The Continuing Value of Ultrastructural Observation in Central Nervous System Neoplasms in Children
Article information
Abstract
Central nervous system (CNS) neoplasms are the second most common childhood malignancy after leukemia and the most common solid organ neoplasm in children. Diagnostic dilemmas with small specimens from CNS neoplasms are often the result of multifactorial etiologies such as frozen or fixation artifact, biopsy size, or lack of knowledge about rare or unfamiliar entities. Since the late 1950s, ultrastructural examination has been used in the diagnosis of CNS neoplasms, though it has largely been replaced by immunohistochemical and molecular cytogenetic studies. Nowadays, pathologic diagnosis of CNS neoplasms is achieved through intraoperative cytology, light microscopy, immunohistochemistry, and molecular cytogenetic results. However, the utility of electron microscopy (EM) in the final diagnosis of CNS neoplasms and investigation of its pathogenetic origin remains critical. Here, we reviewed the distinguishing ultrastructural features of pediatric CNS neoplasms and emphasize the continuing value of EM in the diagnosis of CNS neoplasms.
The general incidence of pediatric central nervous system (CNS) neoplasms is as follows: the most common type is astrocytomas including pilocytic astrocytomas, fibrillary astrocytomas, or brain stem gliomas (46%–48%); followed by medulloblastomas (40%–45%); ependymomas (8%–10%); and others, including germ cell tumors, gangliogliomas, sellar craniopharyngiomas, etc. (2%–5%) [1,2]. Commonly encountered CNS neoplasms in adults, such as oligodendrogliomas, high-grade astrocytic tumors, or meningiomas, are relatively uncommon in children, except for meningiomas with or without neurofibromatosis syndrome or meningioangiomatosis or CNS lymphomas in immunocompromised children with acquired immunodeficiency syndrome or Wiskott-Aldrich syndrome [3-5]. Treatments for adults differ from those for children due to the unpredictable clinical course and a the greater hazardous effect of aggressive treatments such as radiation on the developing brain. Similar to differences in primary CNS neoplasms between children and adults, metastatic CNS neoplasms in children commonly originate from neuroblastoma, embryonal rhabdomyosarcoma, Wilms tumor, or malignant melanoma arising from neurocutaneous melanosis, whereas cerebral metastatic tumors in adults typically originate from lung, breast, or colon cancers, renal cell carcinoma, or malignant melanomas [6,7].
Regarding incidence, the spectrum of pediatric CNS neoplasms varies, from primitive embryonal tumors to highly differentiated tumors such as meningioma, schwannoma, or glioblastoma [8]. The former group is represented by small round cell tumors, i.e., undifferentiated or poorly differentiated tumors. The 2007 World Health Organization (WHO) classification of CNS neoplasms included angiocentric glioma, papillary glioneuronal tumor (PGNT), rosette-forming glioneuronal tumor of the fourth ventricle, papillary tumor of the pineal region (PTPR), pituicytoma, and spindle cell oncocytoma of the adenohypophysis, which are rarely encountered in either children or adults [9].
The role of electron microscopy (EM) in diagnostic pathology has declined over recent decades due to major advancements in immunohistochemistry, flow cytometry, and molecular cytogenetic analysis, thereby eliminating the need for EM. However, its role in the neuropathology field includes identification of etiologic agents or cellular stored materials or for use as an ancillary method for CNS neoplasms for which light microscopy or immunohistochemistry are inconclusive and ambiguous [10,11]. Although benignity and malignancy cannot be distinguished by EM, pediatric CNS neoplasms require the integration of comprehensive data from light microscopic examination with immunohistochemical, EM, and molecular cytogenetic studies. Actually, starting with the 2000 WHO classification of CNS neoplasms, genetic profiles have been incorporated as an additional tool used in the definition of brain tumors because molecular cytogenetic evaluation has both diagnostic and predictive utility [12]. For example, emerging data from cytogenetic and molecular genetic analyses suggest that some molecular cytogenetic alterations such as isocitrate dehydrogenase-1 (IDH-1) or 1p19q loss of heterozygosity (LOH) provide both diagnostic and prognostic data on CNS neoplasms though IDH-1 mutation or 1p19q LOH are rarely found in pediatric oligodendrogliomas, compared to 40%–80% of adult cases [4,13,14]. IDH-1 mutation-specific immunohistochemistry is diagnostically helpful in recognizing diffuse tumor infiltration of astrocytoma or oligodendroglioma and to distinguishing WHO grade I pilocytic astrocytomas from diffuse astrocytomas as well as astrocytic and oligodendroglial tumors from ependymomas or oligodendrogliomas from other glioneuronal tumors with clear-cell morphology [14]. However, it has diagnostic limitations in terms of specificity or sensitivity, and further evaluation of other diagnostic tools might be needed.
Here, we review the ultrastructural findings and categorize the EM findings of pediatric CNS neoplasms based on light microscopic morphology as follows: spindle cell tumors, round cell tumors including oligodendroglioma and oligodendrogliomalike tumors, papillary tumors, rhabdoid cell tumors, and pleomorphic cell tumors. Although not uncommonly encountered in pediatric CNS neoplasms, germ cell neoplasms showing characteristic light microscopic findings such as germinoma, teratoma, choriocarcinoma, embryonal carcinoma, or endodermal sinus tumor will not be described in this review.
SPINDLE CELL TUMORS OF THE CENTRAL NERVOUS SYSTEM
First, the spindle cell category includes the following entities: meningioma, schwannoma, ependymoma, and astrocytoma including pilocytic astrocytoma, and pilomyxoid astrocytoma. Among these, ependymoma and pilocytic astrocytoma are common in children, while meningioma or schwannoma are not common. Rather, meningioma associated with meningioangiomatosis has been reported mainly in childhood [5]. Previous reports on meningioma arising in meningioangiomatosis describe entrapped neurons in the infiltrating meningioma [15]. Ultrastructural findings of meningioma show its meningothelial arachnoidal cell nature, i.e., epithelial and mesenchymal nature; connective tissue fibers and basal lamina are not usually seen among tumor cells within the syncytium (Fig. 1A). Elongated interdigitating cell processes filled with copious amounts of intracytoplasmic intermediate filaments show an interdigitating and jigsaw pattern, corresponding to the whorls of enwrapping meningioma tumor cells under light microscopy. The epithelial nature, such as well-formed desmosomes, is a characteristic ultrastructural feature. The extracellular space of meningioma sometimes contains fine granular materials, mimicking basal lamina. Meningiomas also show basement membrane separating the syncytium from the fibrous septum or the perivascular connective tissue [16]. Rhabdoid or chordoid meningioma can have a thick continuous basal lamina [17-19]. Schwannoma, also called neurilemmoma, neuroma, or neurinoma, is relatively rare in the brain with or without cranial nerve-relation. Intracerebral schwannomas not related to cranial nerves are rare, and most cases occur in the first two decades of life [20]. Schwannoma can be diagnosed in children who might be associated with neurofibromatosis type 2, while intracerebral schwannomas have a weak relationship with neurofibromatosis type 2. The ultrastructure of schwannoma is almost exclusively cells with characteristics of differentiated Schwann cells of a neuroectodermal origin. Numerous finger-like cytoplasmic processes are lined by continuous basal lamina with occasional duplication (Fig. 1B). Closely apposed stacks of plasma membrane, i.e., reminiscent of myelin lamellae, are characteristic of schwannoma. Compared to normal collagen fibers of 64-nm periodicity, so-called Luse bodies show more widely spaced collagen fibers with up to 150-nm periodicity and are frequently found in schwannoma. The tumor cells themselves are composed of cigar-shaped or elliptical nuclei containing one or two small nucleoli and rare nuclear bodies. The cytoplasm contains well-developed Golgi complexes, scattered mitochondria, short segments of rough endoplasmic reticulums (RERs), small numbers of ribosomes, and scattered polysomes. Absence of pinocytotic vesicles always happens in schwannoma. Microfilaments, microtubules, and small vesicles are often found, but few mitochondria and ribosomes are found. These ultrastructural characteristics of schwannoma provide diagnostic clues, particularly regarding small cell changes in schwannoma or small cell malignant peripheral nerve sheath tumors [21,22]. Ependymoma occurs mainly in the ventricular system, and infratentorial ependymomas occur predominantly in children, although development of ependymomas can occur at any age [23-25]. Ultrastructural findings of a well-developed ependymal tumor, which is likely derived from ependymal cells lining the CNS ventricular system, show both epithelial and astrocytic features, and the tumor cells of grade I and II ependymal tumors resemble typical ependymocytes, while anaplastic ependymomas are poorly differentiated with minimal evidence of such ependymal differentiation. Ependymal differentiation includes well-developed junctions, such as zonula adherens or occludens, villous projections, and cilia in a 9+2 arrangement of microtubules (Fig. 1C). The centriole or blepharoplast is located in the basis of the cilia; in the cytoplasm, microtubules or glial filaments are present in the perikaryal area or cytoplasmic processes. Basal lamina is observed in the ependymoma and around blood vessels but not around tumor cells [26]. However, all of the above-mentioned findings are not specific for ependymomas; these ultrastructural findings are shared by angiocentric glioma, i.e., monomorphous angiocentric neuroepithelial tumor, composed of bipolar spindle cells and epithelioid round cells although the occurrence of angiocentric glioma has not yet been reported in children [9]. Angiocentric glioma is composed of infiltrating round, monopolar epithelioid cells arranged in a perivascular arrangement and bipolar spindle-shaped glial cells. Epithelioid monopolar cells showing microlumina filled with numerous microvilli and some cilia with a 9+2 or abnormal 10+2 configuration. Intermediate junctions of complex interdigitating membranes and basal lamina have also been observed [27]. Ependymomas and angiocentric glioma share molecular cytogenetic alterations, as indicated by shared ultrastructural features [28]. These findings suggest that angiocentric glioma belongs to a lineage of ependymal tumors.
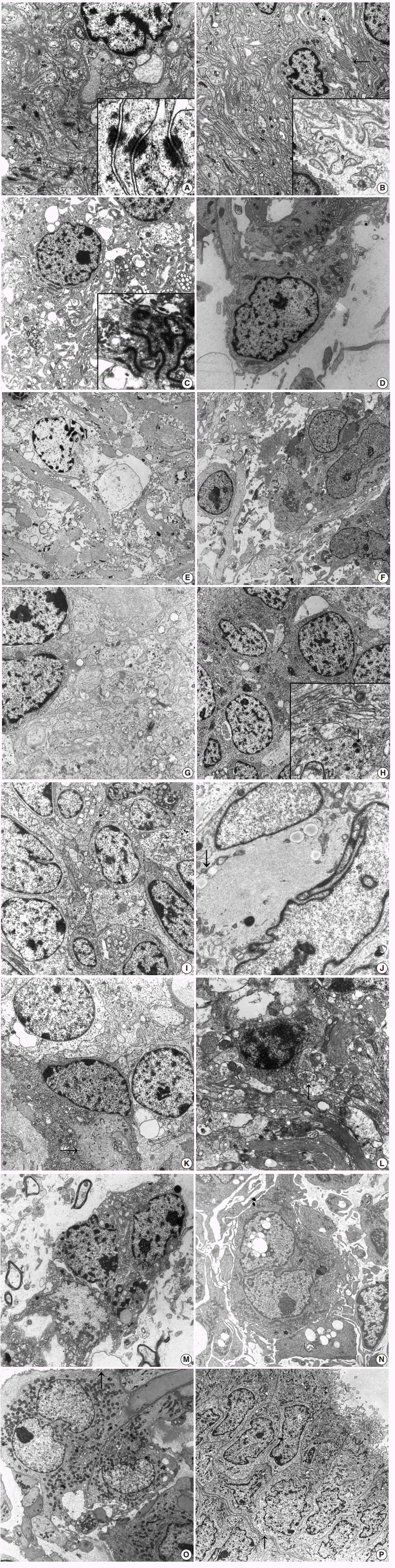
(A) Meningioma shows closely apposed oval- to spindle-shaped tumor cells having interdigitating cell processes lined by well-formed desmosomes (×8,000). Inset indicates tonofibrils attached to the desmosomal plaques (×30,000). (B) Schwannoma shows abundant reduplicated continuous basal lamina surrounding the interdigitating cell processes (×3,500). Note the extracellular long-spacing collagen (arrow) and high power view of reduplicated basal lamina (inset, ×6,500). (C) Ependymoma reveals spindle cells with numerous cytoplasmic processes filled with bundles of intermediate filaments (×4,000). Inset shows well-formed zonula occludens with surface microvilli (×15,000). (D) Pilomyxoid astrocytoma shows bipolar cells with surface microvilli and cilia in the electron-lucent extracellular space (×9,000). Note some intracellular microlumen. (E) Astrocytoma shows loosely scattered round to oval cells having numerous long cytoplasmic processes filled with bundles of glial filaments and scarce other organelles (×2,500). (F) Glioblastoma shows many pleomorphic spindle and oval cells with numerous cytoplasmic processes containing bundles of intermediate filaments with surface microvilli-like differentiation (×2,500). (G) Medulloblastoma shows that loosely arranged oval-shaped tumor cells project cytoplasmic processes forming rosettes filled with glial filaments and dense core granules indicating neuroglial differentiation (×9,000). (H) Neuroblastoma reveals that closely packed polygonal to spherical tumor cells have numerous, thin, electron-lucent cell processes forming an interlacing meshwork between groups of cell bodies (×9,000). Inset shows longitudinally-oriented 20 nm microtubules (white arrow) and dense core granules (black arrow, ×15,000). (I) Primitive neuroectodermal tumor reveals round to closely apposed oval-shaped tumor cells with abundant cytoplasmic processes containing sparse organelles including glycogen particles, mitochondria, and some microtubules (×8,000).(J) Atypical teratoid and rhabdoid tumor reveals paranuclear aggregates of intermediate filaments (arrow) compressing the heterochromatic nuclei (×15,000). (K) Oligodendroglioma presents tumor cells with uniform round nuclei and sparse cytoplasmic organelles as well as some irregularly shaped cell processes (arrow) containing occasional globoid collections of intermediate filaments (×3,500). (L) Neurocytoma reveals round tumor cells with a moderate amount of cytoplasm and numerous long thin cell processes containing microtubules, few electron dense core granules and secretory vesicles (arrow), and glial intermediate filaments (×8,000). (M) Dysembryoplastic neuroepithelial tumor reveals oligodendroglial-like cells with elongated bulbous cell processes forming a neuropil-like structure filled with intermediate filaments in electron-lucent mucoid extracellular spaces (×7,000).(N) Papillary tumor of the pineal region reveals polygonal-shaped tumor cells having short-villous cell surfaces and cytoplasm having abundant rough endoplasmic reticulums with distended cisternae and vacuoles (×3,500). (O) Chordoid glioma of the third ventricle reveals tumor cells with cytoplasmic processes filled with intermediate glial filaments and surrounded by basal lamina (×7,000). Note the numerous surface microvilli (arrow). (P) Choroid plexus papilloma shows ovoid to polygonal tumor cells arranged in glandular patterns joined by well-formed junctional complexes and a continuous basal lamina (arrow) surrounding these glandular structures (×1,500). Note the apical portion of tumor cells lined by numerous microvilli and cilia composed of 9+2 microtubules.
Pilocytic astrocytoma is a commonly encountered CNS tumor in children, and the most common site of occurrence is the sellar region. In contrast, pituitary adenoma, meningioma, and metastatic carcinoma are common tumors in adults but are rarely encountered in children.1 Pilomyxoid astrocytoma and pilocytic astrocytoma share pathogenetic and clinicopathologic features; BRAF oncogene activation through KIAA1549-BRAF fusion most commonly found in pilocytic astrocytoma has also been described in pilomyxoid astrocytoma [29,30]. As with light microscopy, irregularly shaped, amorphous, granular electrondense Rosenthal fibers are commonly found in pilocytic astrocytoma under EM [31]. These Rosenthal fibers are closely located in the glial intermediate filaments [32]. Pilomyxoid astrocytoma showing a monomorphic histologic appearance more commonly occurs in infants and young children compared with pilocytic astrocytoma [33]. Ultrastructurally, pilomyxoid astrocytomas have two morphologically distinct cell types [34]; one is spindle cells showing neurite-like features, and the other is spindle cells showing overt astrocytic morphology (Fig. 1D). Despite shared histologic and molecular cytogenetic findings, pilomyxoid astrocytoma relating to pilocytic astrocytoma and other glioneuronal tumors remain undefined and controversial. With progression of WHO grade in astrocytic tumors, there are sparser glial filaments and more irregular microvillus-like cell projections in anaplastic astrocytoma or glioblastoma, compared to diffuse astrocytoma (Fig. 1E, F). Pediatric glioblastomas are rarely encountered [35-37]. Excluding components of gliosarcoma, pediatric brain sarcomas are rare. However, various types of primary intracranial sarcomas can occur in the absence of radiation history [38,39]. Although rare cases of rhabdomyosarcoma or chondrosarcoma have also been described, most reported cases in children are fibrosarcomas [40]. Proliferation of spindle cells resembling meningioma on immunohistochemistry and light microscopy but with a classical herring-bone pattern suggests fibrosarcoma. Fibrosarcoma is composed of fibroblast-like spindle cells with well-developed, abundant dilated RERs and prominent Golgi complexes [41]. Small patches of basal lamina materials lining some tumor cell surfaces are observed in rare cases. Features of myoid differentiation have not been observed.
SMALL ROUND CELL CATEGORY OF PEDIATRIC CENTRAL NERVOUS SYSTEM TUMORS
In this group, small round cell pediatric CNS tumors include medulloblastoma, CNS primitive neuroectodermal tumor (PNET)/embryonal tumor with abundant neuropils and true rosettes, atypical teratoid and rhabdoid tumor (AT/RT), and neuroblastoma [2,42-48]. Medulloblastoma is a common malignant primary brain tumor in children, categorized as a PNET of the cerebellum, i.e., an undifferentiated tumor exhibiting classic undifferentiated multipotent tumor cells showing myoblastic, neuronal, glial, and melanotic differentiation observed under light microscopy, immunohistochemistry, and EM [48]. A broad spectrum of neural differentiation has been identified in nearly all cases of medulloblastoma [49]. Therefore, there is a spectrum in which the most undifferentiated area might have large undifferentiated cells compared to more differentiated areas exhibiting neuroblastic differentiation. Ultrastructurally, medulloblastoma is composed of primitive cells having a high nuclear-cytoplasmic ratio, and those cells that lack distinguishing ultrastructural findings such as tightly packed poorly differentiated oval to polygonal tumor cells with large nuclei; Rosenthal fibers, i.e., astrocytic differentiation; or neuronal differentiation such as rudimentary synaptic junctions or dense core granules (Fig. 1G). The central acellular area is composed of centrally projected broad cell processes, forming Homer-Wright rosettes. These central acellular rosettes composed of cytoplasmic processes are found in other tumors such as neuroblastoma, neurocytoma, and ependymoma [25,50,51]. Glial intermediate filaments fill the cell processes in an ependymoma or medulloblastoma with glial differentiation, while microtubules are found in tumors of neuronal differentiation such as neurocytoma or neuroblastoma (Fig. 1H) [49,51,52].
PNET was originally described as an undifferentiated neuroepithelial tumor with mesenchymal differentiation and now encompasses tumors showing pluripotentiality toward neuronal, astrocytic, and other cell lines such as those of a mesenchymal lineage (Fig. 1I). However, this entity might be a confusing and controversial factor in the diagnosis and classification of pediatric embryonal tumors. Some cases of PNET mimicking AT/RT have been reported [53]. AT/RT, which occurs mainly in children under the age of 3 years and is associated with frequent recurrence, is a malignant rhabdoid tumor (MRT), i.e., a CNS counterpart of renal MRT, belonging to rhabdoid tumor predisposition syndrome. Mutation or deletion of SMARCB1/INI-1/hSNF5/BAF47-tumor-suppressor gene located on chromosome band 22q11.2 inactivates the tumor suppressor gene, resulting in subsequent negative immunostaining in AT/RT but not in other rhabdoid phenotypes of CNS neoplasms, although mutations of INI-1 in some choroid plexus carcinomas have been reported [47,53,54]. Immunohistochemistry for INI-1 is actually superior to EM or small biopsy of light microscopy because ultrastructural descriptions of AT/RT are few and lack specific CNS cell components of glial or neuronal lineage (Fig. 1J). Histologically, AT/RT shows various mixed histologies of epithelial, mesenchymal, rhabdoid, and primitive neuroepithelial components, suggesting an origin of immature and pluripotent neuroectodermal cells capable of differentiating along multiple lineages, as demonstrated by EM [55].
OLIGODENDROGLIOMA AND OLIGODENDROGLIOMA-LIKE TUMORS
Oligodendroglioma-like tumors, i.e., oligodendroglioma-mimickers, include oligodendroglioma, dysembryoplastic neuroepithelial tumor (DNT), neurocytoma, clear cell ependymoma (CCE), ganglioglioma, and gangliocytoma [25,56-58]. Oligodendroglioma is composed of closely apposed round-shaped tumor cells with euchromatic round nuclei and cytoplasm containing some microtubules and few glial filaments (Fig. 1K). Short broad cell processes might be seen within the tumor cells. Cell junctions or surface microvilli are not found in oligodendroglioma [25,59]. Among oligodendroglioma-mimickers, neurocytomas have distinct immunohistochemical and ultrastructural characteristics compared with oligodendrogliomas [50,51,60]. Both can have microtubules, but the former exhibit neuronal differentiation such as synaptic vesicles or dense core granules. Central and extraventricular neurocytoma has been reported as ependymoma of the foramen of Monro or intraventricular oligodendroglioma due to mimicry of oligodendroglioma-like cells under light microscopy [60]. Characteristic immunohistochemical findings of rosette-like acellular fibrillary areas stained with neuronal markers such as synaptophysin or NeuN might not be identified in extraventricular neurocytomas [61]. Ultrastructurally, neurocytoma has numerous thin neuritic processes filled with dense core granules and microtubules with or without synapses (Fig. 1L). DNT consists of neoplastic oligodendroglial-like cells (OLCs) and elongated processes forming a neuropil-like structure in the mucinous extracellular area [58]. Ultrastructurally, OLCs have round to oval or elongated nuclei and scanty cytoplasm filled with electron-lucent synaptic vesicles, whereas elongated tumor cells have abundant cellular processes containing scanty microtubules and dense-core granules (Fig. 1M). OLCs of DNT can be distinguished from astrocytes by the lack of glial intermediate filaments and from neuronal cells by the presence of microtubules. Some cells of DNT have cytoplasmic ribosome-lamellae complexes, also found in lymphoid neoplasms or glioblastomas [62,63]. These structures have been regarded as astrocytic differentiation but remain elusive. CCE resembling oligodendroglioma and neurocytic tumor under light microscopy can be distinguished from the latter using EM, which shows intracytoplasmic lumen lined by microvilli, complex cell junctions, and cilia. The clear cell morphology of CCE is caused by organellefree areas of the cytoplasm with edema and vacuolization [64,65].
Ganglioglioma and gangliocytoma are composed of several types of tumor cells; neuronal tumor cells have dense core granules, diagnostically salient features, and synaptic junctions. Spherical protein bodies are found in gangliogliomas [56]. Multivesicular bodies, abundant autophagic vacuoles, and dense core vesicles are seen in neoplastic neuronal cells of ganglioglioma and gangliocytomas [66].
PAPILLARY TUMORS OF THE CENTRAL NERVOUS SYSTEM
Papillary tumors of the CNS observable under light microscopy include PGNT, tumors of the suprasellar area such PTPR, pituitary adenoma of papillary configuration, or chordoid glioma of the third ventricle [67-69]. PGNTs can occur at any age, and pediatric cases have been reported [67]. Ultrastructurally, either astrocytic or neuronal differentiation and poorly differentiated or uncommitted primitive cells are found in PGNT [52,69]. PTPR was recently added to the 2007 WHO classification as a rare neuroepithelial tumor of papillary appearance arising in the pineal area [45,46]. This tumor has been named papillary pineocytoma or choroid plexus papilloma due to papillary features and epithelial morphology showing immunopositivity for pancytokeratin and ependymal differentiation. Pediatric occurrence of PTPR is rare. Specialized ependymal cells of the subcommissural organ (SCO) are considered the origin of PTPR because of ultrastructural findings of divergent differentiation of epithelial, ependymal, and neuroendocrine cells. On EM, clear and dark epithelioid cells have an ovoid nuclei and abundant cytoplasm filled with abundant organelles including RERs, dense core vesicles, microtubules, numerous clear and coated vesicles, mitochondria, and intermediate filaments [70]. Abundant microvilli and less frequent cilia are found at the apical pole (Fig. 1N). Well-formed junctional complexes are found at the apical portion, and the basal portion is surrounded by a basement membrane. Chordoid glioma of the third ventricle is characterized by clusters and cords of epithelioid cells in a mucinous chordoid matrix. It is a slowly growing glial tumor occurring mainly in adults, although rare cases of pediatric choroid glioma have been reported [68,71]. Chordoid glioma and PTPR share an anatomic location, i.e., circumventricular organ occurrence and subsequent shared ultrastructural findings; secretory vesicles as well as cilia, microvilli, and junctional complexes such as hemidesmosomes, are suggestive of the origin of modified specialized ependymal, i.e., tanycytes of the SCO such as lamina terminalis (Fig. 1O) [68,69]. Choroid plexus papilloma shows cuboidal-shaped tumor cells with apical-basal orientation with varying sizes of apical club-like or roundish microvilli filled with glycogen particles and rare cilia with a 9+2 arrangement of microtubules (Fig. 1P) [25,72,73]. The neoplastic cells contain numerous free ribosomes, glycogen granules, and RERs. Elongated junctional complexes were occasionally seen near the apical ends. The basal portions of the tumor cells are lined with a continuous basal lamina. These apical cilia or glycogen particles are more often seen in infantile choroid plexus papillomas than in those of older age [72].
RHABDOID CELL TUMORS
Rhabdoid cells are designated as large cells with eccentrically located nuclei and well-demarcated abundant, eosinophilic globular inclusions with characteristic immunoreactivity for vimentin, keratin, and epithelial membrane antigen, which ultrastructurally correspond to paranuclear whorls of intermediate filaments. These rhabdoid changes can appear in various neoplasms from diverse organ sites [54,73-75]. In the CNS, most rhabdoid tumors occur in the infratentorial posterior fossa of very young children. Histological diagnosis of an MRT depends on identification of characteristic rhabdoid choroid plexus carcinoma, an extremely rare variant of choroid plexus carcinoma, belonging to choroid plexus tumor; however, carcinoma can lose its papillary pattern, and this ill-defined growth pattern with small foci of epithelial differentiation can make diagnosis challenging and mimic AT/RT due to overlapping morphological, immunohistochemical, and ultrastructural features, except for INI-1 expression [54,55,74]. Like other MRT, EM shows the typical rhabdoid cells with paranuclear whorls or bundles of intermediate filaments as well as reminiscent retention of choroid plexus differentiation [73]. These rhabdoid features can be seen in rhabdoid meningioma, ependymoma, glioma, or germ cell tumors and even in metastatic rhabdoid melanoma [19,75,76].
PLEOMORPHIC CELL TUMORS
CNS tumors showing pleomorphic features under light microscopy include pleomorphic xanthoastrocytoma (PXA), glioblastoma, AT/RT, or undifferentiated primitive tumors [63,77,78]. Tumors such as AT/RT or undifferentiated primitive tumors were previously discussed in the section on small round cell tumors. PXA that is prevalent in children belongs to an astrocytic lineage and appears as irregularly-shaped heterochromatic nuclei and abundant cytoplasm filled with lysosomes, lipid droplets, and glial filaments [63]. Ultrastructurally, PXA is composed of two types of cells; spindle-shaped astrocytic cells and bizarre giant cells filled with glial filaments and lipid droplets; the astrocytic tumor undergoes neuronal differentiation. Basal lamina surrounds the individual tumor cells or in groups, in contrast to other astrocytic tumors. Cytoplasmic lysosomes and ribosomelamellae complexes are predominant in PXA, as in DNT, lymphoid leukemia and glioblastoma [62,63].
CONCLUSION
Despite declining usage, EM retains its diagnostic usefulness in neuropathology, particularly in distinguishing histological look-alikes, poorly differentiated or undifferentiated CNS neoplasms. When there is an overlap in the immunohistochemical profiles between CNS tumors, ultrastructural examination serves to confirm the concrete diagnosis even if the components of surrounding non-neoplastic cells may be misinterpreted as those of the tumor cells.
In this article, we summarized and reviewed diagnostic ultrastructural findings of CNS neoplasms commonly found in children.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The authors are very grateful to Professor Je G. Chi, who passed away last winter. He was a great mentor and friend to all of us. This work is dedicated to his memory.