Indeterminate Dendritic Cell Tumor: A Case Report of a Rare Langerhans Cell Lineage Disease
Article information
Indeterminate dendritic cell tumor (IDCT) is a very rare neoplastic disorder composed of so-called indeterminate cells [1]. Although it was first described in the early 1980s [2], its pathogenesis and cellular origin have not been completely defined. These indeterminate cells morphologically and immunophenotypically resemble Langerhans cells, as they are immunopositive for S100 protein and CD1a. However, they typically lack both Birbeck granules and langerin expression [1,3].
Due to its rarity and similarity to Langerhans cell lesions (LCL), IDCT can be misdiagnosed; however, the pathobiology of IDCT is different from that of LCL. Even though the clinical behavior of IDCT has not yet been firmly established, no direct mortality due to IDCT has been reported so far [1,4]. Recently, we experienced a case of IDCT with typical histologic features. This is the first reported case of IDCT in Korea, which pathologists should include in the differential diagnosis of Langerhans cell-like lesions.
CASE REPORT
A 29-year-old female patient presented with a 1.2-cm, ill-defined, erythematous subcutaneous nodule on the left flank (Fig. 1A), which appeared a month ago and increased in size over time. She had no cutaneous or extracutaneous symptoms. The patient was otherwise healthy with no remarkable medical history. Skeletal survey and bone scan revealed no bone lesions.
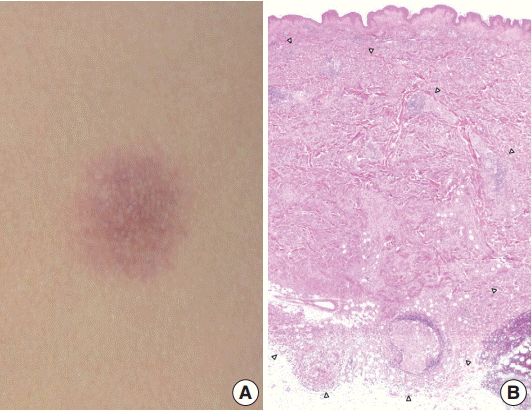
(A) An ill-defined, 1.2-cm, round, erythematous subcutaneous nodule on the left flank. (B) The ill-defined tumor is mainly located in the dermis and subcutis. The margin of the tumor is marked by arrowheads.
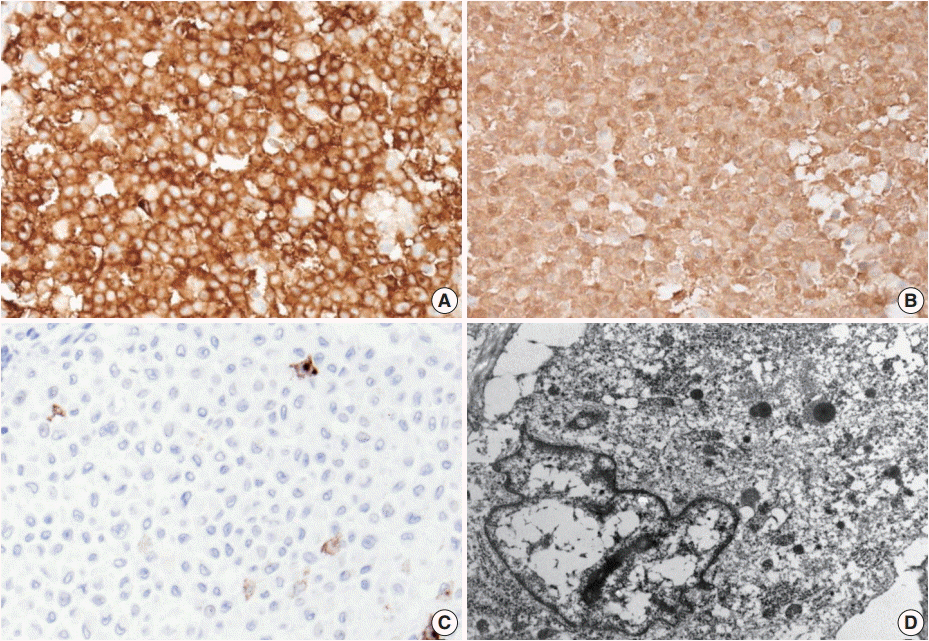
Excision of the nodule was performed. Microscopically, the lesion showed poorly circumscribed infiltrates of tumor cells forming mostly solid sheets in the dermis and subcutis (Fig. 1B). No necrosis or adnexal invasion was noted. A small amount of peritumoral inflammatory cell infiltration, composed mostly of lymphocytes, was observed. Eosinophils were notably absent (Fig. 2A). Tumor cells showed monotonous morphology with indistinct cell borders. They had moderately abundant and lightly eosinophilic cytoplasm (Fig. 2B). The tumor cells had enfolded or reniform vesicular nuclei with inconspicuous nucleoli, and some of the tumor cells showed longitudinal nuclear grooves. Although occasional neoplastic cells had a large nucleus, mitotic figures were rarely seen (Fig. 2C). Characteristically, there was no epidermal involvement by tumor cells (Fig. 2D). The neoplastic cells in the tumor were diffusely immunopositive for S100 and CD1a (Fig. 3A, B). Langerin (CD207) was clearly negative in the tumor cells. Scattered langerin-positive bystander Langerhans cells serve as internal positive controls (Fig. 3C). Despite intensive scrutiny, no Birbeck granules were identified on electron microscopy (Fig. 3D).
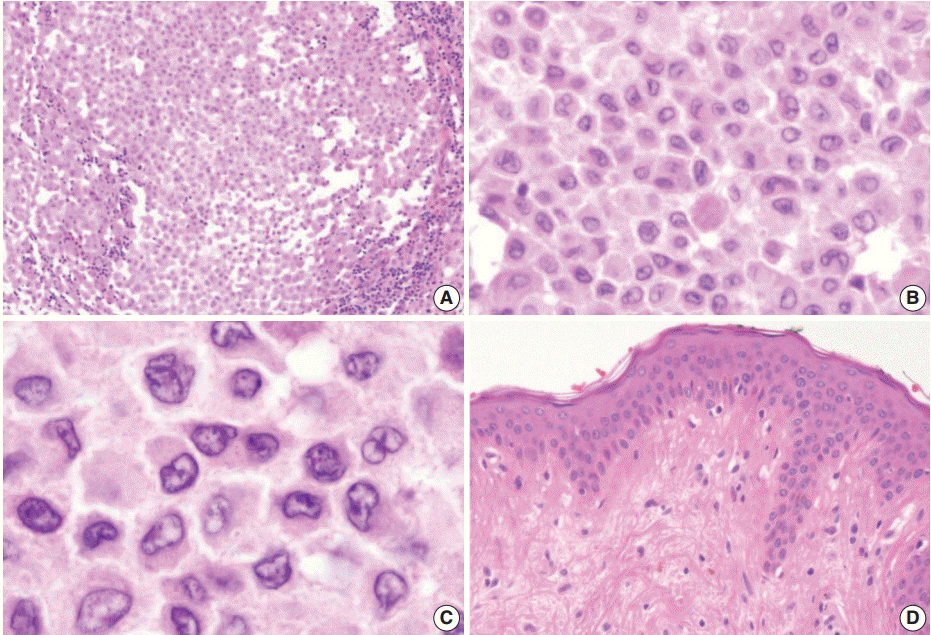
(A) There is patchy infiltration by aggregates of the tumor cells. Tumor cells are usually monotonous and have indistinct cell borders. Clusters of lymphocytes are admixed with tumor cells in focal areas without eosinophils. (B) The constituent cells have ovoid cell morphology with abundant eosinophilic cytoplasm. (C) Their nuclei are oval and sometimes indented. Nuclear grooves are frequently seen. Although occasional enlarged nuclei are identified, mitoses are rarely seen. (D) There is no epidermal involvement.
DISCUSSION
In this paper, we report a case of IDCT, an extraordinarily rare neoplasm. Most IDCTs occur in adults without predilection for either sex [3,5]. IDCT is almost always restricted to the skin without systemic symptoms [2,6]. Its rarity and resemblance to LCL often cause as diagnostic challenge for pathologists who are not familiar with this entity. Uniform expression of CD1a and S100 protein enables distinction of IDCT from other forms of non-Langerhans cell neoplasms, such as juvenile xanthogranuloma, xanthoma, histiocytic sarcoma, and reticulohistiocytosis [7]. The differential diagnosis also includes Langerhans cell lineage tumors, such as Langerhans cell histiocytosis (LCH) and Langerhans cell sarcoma (LCS). LCH is a rare condition and mostly occurs in childhood [7]. In cutaneous LCH, the lesions present as papules or plaques [3]. Tumor cells show typical morphology of Langerhans cells, which are indistinguishable from those of IDCT [5]. However, unlike IDCT, LCH shows evident epidermotropism with intraepidermal Langerhans cell microabscesses [3]. Presence of eosinophilic infiltration is another important difference from IDCT [3]. Immunopositivity for S100 protein, CD1a, and langerin (CD207), and the presence of Birbeck granules confirm the diagnosis of LCH [2]. Although LCH shows variable outcomes, most of the cases require long-term follow-up. LCS is another important tumor in the differential diagnosis. LCS is a very rare high-grade neoplasm [8]. Unlike in IDCT, epidermotropism and eosinophil infiltration are not usually evident in LCS [5]; however, most of the tumor cells show overtly malignant cytology including pleomorphic and hyperchromatic nuclei with frequent mitotic figures [8]. Langerhans cell differentiation of neoplastic cells of LCS must be confirmed by immunohistochemistry (IHC) or electron microscopy. LCS is an aggressive neoplasm and has very poor prognosis [8]. In the present case, langerin (CD207) immunonegativity of the tumor cells and the absence of Birbeck granules on electron microscopy were crucial for definitive diagnosis of IDCT [5].
Regarding the utility of IHC in comparison with electron microscopy, langerin IHC is important in the differentiating between true Langerhans cells and Langerhans cell-like lesions [2]. Langerin is an antibody to a transmembrane C-type lectin that associates with Birbeck granule formation. Previous studies revealed high sensitivity and specificity of langerin for Langerhans cell differentiation [9,10].
In summary, IDCT should be included in the differential diagnosis for a lesion composed of Langerhans cell-like cells without epidermotropism and eosinophilic infiltration. To our knowledge, the present study is the first report of IDCT in Korean pathologic literature. Making an accurate diagnosis of IDCT is important to avoid overly aggressive management.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The authors thank Dr. John K.C. Chan, Department of Pathology at Queen Elizabeth Hospital, HK for his expert diagnostic opinion and the courtesy of performing the langerin immunohistochemistry.