Characteristic Changes in Decidual Gene Expression Signature in Spontaneous Term Parturition
Article information
Abstract
Background
The decidua has been implicated in the “terminal pathway” of human term parturition, which is characterized by the activation of pro-inflammatory pathways in gestational tissues. However, the transcriptomic changes in the decidua leading to terminal pathway activation have not been systematically explored. This study aimed to compare the decidual expression of developmental signaling and inflammation-related genes before and after spontaneous term labor in order to reveal their involvement in this process.
Methods
Chorioamniotic membranes were obtained from normal pregnant women who delivered at term with spontaneous labor (TIL, n = 14) or without labor (TNL, n = 15). Decidual cells were isolated from snap-frozen chorioamniotic membranes with laser microdissection. The expression of 46 genes involved in decidual development, sex steroid and prostaglandin signaling, as well as pro- and anti-inflammatory pathways, was analyzed using high-throughput quantitative real-time polymerase chain reaction (qRT-PCR). Chorioamniotic membrane sections were immunostained and then semi-quantified for five proteins, and immunoassays for three chemokines were performed on maternal plasma samples.
Results
The genes with the highest expression in the decidua at term gestation included insulin-like growth factor-binding protein 1 (IGFBP1), galectin-1 (LGALS1), and progestogen-associated endometrial protein (PAEP); the expression of estrogen receptor 1 (ESR1), homeobox A11 (HOXA11), interleukin 1β (IL1B), IL8, progesterone receptor membrane component 2 (PGRMC2), and prostaglandin E synthase (PTGES) was higher in TIL than in TNL cases; the expression of chemokine C-C motif ligand 2 (CCL2), CCL5, LGALS1, LGALS3, and PAEP was lower in TIL than in TNL cases; immunostaining confirmed qRT-PCR data for IL-8, CCL2, galectin-1, galectin-3, and PAEP; and no correlations between the decidual gene expression and the maternal plasma protein concentrations of CCL2, CCL5, and IL-8 were found.
Conclusions
Our data suggests that with the initiation of parturition, the decidual expression of anti-inflammatory mediators decreases, while the expression of pro-inflammatory mediators and steroid receptors increases. This shift may affect downstream signaling pathways that can lead to parturition.
Parturition is a complex process tightly regulated by the cooperation of the neuroendocrine and immune systems [1,2]. Among the numerous hormonal factors implicated in the regulation of uterine quiescence and parturition (e.g., corticotropin-releasing hormone, cortisol, oxytocin), the changes in the bioavailability of estrogen and progesterone seem to be key in parturition [2,3]. While estrogen promotes labor by stimulating biochemical and physiological changes in myometrial cells that augment uterine contractility and excitability [4], the actions of progesterone support pregnancy maintenance and prevent parturition by promoting myometrial quiescence [5,6]. In humans, the changes in the relative expression of various progesterone receptors (elevation in the progesterone receptor A [PR-A]/PR-B ratio) in uterine tissues and fetal membranes are responsible for decreasing progesterone actions (functional progesterone withdrawal) [7-9]. A strong positive association between the PR-A/PR-B ratio and estrogen receptor α (ERα) expression in the myometrium of term pregnant women has been demonstrated [7], suggesting that functional progesterone withdrawal generates “functional estrogen activation [3].” All of these changes can then lead to the stimulation of pro-inflammatory pathways in the cervix, decidua, and fetal membranes [10-12].
The pro-inflammatory microenvironment in various gestational tissues [10,13,14], generated by the influx and local activation of maternal leukocytes [15,16] and the increased production of soluble immune mediators, is essential for the onset of labor [17,18]. The selective influx of leukocytes into gestational tissues [19-21] and various layers of the fetal membranes [22,23] is driven by chemokines [24,25], a subset of cytokines with chemotactic properties [26]. The infiltrating monocytes and differentiated macrophages, neutrophils, and T cells as well as decidual stromal cells [27,28] are rich sources of proinflammatory cytokines, including interleukin 1 (IL-1), IL-6, and tumor necrosis factor α (TNFα) [29-32]. These cytokines have been shown to regulate inflammatory processes leading to labor [33] by promoting myometrial contractility, cervical ripening, and membrane rupture [34,35]. For example, pro-inflammatory cytokines induce the expression of prostaglandin-endoperoxide synthase 2 (PTGS2) in the chorioamniotic membranes and decidua, leading to the increase in local and amniotic fluid concentrations of prostaglandins [36-40] that contribute to the transition from myometrial quiescence to labor initiation [41]. These data suggest that cytokines and chemokines orchestrate the activation of decidual tissue during labor [20].
To promote labor-associated inflammation, the actions of anti-inflammatory mechanisms, at least in part, need to be attenuated in gestational tissues [5]. Among anti-inflammatory molecules, soluble tumor necrosis factor receptors (TNF-R), IL-1 receptor antagonist (IL-1RA), IL-4, and IL-10 have been previously examined in the amniotic fluid in relation to labor. The concentrations of soluble TNF-R1 and TNF-R2 [42] were lower in the amniotic fluid of women in spontaneous labor at term than in women who were not in labor at term, while there was no difference in amniotic fluid IL-1RA [43,44] concentrations regardless of the labor status, either at term or preterm. Data on amniotic fluid IL-10 and IL-4 concentrations were inconsistent [45], since IL-10 [46,47] or IL-4 [47] concentrations were not different or even higher (IL-10 [48] and IL-4 [49]) in term or preterm labor than in gestational age-matched groups of non-laboring women. The expression of these anti-inflammatory molecules in decidual cells during term parturition has not been widely investigated. In this context, it is of importance that some other anti-inflammatory mediators (e.g., cytokines, galectins [50-52], and progestogen-associated endometrial protein [PAEP] [53]) have their strongest expression in the decidua within the uterus [53,54] and may be involved in the regulation of local inflammatory pathways.
The decidua is localized between the chorioamnion and the myometrium and, thus, may play a key role in the cross-talk between the maternal and fetal compartments and in the regulation of labor. Maternal leukocyte populations in the decidua and their interaction with fetal trophoblasts as well as the network of soluble factors produced by the decidua involved in the regulation of implantation are increasingly characterized [55-60]. However, the complex and multifaceted roles of the decidua in the mechanisms leading to the onset of labor have been relatively understudied [61-63]; in spite of that, the activation of the decidual layer of the chorioamniotic membranes is one of the earliest events in spontaneous term parturition. This is supported by a recent concept stating that there is a “decidual clock [64],” one of the multiple gestational clocks that regulates the timing of birth [65]. This suggests that advancing gestational age is associated with the withdrawal of active suppression and/or an enhanced sensitivity of the decidua to signals capable of inducing inflammation, which then promotes the release of a variety of biologically active inflammatory mediators, such as prostaglandins, cytokines, and chemokines, leading to the onset of labor [64].
In relation to the “decidual clock” concept, it is tempting to hypothesize that the entire decidual developmental program may also play a role in the regulation of the local micro-environmental balance, controlling the maintenance of pregnancy and then the activation of labor. This developmental program may involve the key action of homeobox (HOX) gene expression in the uterus [66]. So far, it has been demonstrated that steroids regulate the expression of HOX genes in a tissue-specific manner [67], and the changing steroid responsiveness of the decidua during the perilabor period may also influence their expression. Although several publications outline the importance of HOX genes in uterine development, the menstrual cycle, and implantation [68-73], their involvement in the labor process is poorly understood.
Taken together, these data show that the importance of uncovering molecular events in the decidua seems pivotal to our better understanding of the regulation of term labor. To date, most of the studies that focus on gene, protein, hormonal, or other molecular changes in relation to labor utilize whole chorioamniotic membranes but fail to pinpoint the involvement of the decidua in these changes. Therefore, the aim of this study was to compare decidual gene expression patterns in normal term delivery with or without labor. Based on the current state of knowledge about the cellular pathways involved in the activation of the decidua during labor, genes in the following categories were of interest to our study: (1) estrogen, progesterone, and prostaglandin signaling; (2) cytokines, chemokines, and chemokine receptors; (3) anti-inflammatory molecules with predominant decidual expression; (4) transcription factors involved in decidual development and immune cell differentiation; and (5) additional genes with predominant decidual expression.
MATERIALS AND METHODS
Ethics statement
Chorioamniotic membrane samples and maternal plasma specimens were retrieved from the Bank of Biological Specimens at Wayne State University, the Detroit Medical Center, and the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS). Approval from the Institutional Review Boards of NICHD and Wayne State University for the collection and utilization of biological materials as well as written informed consent from all human subjects was obtained prior to the collection of samples.
Human subjects, clinical specimens, and definitions
Women were included in the following gestational age-matched groups: (1) spontaneous term labor and delivery (TIL) (n = 14); and (2) elective Cesarean section at term without labor (TNL) (n = 15). There were no significant differences among the study groups regarding demographic and clinical characteristics (Table 1). Patients with multiple pregnancies or neonates having congenital or chromosomal abnormalities were excluded. No women had any medical or obstetrical complications or clinical or histological signs of chorioamnionitis, and they delivered neonates of appropriate birth weight for their gestational age, according to the national birth weight distribution curve [74]. Labor was defined by the presence of regular uterine contractions at a frequency of at least two contractions every 10 minutes and cervical changes that resulted in delivery [74].
Placental histopathological examinations
Five-micrometer-thick sections of formalin-fixed, paraffin-embedded chorioamniotic membrane specimens (n = 29) were cut and mounted on Superfrost slides (Erie Scientific LLC, Portsmouth, NH, USA). After deparaffinization, the slides were rehydrated and stained with hematoxylin and eosin. Two pathologists, who were blinded to the clinical outcome, evaluated the slides according to published criteria [75,76].
Sample preparation
Fifteen-micrometer-thick sections of snap-frozen chorioamniotic membranes were embedded in Tissue-Tek O.C.T. Compound (Tissue-Tek 4538, Sakura Finetek USA, Inc., Torrance, CA, USA), sectioned with a Leica CM3050S Cryostat (Leica Micro-systems, Wetzlar, Germany), and then mounted on polyethylene membrane glass slides (Leica) and stored at –80°C until use. All further steps were carried out at room temperature. Slides were quickly treated with graded ethanol solutions (Val Tech Diagnostics, Inc., Pittsburgh, PA, USA), and then cleared by immersion in xylene (Dynamic Diagnostics Inc., Livonia, MI, USA). All steps were carried out under RNase-free conditions.
Laser microdissection
Decidual cell populations were collected from snap-frozen chorioamniotic membranes (n = 29, ~18 mm2/sample) using laser microdissection with an LMD 6000 laser microdissection microscope (Leica Microsystems). To compare decidual and non-decidual gene expression, chorioamniotic cell populations were also collected for three cases. Dissections were completed within 60–90 minutes after the slides were thawed to ensure minimal RNA degradation. Cell populations were cut and collected in extraction buffer (Arcturus Picopure RNA Isolation Kit, Applied Biosystems, Life Technologies Corporation, Foster City, CA, USA), and then incubated at 42°C for 30 minutes. Cell extracts were vortexed, centrifuged at 800 × g, and stored at –80°C until use.
Total RNA isolation
Total RNA was isolated from laser-microdissected and digested samples using the Arcturus Picopure RNA Isolation Kit (Applied Biosystems), according to the manufacturer’s protocol. The Ambion DNA-free Kit (Life Technologies) was used to remove genomic DNA. Total RNA concentrations were measured using the NanoDrop ND-1000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA) was used to determine RNA integrity and quality. RNA samples were then stored at –80°C until use.
Complementary DNA preparation and quantitative real-time PCR
The SuperScript III First-Strand Kit (Life Technologies) was used to generate cDNA. TaqMan gene expression assays (Applied Biosystems) (Table 2), the Biomark System (Fluidigm, San Francisco, CA, USA), and Fluidigm’s 96.96 Dynamic Array Chip were used for high-throughput quantitative real-time polymerase chain reaction (qRT-PCR). Pre-amplification procedures included combining cDNA with TaqMan PreAmp Master Mix (Applied Biosystems) and Pooled Assay Mix (Applied Biosystems), according to the manufacturer’s instructions. The reaction was performed with a thermal cycler for one cycle at 95°C for 10 minutes followed by another 14 cycles at 95°C for 15 seconds and at 60°C for 4 minutes. The Fluidigm 96.96 Dynamic Array Chip (Fluidigm) was used to perform the qRT-PCR assays. The 96.96 Array Chip was primed in an Integrated Fluidic Circuit Controller with control fluid. The TaqMan gene expression assays were mixed with 2 × assay loading reagent (Fluidigm) and loaded into the assay inlet on the chip after priming. The sample inlet was filled with a mixture of preamplified cDNA, TaqMan Universal PCR Master Mix (Applied Biosystems), and 20 × sample loading reagent (Fluidigm), and then the chip was loaded into the BioMark System. The cycle threshold (Ct) value of each reaction was obtained with the Fluidigm qRT-PCR analysis software.
Immunohistochemistry
Five-micrometer-thick sections of formalin-fixed, paraffin-embedded chorioamniotic membrane specimens were cut and placed on salinized TruBond 380 adhesive microscope slides (Tru Scientific, Bellingham, WA, USA). Immunostainings were performed using the Leica Bond-Max (Leica Microsystems) and the Ventana Discovery (Ventana Medical Systems, Inc., Tucson, AZ, USA) autostainer systems, as detailed in Supplementary Table S1. The Bond Polymer Refine Detection Kit (Leica Microsystems) and DAB-MAP HRP Kit (Ventana Medical Systems) were used to detect the chromogenic reaction of horseradish peroxidase. Negative control immunostainings were performed by omitting primary antibodies and using either rabbit IgG or mouse IgG isotype control antibodies (Supplementary Fig. S1).
Semi-quantitative immunoscoring
Semi-quantitative immunoscoring was carried out by scoring five random fields in each tissue section with an immunoreactive score modified from a previous publication [77]. The two examiners were blinded to the clinical information. Immunostaining intensity was graded as follows: 0, negative; 1, weak; 2, intermediate; and 3, strong.
Immunoassays
Maternal plasma concentrations of CCL2 (chemokine C-C motif ligand-2), CCL5, and IL-8 were determined by sensitive and specific immunoassays (Meso Scale Discovery, Rockville, MD, USA), according to the manufacturer’s protocol. Briefly, 1% (w/v) Blocker B Solution was dispensed into each well of the pre-coated plates and incubated with vigorous shaking for 1 hour at room temperature. Plates were then washed three times with phosphate buffered saline (PBS) and 0.05% Tween 20 (Sigma-Aldrich Corporation, St. Louis, MO, USA). Subsequently, samples or calibrators were dispensed into separate wells and incubated for 2 hours with vigorous shaking at room temperature. Plates were then washed three times with PBS and 0.05% Tween-20, followed by the addition of the 1 × Detection Antibody Solution (Meso Scale Discovery) into each well and incubation for 2 hours with vigorous shaking at room temperature. The plates were washed three times, and then 2 × Read Buffer T (Meso Scale Discovery) was added to each well; the signals were read by the SECTOR 2400 Imager (Meso Scale Discovery). Standard curves were generated and the assay values of the samples were interpolated from the curves. The sensitivity of the assays for CCL2, CCL5, and IL-8 was 0.769 pg/mL, 0.514 pg/mL, and 0.213 pg/mL, respectively.
Statistical analysis
Demographic and clinical data were analyzed using SPSS ver. 19.0 (SPSS Inc., Chicago, IL, USA). All other data analysis was conducted within the R statistical environment [78]. When analyzing qRT-PCR data, group comparisons were evaluated via a genewise linear model where gene expression was treated as a response and explained by the group variable while adjusting for gestational age. The derived p-values were further adjusted for false discovery rate (FDR) [79] to account for simultaneous testing on multiple genes. An FDR-adjusted p-value (pFDR value) of .1 and a 1.5-fold change were used as thresholds for significance.
Immunoassay data were log-transformed, matched with the qRT-PCR data for samples from the TIL and TNL groups, and represented by a scatter plot. Pearson correlations and their p-values were computed to assess the linear associations of measurements between enzyme-linked immunosorbent assay and qRT-PCR. To account for heterogeneous sample groups, an ANOVA comparison between the linear models was also conducted.
Immunoreactive scores generated by two independent examiners were averaged to represent the quantity of a given protein in a given patient sample. The two patient groups were compared by using a t-test. A p-value of < .05 was considered statistically significant.
RESULTS
Genes with the highest expression in the decidua at term gestation
Genes included in this expression profiling study (Table 2) were selected according to their biological plausibility and/or their implication in decidual physiology. As a good concordance with published evidence, insulin-like growth factor-binding protein 1 (IGFBP1) and galectin-1 (LGALS1), genes with predominant endometrial expression among all human tissues [80,81], had their highest expression in the decidua at term gestation among the 46 tested genes (Fig. 1A). The expression of IGFBP1 was 28-fold higher (p = .01) in cells microdissected from the decidua than in those collected from the chorioamnion as an internal control in our study (Fig. 1B). This is in accord with the strong IGFBP-1 immunoreactivity in the decidual layer compared to the faint IGFBP-1 immunostaining in other layers of the membranes (Fig. 1C), confirming the accurate isolation of decidual cell populations by laser microdissection.
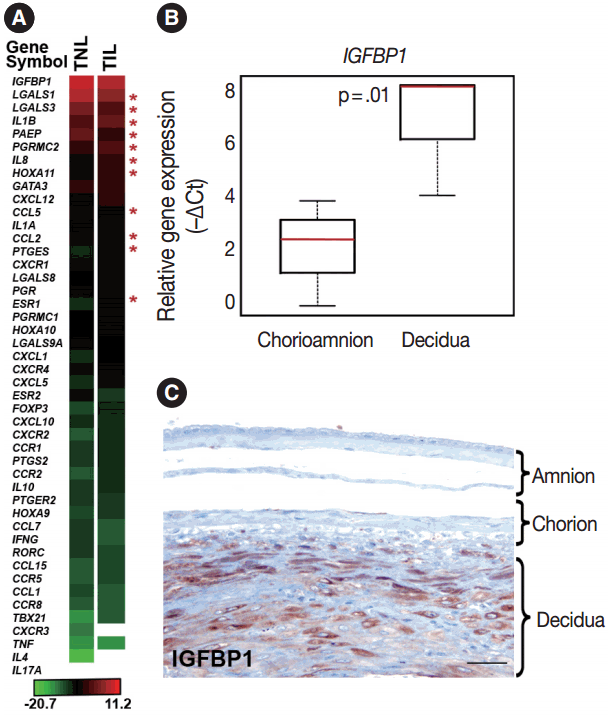
Decidual gene expression in healthy term gestation. (A) The heatmap depicts the mean gene expression levels in the decidua of women with no labor at term (TNL, n=15) or those in term labor (TIL, n=14). Bar denotes color coding for gene expression levels (–∆Ct). Out of all genes sorted by their expression levels, the expression of insulin-like growth factor-binding protein 1 (IGFBP1) was the highest and interleukin 17A (IL17A) expression could not be detected with our method. Stars depict the differentially expressed genes in the decidua between TIL and TNL cases. White areas represent nondetectable gene expression. (B) The relative expression of IGFBP1 was 28-fold higher in the decidua compared to the chorioamnion as visualized in box-plots (p=.01). (C) A representative micrograph shows that IGFBP1 immunostaining was strong in the decidua, while it was weak in the chorion and amnion layers of the membrane.
Genes with differential expression in the decidua in term labor
The expression of 11 genes was significantly different in TIL cases compared to TNL cases. These genes encode for sex steroid receptors, chemokines, cytokines, and galectins as well as an endometrial protein, a prostaglandin synthesis enzyme, and a transcription factor involved in uterine development.
Genes involved in sex steroid signaling and decidual development
Among the sex steroid receptor genes, estrogen receptor 1 (ESR1) and the progesterone receptor membrane component 2 (PGRMC2) had differential expression between the groups (Fig. 1A). ESR1 had an 8.1-fold (pFDR = .032) while PGRMC2 had a 1.7-fold (pFDR = .096) higher expression in TIL cases compared to TNL cases (Fig. 2A, B). Among the tested HOX genes involved in decidualization, HOXA11 had the highest expression in the decidua (Fig. 1A), and it had higher expression in TIL cases compared to TNL cases (4-fold, pFDR = .075) (Fig. 2C).

Differential expression of genes involved in sex steroid signaling and decidual development. Box-plots represent gene expression levels (–∆Ct) in the decidua of women with no labor at term (TNL, n = 15) or those in term labor (TIL, n = 14). Estrogen receptor 1 (ESR1) (A), progesterone receptor membrane component 2 (PGRMC2) (B), and homeobox A11 (HOXA11) (C) expression was higher in TIL cases compared to TNL cases (8.1-fold, false discovery rate–adjusted p-value [pFDR]=.032; 1.7-fold, pFDR=.096; 4-fold, pFDR=.032, respectively).
Chemokine, cytokine, and prostaglandin signaling genes
Among the investigated chemokine genes, IL8, CCL5, and CCL2 were the most abundantly expressed genes in the decidua (Fig. 1A). Two of these genes had differential expression between TIL cases and TNL cases (Fig. 3A–C). IL8 expression was higher in TIL cases compared to TNL cases at the RNA (7.3-fold, pFDR = .004) and protein (immunohistochemistry [IHC] score, TIL 1.5 vs TNL 0.9; p = .027) levels (Fig. 3A). In contrast, there were low expressions of CCL2 in TIL cases compared to TNL cases at the mRNA (8.7-fold, pFDR = .013) and protein (IHC score, TIL 1.9 vs TNL 2.7; p < .0001) levels (Fig. 3B). Decidual CCL5 mRNA expression levels were lower in TIL cases compared to TNL cases (3.5-fold, pFDR = .052) (Fig. 3C).
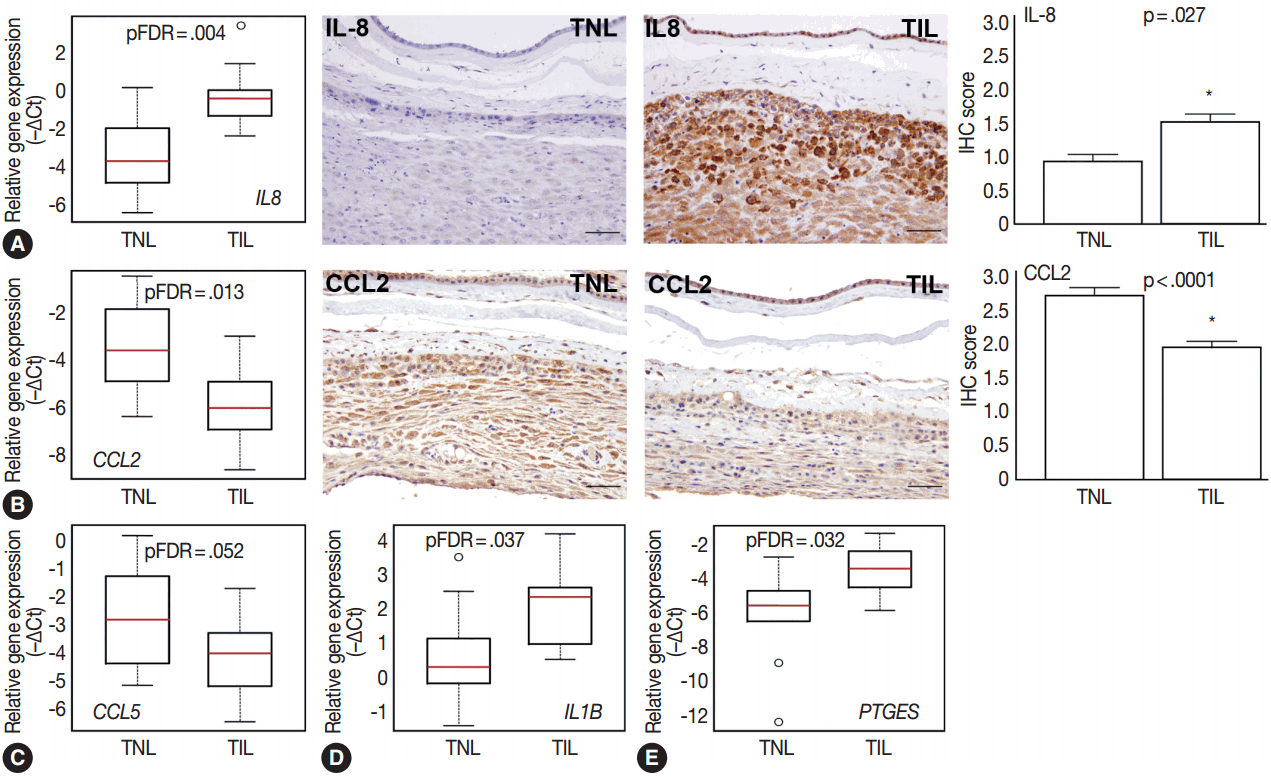
Differential expression of chemokines, cytokines, and prostaglandin signaling genes. Box-plots represent gene expression levels (–∆Ct), while immunohistochemical (IHC) staining and immunoscoring show protein abundance in the decidua of women with no labor at term (TNL, n =15) or those in term labor (TIL, n=14). (A) Relative mRNA expression of interleukin 8 (IL8) in the decidua was higher in TIL cases than in TNL cases (7.3-fold, false discovery rate–adjusted p-value [pFDR]=.004; left panel). IHC staining confirmed higher expression of IL-8 in TIL cases than in TNL cases (middle panels), also quantified by immunoscoring (right panel; TIL 1.5 vs TNL 0.9; p=.027). (B, C) Relative mRNA expression of chemokine C-C motif ligand 2 (CCL2) and CCL5 in the decidua was lower in TIL cases than in TNL cases (8.7-fold, pFDR=.013; 3.5-fold, pFDR=.052, respectively; left panel). IHC staining confirmed lower CCL2 protein expression in TIL cases than in TNL cases (middle panels), also quantified by immunoscoring (right panel; TIL 1.9 vs TNL 2.7; p<.0001). (D, E) Relative decidual mRNA expression of IL1B and PTGES was higher in TIL cases than in TNL cases (2.8-fold, pFDR=.037; 6.7-fold, pFDR=.032, respectively).
Compared to the tested cytokine genes, IL1B had the strongest expression in the decidua (Fig. 1A) and had an increased expression in TIL cases compared to TNL cases (2.8-fold, pFDR = .037) (Fig. 3D). Furthermore, among the prostaglandin signaling pathway genes, prostaglandin E synthase (PTGES) had higher expression levels in TIL cases compared to TNL cases (6.7-fold, pFDR = .032) (Fig. 3E).
Genes encoding anti-inflammatory proteins
Among the tested galectin genes, LGALS1 and LGALS3 had lower expression levels in TIL cases compared to TNL cases (LGALS1: 5.2-fold, pFDR = .039; LGALS3: 4.1-fold, pFDR = .075) (Fig. 4A, B). These findings were confirmed by immunostaining, as galectin-1 and galectin-3 proteins had decreased expression levels in TIL cases compared to TNL cases (galectin-1 IHC score, TIL 2.1 vs TNL 2.6, p = .016; galectin-3 IHC score, TIL 1.4 vs TNL 2.0, p = .041) (Fig. 4A, B).
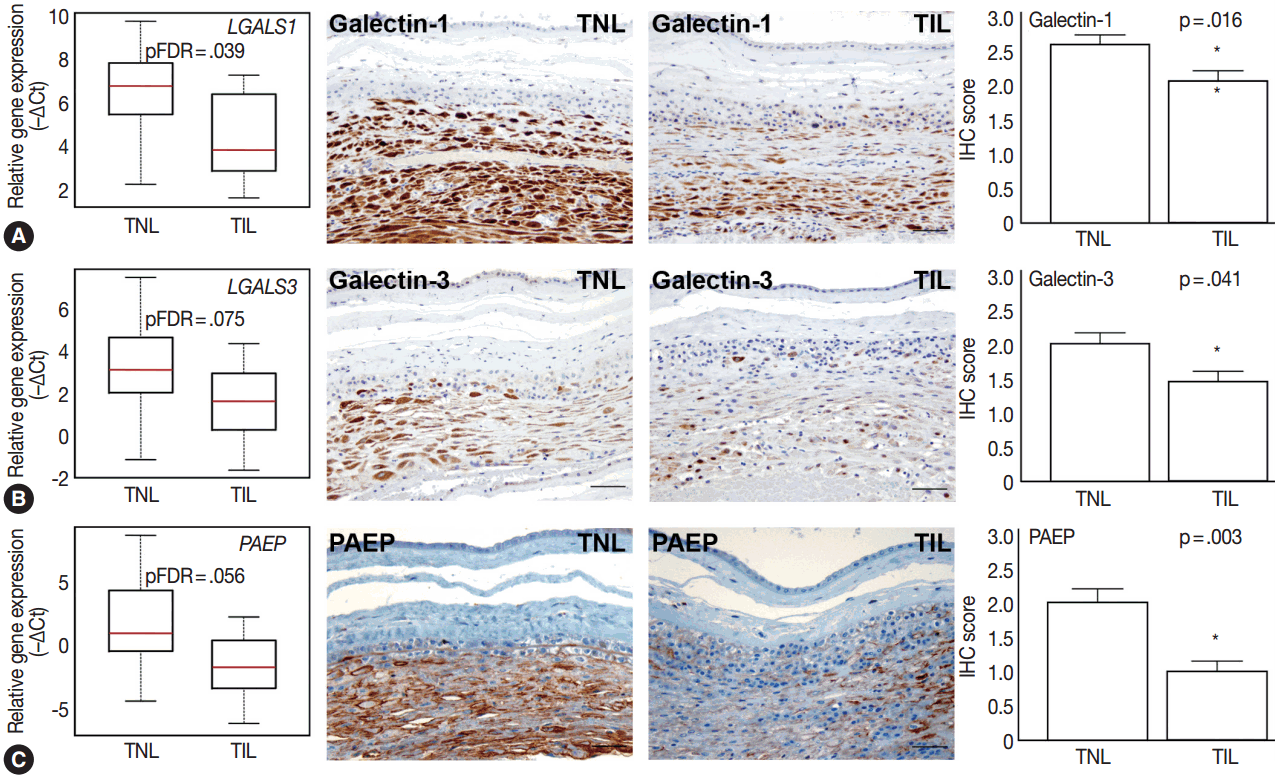
Differential expression of anti-inflammatory mediators in the decidua. Box-plots represent gene expression levels (–∆Ct), while immunohistochemical (IHC) staining and immunoscoring show protein abundance in the decidua of women with no labor at term (TNL, n =15) or those in term labor (TIL, n=14). Galectin-1 (LGALS1) (A), galectin-3 (LGALS3) (B), and progestagen-associated endometrial protein (PAEP) (C) gene expression was lower in TIL cases than in TNL cases (5.2-fold, false discovery rate–adjusted p-value [pFDR]=.039; 4.1-fold, pFDR= .075; 12.6-fold, pFDR=.056, respectively). IHC staining confirmed lower galectin-1 (TIL 2.1 vs TNL 2.6, p=.016) (A), galectin-3 (B) (TIL 1.4 vs TNL 2.0, p=.041), and PAEP (C) (TIL 1.0 vs TNL 2.0, p=.003) expression in TIL cases than in TNL cases.
Among the genes encoding for endometrial proteins, PAEP, one of the most abundantly expressed genes in the decidua [53], had reduced expression levels in TIL cases compared to TNL cases (12.6-fold, pFDR = .056) (Fig. 4C). PAEP protein levels were also weaker in TIL cases compared to TNL cases (IHC score, TIL 1.0 vs TNL 2.0; p = .003) (Fig. 4C).
Comparison of decidual gene expression and maternal plasma concentrations of chemokines
In order to determine whether the local, decidual differential expression of chemokines is also reflected by changes in their systemic concentrations, maternal plasma samples collected at the time of delivery were analyzed with immunoassays for CCL2, CCL5, and IL-8. There was no difference in the concentrations of these chemokines between patient groups, and there was no correlation between the mRNA expression of these chemokines in the decidua and their protein concentrations in maternal plasma samples obtained from the same patients (Fig. 5).

Correlation analyses between decidual gene expression and maternal plasma concentrations of chemokines. (A–C) The scatterplots demonstrate the absence of correlation between the decidual gene expression and maternal plasma concentrations of secreted chemokines chemokine C-C motif ligand 2 (CCL2) (r=.10, p=.66), CCL5 (r=.27, p=.21), and interleukin 8 (IL-8) (r=.03, p=.88) in women with no labor at term (TNL, n=15) or those in term labor (TIL, n=14). ELISA, enzyme-linked immunosorbent assay; qRT-PCR, quantitative real-time polymerase chain reaction.
DISCUSSION
Principal findings of the study
(1) Transcriptomic analysis revealed differences in the expression of genes involved in developmental, signaling, and inflammatory pathways between decidual samples collected from women who delivered at TIL and TNL; (2) genes with the highest expression in the decidua at term included IGFBP1, LGALS1, and PAEP; (3) the expression of ESR1, HOXA11, IL1B, IL8, PGRMC2, and PTGES was higher in TIL cases compared to TNL cases; (4) the expression of CCL2, CCL5, LGALS1, LGALS3, and PAEP was lower in TIL cases compared to TNL cases; (5) immunostaining confirmed a high expression of IL-8 and a low expression of CCL2, galectin-1, and galectin-3 in TIL cases compared to TNL cases; and (6) no correlation between decidual gene expression and maternal plasma protein concentrations of CCL2, CCL5, and IL-8 was found.
Significance of differential gene expression in the decidua
Parturition is a result of pro-inflammatory processes in various gestational tissues, which interact with and orchestrate the terminal pathway of spontaneous labor [82]. This common pathway includes anatomical, physiological, biochemical, endocrinological, immunological, and clinical events that occur in the mother and her fetus. Based on targeted and high-dimensional studies, a growing body of evidence has shown that all uterine components of this terminal pathway—the increased myometrial contractility and cervical ripening as well as the activation of the decidua/fetal membranes—undergo pro-inflammatory changes during labor [82,83]. In spite of the similarities in gene expression signatures, the activation of certain biological pathways in various gestational tissues may be different. Unfortunately, the biological processes taking place in the decidua during spontaneous term parturition have been relatively underinvestigated. This is corroborated by a recent meta-analysis focusing on the transcriptomics of all human gestational tissues in the context of term and preterm birth [84]. This study found that the most commonly studied tissues are the placenta (61%), the fetal membranes (12%), and the myometrium (12%), while the decidua had been the focus of only less than 5% of all investigations [84]. Therefore, our study aimed to fill this gap in our understanding of the role of the decidua in the orchestration of pro-inflammatory pathways during spontaneous term parturition.
Hormonal regulation of the decidua during labor
The appropriate hormonal regulation and development of the decidua are fundamental in the establishment and maintenance of pregnancy. Among hormones, estrogen and progesterone play the most important roles in these processes mediated by estrogen and progesterone receptors [6], while HOX genes are essential for endometrial development, growth, and differentiation under the regulation of estrogen and progesterone [85,86]. Therefore, we studied the most relevant estrogen and progesterone receptors as well as HOX genes to gain insights into their decidual expression changes during term parturition. Members of all of these gene groups were found to have transcriptional changes, since ESR1, PGRMC2, and HOXA11 mRNA expression was increased in TIL cases compared to TNL cases.
ERα (ESR1) is an estrogen receptor predominantly expressed in the uterus, and its expression elevates in the human myometrium during labor [87,88]. Interestingly, the increased relative abundance of ERα, but not ERβ (ESR2) mRNA, is associated with increased PR-A expression and PR-A/PR-B expression ratios in the human myometrium, which, in turn, is responsible for the inhibition of progesterone’s anti-inflammatory actions mediated by PR-B [7,8]. A similar increase in the PR-A/PR-B expression ratio was found in the decidua during term labor [8], which, in conjunction with our finding on the increased decidual ESR1 expression, may suggest that a similar regulatory process is effective in the decidua during term labor.
The decidualization of the human endometrium depends on the actions of progesterone [89] promoted by the expression of nuclear PR isoforms in decidual cells [90], which are central to the genomic actions of progesterone. Moreover, the cellular responsiveness to this hormone also depends on the expression of the membrane progesterone receptors (mPRα, mPRβ, and mPRγ) and their adaptor proteins (PGRMC1 and PGRMC2), which act via a fast, “non-genomic pathway [91].” Several studies support the idea that membrane PRs actually suppress the actions of nuclear PRs and promote myometrial contractility [92] and that the PGRMC adaptor proteins are pivotal in enhancing the cell surface expression and receptor functions of membrane PRs [93]. A recent proteomics study on choriodecidual tissues revealed PGMRC2 to be up-regulated in samples from term and preterm labor samples compared to samples obtained from non-laboring women [94], implicating this protein in the hormonal regulation of parturition. Our finding of the higher decidual expression of PGRMC2 in laboring women, compared to non-laboring women, corroborates these earlier results and may suggest that this “non-genomic pathway” may be involved in the functional progesterone withdrawal in the decidua.
Previous in vitro studies showed that estrogen and progesterone facilitated the expression of developmental HOX genes (HOXA9, HOXA10, and HOXA11) in human decidual cells at term, among which HOXA10 and HOXA11 are expressed in the decidua from early pregnancy [85,86] throughout gestation [95,96]. Furthermore, it has been shown that the interactions of HOXA11 with CCAAT/enhancer binding protein beta (CEBPB) and forkhead box O1 (FOXO1) are especially necessary for the regulation of decidual gene expression during the decidualization of endometrial fibroblasts [97,98]. In this context, it is of interest that FOXO1 can promote IL-1β, IL-6, IL-8, and cyclooxygenase-2 expression and the release of prostaglandins as well as matrix metalloproteinase 9 (MMP-9) expression and MMP activity as detected in pregnant human myometrial cells [99]. It is possible that the increased HOXA11 expression in the decidua of the TIL patients, compared to the TNL patients, who participated in our study may be related to the upstream regulatory events including ESR1 up-regulation and estrogen-mediated actions as well as downstream pro-inflammatory pathways involving the cooperation of HOXA11 with FOXO1 in the TIL cases.
Chemokines, cytokines, and pro-inflammatory mediators in labor
Among the mediators of pro-inflammatory pathways, chemokines are essential for the recruitment and activation of various immune cell populations into distinct tissue compartments at the maternal-fetal interface [100-102]. These infiltrating immune cells are involved in the orchestration of local inflammation, partly by releasing cytokines that regulate prostaglandin production [45]. Members of these gene groups of chemokines, cytokines, and receptors (CCL2, CCL5, IL8, IL1B, and PTGES) that we studied had transcriptional changes in TIL cases compared to TNL cases.
CCL2 and CCL5 have been extensively studied in different gestational tissues as important inducers of immune cell infiltration. CCL2, a monocyte chemoattractant, has been studied in the context of pro-inflammatory changes and monocyte influx/activation in the myometrium, cervix, and fetal membranes during term and preterm parturition [103-105]. CCL5, another monocyte chemoattractant that has additional activity toward T cells, has been implicated in the regulation of inflammatory responses and in the recruitment of macrophages to the implantation site in early pregnancy [106-108]. The role of CCL5 in the mechanisms of human parturition is demonstrated by the increased concentration of this chemokine in the amniotic fluid of women who undergo term labor compared to those who deliver at term without labor [109]. The concentration of CCL5 is also higher in the amniotic fluid of women who undergo term or preterm labor with microbial invasion of the amniotic cavity than in those without this complication [107], supporting the role for CCL5 in the regulation of the host response against intrauterine infection. Of note, monocytes/macrophages are the second most abundant leukocyte population in the human decidua that contributes to parturition by expressing pro-inflammatory mediators (e.g., IL-1β, IL-6, TNFα, and prostaglandins) and that plays a key role in tissue remodeling [15]. Our findings demonstrate higher CCL2 and CCL5 mRNA expression in the decidua prior to labor, compared to TIL cases, which was validated for CCL2 expression at the protein level. These results are in agreement with rodent experiments showing pre-partum recruitment of macrophages into the uterine/decidual tissues in rats and mice [110-113] and with the increase in the proportion of human choriodecidual macrophages from preterm to term gestation [114]. Although the proportion of these macrophages among human choriodecidual leukocytes does not change before and after term labor [114], it was recently demonstrated that macrophages undergo a pro-inflammatory M1-like polarization in the decidua during spontaneous term labor [115]. Therefore, it is likely that macrophages infiltrate the human decidua prior to term gestation, and they acquire an M1 phenotype prior to parturition [115]. Concentrations of CCL2 and CCL5 in the maternal blood of women who undergo term labor are similar to those in women who undergo term delivery without labor [116]. In accord with this earlier finding, there was no correlation between decidual mRNA abundance and concentrations of CCL2 and CCL5 in maternal plasma, which further confirms their importance in the local inflammatory process. Overall, these data suggest that monocyte recruitment into the decidua and macrophage-mediated decidual inflammatory processes are early events during parturition and precede the CCL2- and CCL5-mediated recruitment of monocytes into other uterine tissue compartments.
The local elevated expression of IL-8, a chief chemokine and activator of neutrophils [117,118], fulfills multifaceted roles, both in humans and other examined mammals during term and preterm parturition [119,120], and may determine the timing of neutrophil infiltration and activation in various compartments in the uterine cavity [121], where these cells release cytokines and MMPs to contribute to labor and post-partum wound sealing and healing [122]. Accordingly, the expression of IL-8 by a variety of cells—including monocytes/macrophages, fibroblasts, decidual stromal cells, cervical epithelial cells, and amnion cells [27,123]—have been reported to be elevated in gestational tissues and amniotic fluid in term and pretem parturition [124-133]. Our findings of the higher expression of IL-8 mRNA and protein in the decidua in TIL cases compared to TNL cases support these previous reports. Interestingly, studies that focus on IL-8 concentrations in maternal blood are controversial. One study found significantly higher IL-8 plasma concentrations in laboring women compared to non-laboring women who delivered at term or preterm [116], while another study showed no difference between these groups [134]. Our study agrees with the latter, since we found no difference between the TNL and TIL groups and no correlation between decidual mRNA expression and maternal plasma concentrations of IL-8.
IL-1β and its receptor IL1R are widely expressed in human gestational tissues, where they are involved in the pro-inflammatory response during labor [135-137]; this is supported by the higher IL-1β concentrations in the amniotic, choriodecidual, and placental tissues [31] as well as in the amniotic fluid [138] of patients with spontaneous labor compared to those who delivered at term without labor. Our data suggests that the increased decidual IL1B expression in TIL cases compared to TNL cases is consistent with these earlier findings and further highlights the key role of IL-1β in parturition. As functional evidence, in vitro experiments with human decidual cells revealed that IL-1β treatment alters the expression of genes and microRNAs that function in pro-inflammatory signaling, including the induction of numerous cytokines and chemokines [139], such as IL-8 [140], as well as prostaglandin production [141].
Prostaglandins have central roles in the separate but integrated physiological events of parturition: fetal membrane rupture, cervical dilatation, myometrial contractility, placental separation, and uterine involution [142-144]. There is abundant evidence showing that the decidua, among other gestational tissues, is a major source of prostaglandins in parturition [144]. PGF2α is the most abundant prostaglandin in the decidua, but decidual cells can also produce detectable amounts of prostaglandin E2 (PGF2) [145], which was increased in decidual cells obtained during spontaneous vaginal delivery at term compared to those obtained before the onset of labor [146].
Among the prostaglandin-synthesizing enzymes, PTGS2, the rate-limiting enzyme of the synthesis of prostaglandins, was found to be up-regulated in the human myometrium [102], but not in the decidua [147], for TIL cases compared to TNL cases. Among other key enzymes of prostaglandin synthesis, PTGES, involved in PGF2 synthesis, was elevated in the amnion in TIL cases [148]. The same study found increased expression levels of AKR1C3 (aldo-keto reductase), involved in PGF2α synthesis, in the choriodecidua in TIL cases [148]. The expression of CBR1 (carbonyl reductase), responsible for the conversion of PGF2 to PGF2α and thereby favoring PGF2α elevation, was also detected in the decidua [148], but no difference was found in its expression between TIL and TNL cases. Our study only investigated PTGES from these three genes and found its increased expression in TIL cases compared to TNL cases. Since various pro-inflammatory cytokines [149] and estrogen can induce PTGES expression requiring nuclear factor κB and estrogen receptor signaling pathways, respectively [150], the elevated expression of PTGES during labor in the decidual inflammatory microenvironment is not surprising. All of these results may suggest that local inflammatory pathways induce PTGES, leading to heightened PGF2 production as well as increased production of PGF2α via enzymatic conversion.
Transcription factors for T-cell polarization in labor
The proper balance between innate and adaptive immune cells in gestational tissues is required to sustain pregnancy, and an alteration of this balance may lead to labor at term or preterm gestation [121]. Most studies investigated the roles of macrophage and neutrophil infiltration into gestational tissues during labor; however, the importance of adaptive immune responses in parturition has only recently been recognized [114]. In human pregnancies, almost one-half of the leukocytes in the decidua basalis are CD3+ T cells in term cases compared to the low decidual percentage (6%–30%) of these immune cells observed in the first trimester [151]. It has been proposed that the high proportion of T cells concentrated in the choriodecidua is a result of the expansion of the total number of CD4+ CD25+ regulatory T cells (Tregs) [114] induced by pregnancy [152,153]. It was suggested that these T cells are recruited during term gestation, particularly during parturition, to participate in the cascade of inflammatory mediators at the maternal-fetal interface [154]. Choriodecidual CD4+ T cells display a memory-like phenotype and express high levels of IL-1β, TNFα, and MMP-9 in spontaneous labor at term [114], suggesting that these immune cells have an effector-memory phenotype.
The finding that different T helper (Th) lymphocyte populations (Th1, Th2, Treg, and Th17) could be identified in the early human decidua based on the expression of their lineage-specific differentiation transcription factors (TBX21, GATA3, FOXP3, and RORC) [155] gave us the idea to investigate various Th subsets in term decidua. The mRNAs for all of these transcription factors were found to be expressed, showing the presence of the four Th subsets in term decidua; however, no differences were found between the two groups. It is possible that certain differences could have been detected using phenotypic T-cell markers as previously reported [156] and also that the functional state of these cells may change during parturition.
Anti-inflammatory molecules in labor
While we detected an increased decidual expression among key pro-inflammatory mediator genes, the mRNA and protein expression levels of some anti-inflammatory molecules (galectin-1, galectin-3, and PAEP) were lower in TIL cases compared to TNL cases.
Galectins are secreted proteins, multifunctional regulators of fundamental cellular processes. This is due to their high affinity for β-galactosides, which allow their binding to a broad range of glycosylated proteins critical for cell-cell and cell-extracellular matrix interactions [157] and the regulation of inflammation and immune responses [158]. Some galectins with strong expression at the maternal–fetal interface have been implicated in key functions of pregnancy maintenance in eutherian mammals [80,159,160]. Among these, galectin-1 and galectin-3 are expressed in the decidua under the regulation of sex steroids both in humans and mice [54,80,161,162] and are involved in decidual embryo implantation [163]. Of importance, decidual galectin-1 is one of the most potent mediators of maternal-fetal immune tolerance acting through multiple mechanisms, including the induction of tolerogenic dendritic cells, which, in turn, promote the expansion of IL-10–secreting regulatory T cells, as evidenced in mice [164]. Galectin-1 also has the ability to induce the apoptosis of Th1 cells, thereby causing a shift toward the Th2-type immune response, which is favorable during pregnancy [165]. Extracellular galectin-3 also has pro-apoptotic activity [166] and, thus, may have similar apoptotic functions compared to galectin-1 in the decidua. Our findings show decreased decidual LGALS1 and LGALS3 expression, which suggests that galectin-1 and galectin-3 may have important roles in the regulation of decidual immune cell populations and in the maintenance of a local microenvironment that favors progesterone production and pregnancy maintenance.
PAEP, also known as glycodelin, placental protein 14, and progestagen-dependent endometrial protein, is a glycoprotein that has several isoforms according to its glycosylation pattern [167,168]. The most abundant glycodelin isoform, glycodelin-A, is highly expressed in the decidua [53] and has major anti-inflammatory properties [169]. Decidual PAEP expression is regulated by progesterone [170], and its immunoregulatory actions require estrogen priming [171]. This is in agreement with the role of PAEP as a potent regulator of predominant immune cell lineages at the maternal-fetal interface [172] and the capability of PAEP to induce the apoptosis of activated T cells [173], the inhibition of monocyte chemotaxis [174], and the induction of monocyte apoptosis [175]. Our results demonstrate decreased PAEP mRNA abundance and protein expression in TIL cases compared to TNL cases, which may suggest that glycodelin-A, similar to galectins, is involved in the maintenance of a local immune balance in the decidua.
Our study also targeted the investigation of anti-inflammatory cytokines, but neither the decidual mRNA expression of IL4 nor IL10 changed with labor status at term. This may support other findings demonstrating no differences between IL-10 protein levels in decidual cells isolated from women who underwent labor compared to those who did not undergo labor at term [47]. However, the basal choriodecidual production rates of IL-10 have been found to be significantly decreased with labor [176], which relates to our findings on PAEP and galectins. This suggests that changes may also occur in IL-10 mRNA/protein expression at certain time-points during term labor, and that IL-10 may also be a part of the local molecular mechanisms that maintain decidual quiescence during pregnancy.
Strengths and limitations
The strengths of our study include the precise collection of decidual cells using laser microdissection, which allowed insights into decidual transcriptomic changes during spontaneous term parturition. High-throughput qRT-PCR enabled the parallel investigation of 46 genes involved in a large number of pathways. Immunostaining and semiquantitative immunoscoring of tissue sections from the same chorioamniotic membranes supported the decidual investigation of differentially expressed genes at the protein level. The comparisons between local decidual and maternal systemic expression of certain genes and their protein products in the same patients provided an additional strength of our study. This approach is unique since previous studies mainly focused on the investigation of the fetal membranes as a whole without illustrating which part of the membranes is responsible for the parturition-specific changes.
Limitations of our study included the relatively small sample sizes due to the technical limitations (low yield, high labor intensity, and slow workpace) of laser microdissection. Many parturition-related molecules as well as alternative splicing variants of the examined genes have not been investigated since the tiny amount of RNA samples, which could be obtained with our methodology, only allowed the study of a maximum of 48 genes in our high-throughput qRT-PCR system. That is why we selected several key parturition-related genes with well-characterized decidual expression in spontaneous term labor as positive controls for our study as well as many other genes not yet characterized in the context of term labor. Since we examined gene expression signatures at two time-points, it was not possible to analyze in detail the exact temporal changes in the cascade of decidual gene expression changes as term parturition progressed.
Conclusions
Our data proposes that, with the initiation of parturition, the decidual expression of anti-inflammatory mediators decreases while the expression of pro-inflammatory mediators and steroid receptors increases, affecting downstream signaling pathways that lead to chorioamniotic membrane weakening and myometrial contractions (Fig. 6). Our results agree with earlier findings that suggest that the decidua is one of the earliest gestational tissues that becomes primed during parturition. Nevertheless, functional studies are required to further explore and establish a causal link between the pathways that were found in our descriptive study during term labor.
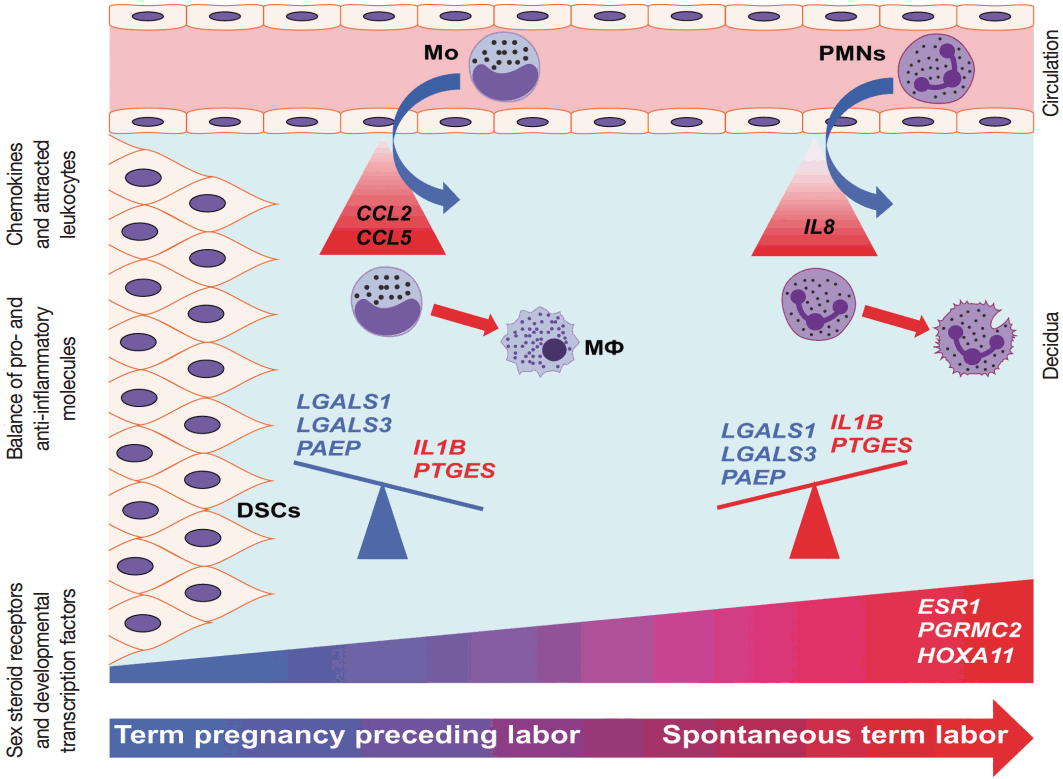
Conceptual framework. The increased decidual expression of a signaling factor responsible for decidual maturation and development (HOXA11) is preceded by the increased expression of chemokines (CCL2 and CCL5), which may stimulate the early recruitment of monocytes into the decidua as the onset of labor approaches. These immune cells will be activated by the local microenvironment and contribute to the orchestration of inflammation. With the initiation of parturition, the decidual expression of anti-inflammatory mediators (LGALS1, LGALS3, and PAEP) decreases, while the expression of pro-inflammatory mediators (IL1B and PTGES) and steroid receptors (ESR1 and PGRMC2) increases, contributing to “functional progesterone withdrawal” and heightened inflammation, eventually leading to uterine contractions, cervical ripening, and membrane rupture. The relatively late increase of IL8 expression will be followed by the recruitment of neutrophils, which plays a key role in tissue repair. These results strengthen earlier findings on the decidua being the earliest among gestational tissues that get primed during parturition. CCL2, chemokine C-C motif ligand 2; CCL5, chemokine C-C motif ligand 5; DSCs, decidual stromal cells; ESR1, estrogen receptor 1; HOXA11, homeobox A11; IL1B, interleukin-1β; IL8, interleukin 8; LGALS1, galectin-1; LGALS3, galectin-3; Mo, monocytes; MΦ, macrophages; PAEP, progestogen-associated endometrial protein; PGRMC2, progesterone receptor membrane component 2; PMNs, neutrophil granulocytes; PTGES, prostaglandin E synthase.
Electronic Supplementary Material
Supplementary materials are available at the Journal of Pathology and Translational Medicine (http://jpatholtm.org).
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The authors thank Lorraine Nikita, Newaj Abdullah, Ryan Cantarella, Ruowen Liu, Dayna Sheldon, and the nursing staff of the Perinatology Research Branch for their technical assistance, and Andrea Bernard, Maureen McGerty, and Sara Tipton for their critical readings of the manuscript.
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, by Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C. Data analysis, interpretation, and manuscript writing were also supported by the Hungarian Academy of Sciences Momentum Grant “LP2014-7/2014” (to N.G.T.) and the OTKA-PD “104398” Grant (to A.B.). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.

