), germ cell tumor markers (alpha-feto protein [AFP], CD30, human chorionic gonadotropin [HCG], placental-like alkaline phosphatase [PLAP], CD10), vimentin, and CD34. The final pathological diagnosis was ASPS of the uterine cervix.
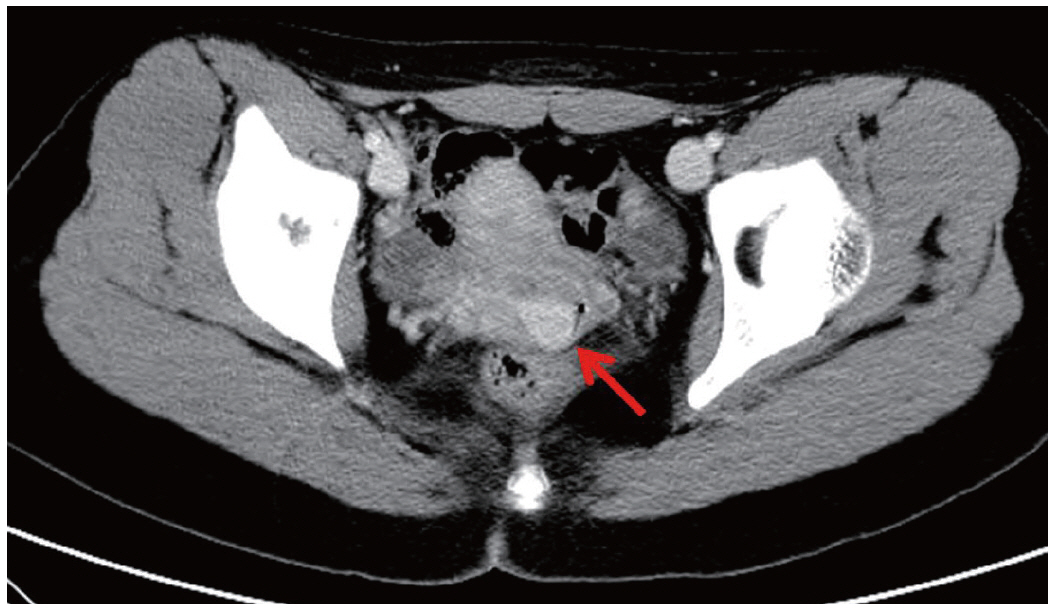
Computed tomography examination reveals a mass lesion (red arrow), 1.6 cm in diameter, mainly located in the vagina originating in the uterine cervix.
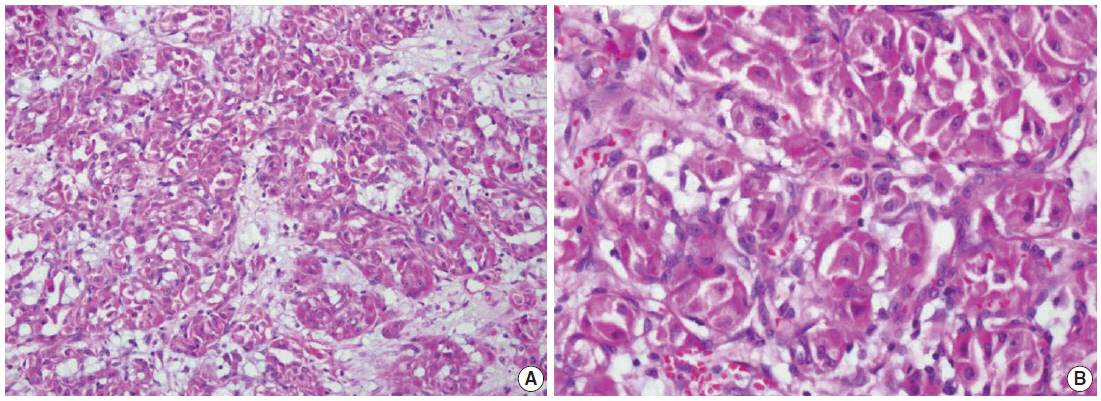
Representative histologic features of an alveolar soft part sarcoma. (A) Solid nest arrangement with stromal hyalinization. (B) Groups of tumor cells are outlined by delicate connective tissue septa. The tumor cells show prominent nucleoli and abundant eosinophilic cytoplasm. No mitotic activity is found.
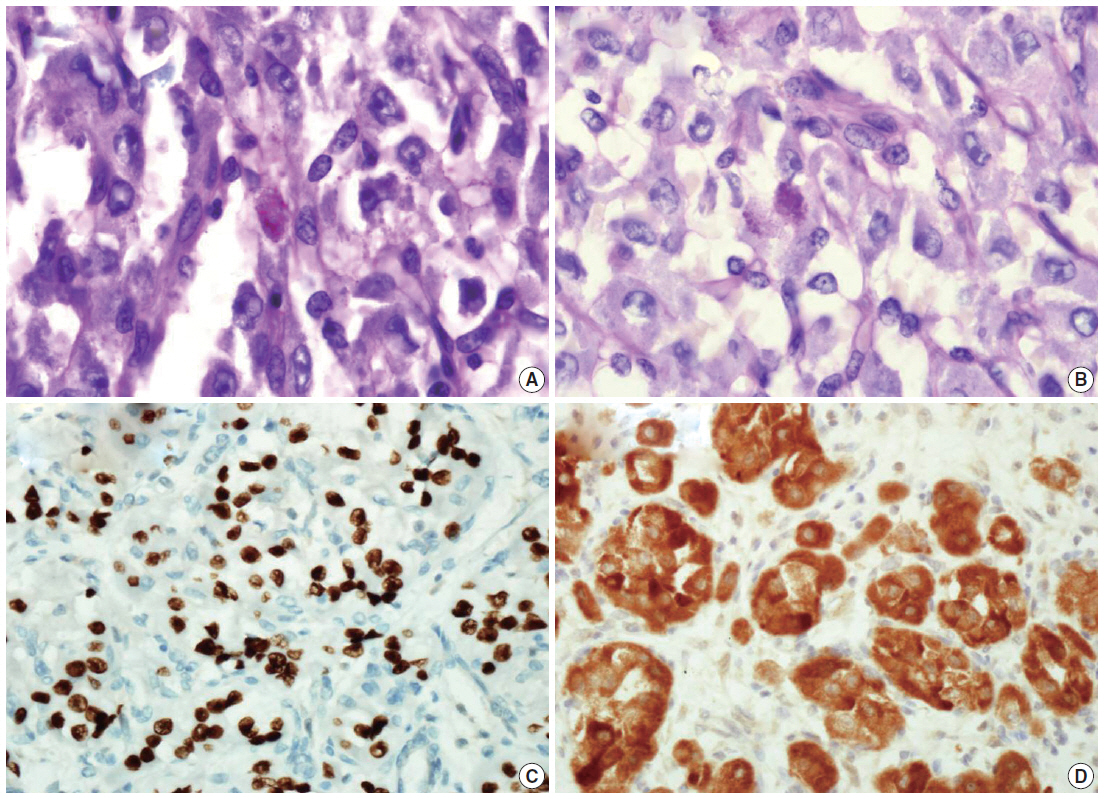
Periodic acid–Schiff (A) and periodic acid–Schiff with diastase (B) staining shows only poorly defined granules in the cytoplasm of the tumor cells. Immunohistochemically, the tumor cells are strongly nuclear positive for transcription factor E3 (C), positive for MyoD1 (D).
The patient was readmitted for a wide excision of the cervix and further examination after the surgery. There were no metastatic lesions in the lymph nodes, uterine cavity, ovary, or lung. The patient has remained disease free for 24 months without adjuvant therapy.
DISCUSSION
ASPS originating from the uterine cervix is exceedingly rare, with only 20 cases previously reported in the literature of all languages[2-11]. Involvement of the uterine cervix is unusual, although the uterine cervix is the most common site if ASPS occurs in the female genital tract[2].
The age of patients with cervical ASPS ranges from 8 to 57 years, with an average age of 32.4 years. Abnormal uterine bleeding is the most common clinical symptom of cervical ASPS. The tumor size ranges from 0.16 cm to 8 cm with an average of 2.49 cm and generally has a well-circumscribed boundary.
Microscopically, cervical ASPS display a classical appearance characterized by tumor cells forming distinct nests often with central necrosis or loss of cohesion. This causes a pseudoalveolar appearance composed of large polygonal cells with granular eosinophilic cytoplasm, vesicular nuclei, prominent nucleoli, particularly no/rare mitotic figures, and minimal pleomorphism. ASPS are also characterized by a solid growth pattern[1]. Vascular invasion is a constant and striking finding in ASPS of soft tissue, but vascular invasion is not found in cervical ASPS.
Mild cytologic atypia, low mitotic activity, absence of necrosis, and abundant eosinophilic cytoplasm (low N/C ratio) usually suggest a benign nature and are findings reminiscent of a decidualized polyp of the endometrial stroma or luteinized granulosa cells of the corpus luteal cyst. However, there are some differences in morphology and location between the tumor cells and luteinized granulosa cells of a corpus luteal cyst or decidualized polyp of the endometrial stroma. Although this case showed no mitotic activity and mild nuclear pleomorphism, the marked prominent macronucleoli could have raised the potential for malignancy
Although ASPS has a characteristic light microscopic appearance, in some cases it can be mistaken for other neoplasms. In the uterine cervix, the differential diagnosis includes endocervical clear cell carcinoma, glassy cell carcinoma, sarcoma, malignant melanoma, neuroendocrine tumor, paraganglioma, malignant granular cell tumor, PEComa (perivascular epithelioid cell tumor), parachordoma, yolk sac tumor, metastatic clear cell renal cell carcinoma, alveolar rhabdomyosarcoma, metastatic hepatocellular carcinoma, and adrenal cortical carcinoma.
Of the 21 reported cases[2-11], 11 (52%) showed PAS-positive diastase-resistant crystals. PAS-positive diastase-resistant granules were identified in 16 cases (76%). In our case, well defined crystals were not prominently identified and only poorly defined granules were demonstrated in the cytoplasm of the tumor cells. The organoid structure and granular PAS-positive and diastaseresistant cytoplasm were the hallmarks of this tumor. In a series by Enzinger and Weiss, the intracytoplasmic granular material was interpreted as possible crystal precursors[1]. While highly characteristic and virtually diagnostic, the PAS-positive, diastase-resistant crystals are not observed in every case[1].
Immunohistochemical staining was performed with variable results in the reported cases, usually in an attempt to rule out other tumors in the differential diagnosis (Table 1)[4,7-10]. Cervical ASPS showed 100% nuclear positive staining for TFE3 in six cases and 100% positivity for MyoD1 in five cases[7-10]. Cervical ASPS was sometimes positive for myoglobin (4/6, 67%), SMA (4/8, 50%), desmin (3/9, 33%), NSE (5/5, 100%), S-100 protein (2/10, 20%), CK8/18 (1/3, 33%), vimentin (2/8, 25%), HMB45 (2/7, 29%), and CD34 (1/2, 50%). Cervical ASPS were typically negative for epithelial markers, such as cytokeratin (9 cases), HMWCK, (1 case), and epithelial membrane antigen (3 cases), negative for neuroendocrine markers, such as synaptophysin (4 cases), chromogranin A (5 cases), and CD56 (2 cases), and negative for CD10 (3 cases). Tumors were also negative for glial fibrillary acidic protein.
Recently, the discovery of an unbalanced X; 17 chromosome translocation resulting in a fusion of the ASPL gene on chromosome 17 to the TFE3 (transcription factor 3) gene on chromosome X, translocation (X;17) (p11;q25), has been demonstrated in ASPS[12]. It is assumed that the detection of this fusion gene by fluorescence in situ hybridization, reverse transcriptase-polymerase chain reaction as well as immunohistochemical staining for TFE3 is useful for the diagnosis of ASPS due to its high sensitivity (97.5%) and specificity (99.6%)[13]. Three cases of cervical ASPS showed an unbalanced X; 17 translocation (X;17) (p11; q25)[8,10].
The first treatment of choice for cervical ASPS is a radical excision of the mass, typically by hysterectomy. Of the 21 reviewed cases[2-11], 18 received a follow-up ranging in time from 6 months to 6 years (average, 37 months). Metastasis or recurrence was seen in only one case. This suggests a good prognosis once the lesion has been completely resected.
The relatively favorable prognosis of ASPS in the uterine cervix might reasonable due to the relatively small tumor size (<5 cm) of the tumors in almost all cases and the fairly early detection due to bleeding. Higher age of the patient, larger tumor size, and obviously metastatic disease at presentation have been reported as poor prognostic factors[1]. The role of chemotherapy has not yet been defined. A lifelong clinical follow-up is required due to the possibility of late metastases.
In conclusion, ASPS may rarely occur in the uterine cervix and should be considered in the differential diagnosis of an unusual epithelioid neoplasm showing organoid appearance with mild cytologic atypia and no/rare mitotic figures, particularly in young women. Pathologists should be aware of those unusual locations where ASPS may originate.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the Soonchunhyang University Research Fund.
