Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 51(3); 2017 > Article
-
Original Article
Characteristic Changes in Decidual Gene Expression Signature in Spontaneous Term Parturition - Haidy El-Azzamy1,*, Andrea Balogh1,2,*, Roberto Romero,1,3,4,5, Yi Xu1, Christopher LaJeunesse1, Olesya Plazyo1, Zhonghui Xu1, Theodore G. Price1, Zhong Dong1, Adi L. Tarca1,6, Zoltan Papp7, Sonia S. Hassan1,6, Tinnakorn Chaiworapongsa1,6, Chong Jai Kim1,8,9, Nardhy Gomez-Lopez1,6, Nandor Gabor Than,1,6,7,10,11
-
Journal of Pathology and Translational Medicine 2017;51(3):264-283.
DOI: https://doi.org/10.4132/jptm.2016.12.20
Published online: February 22, 2017
1Perinatology Research Branch, NICHD/NIH/DHHS, Bethesda, MD, and Detroit, MI, USA
2Department of Immunology, Eotvos Lorand University, Budapest, Hungary
3Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA
4Department of Epidemiology and Biostatistics, Michigan State University, East Lansing, MI, USA
5Center for Molecular Medicine and Genetics, Wayne State University, Detroit, MI, USA
6Department of Obstetrics and Gynecology, Wayne State University, School of Medicine, Detroit, MI, USA
7Maternity Private Department, Kutvolgyi Clinical Block, Semmelweis University, Budapest, Hungary
8Department of Pathology, Wayne State University, School of Medicine, Detroit, MI, USA
9Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
10Systems Biology of Reproduction Lendulet Research Group, Institute of Enzymology, Research Centre for Natural Sciences, Hungarian Academy of Sciences, Budapest, Hungary
11First Department of Pathology and Experimental Cancer Research, Semmelweis University, Budapest, Hungary
-
Corresponding Author Nandor Gabor Than, MD, PhD Systems Biology of Reproduction Lendulet Research Group, Institute of Enzymology, Research Centre for Natural Sciences, Hungarian Academy of Sciences, 2 Magyar Tudosok korutja, H-1117 Budapest, Hungary Tel: +36-1-382-6788 E-mail: than.gabor@ttk.mta.hu
Roberto Romero, MD, DMedSci Perinatology Research Branch, NICHD/NIH/DHHS, Hutzel Women’s Hospital, 3990 John R Street, 4 Brush, Detroit, MI 48201, USA Tel: +1-313-993-2700 Fax: +1-313-993-2694 E-mail: prbchiefstaff@med.wayne.edu - *Haidy El-Azzamy and Andrea Balogh contributed equally to this work.
© 2017 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- The decidua has been implicated in the “terminal pathway” of human term parturition, which is characterized by the activation of pro-inflammatory pathways in gestational tissues. However, the transcriptomic changes in the decidua leading to terminal pathway activation have not been systematically explored. This study aimed to compare the decidual expression of developmental signaling and inflammation-related genes before and after spontaneous term labor in order to reveal their involvement in this process.
-
Methods
- Chorioamniotic membranes were obtained from normal pregnant women who delivered at term with spontaneous labor (TIL, n = 14) or without labor (TNL, n = 15). Decidual cells were isolated from snap-frozen chorioamniotic membranes with laser microdissection. The expression of 46 genes involved in decidual development, sex steroid and prostaglandin signaling, as well as pro- and anti-inflammatory pathways, was analyzed using high-throughput quantitative real-time polymerase chain reaction (qRT-PCR). Chorioamniotic membrane sections were immunostained and then semi-quantified for five proteins, and immunoassays for three chemokines were performed on maternal plasma samples.
-
Results
- The genes with the highest expression in the decidua at term gestation included insulin-like growth factor-binding protein 1 (IGFBP1), galectin-1 (LGALS1), and progestogen-associated endometrial protein (PAEP); the expression of estrogen receptor 1 (ESR1), homeobox A11 (HOXA11), interleukin 1β (IL1B), IL8, progesterone receptor membrane component 2 (PGRMC2), and prostaglandin E synthase (PTGES) was higher in TIL than in TNL cases; the expression of chemokine C-C motif ligand 2 (CCL2), CCL5, LGALS1, LGALS3, and PAEP was lower in TIL than in TNL cases; immunostaining confirmed qRT-PCR data for IL-8, CCL2, galectin-1, galectin-3, and PAEP; and no correlations between the decidual gene expression and the maternal plasma protein concentrations of CCL2, CCL5, and IL-8 were found.
-
Conclusions
- Our data suggests that with the initiation of parturition, the decidual expression of anti-inflammatory mediators decreases, while the expression of pro-inflammatory mediators and steroid receptors increases. This shift may affect downstream signaling pathways that can lead to parturition.
- Ethics statement
- Chorioamniotic membrane samples and maternal plasma specimens were retrieved from the Bank of Biological Specimens at Wayne State University, the Detroit Medical Center, and the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS). Approval from the Institutional Review Boards of NICHD and Wayne State University for the collection and utilization of biological materials as well as written informed consent from all human subjects was obtained prior to the collection of samples.
- Human subjects, clinical specimens, and definitions
- Women were included in the following gestational age-matched groups: (1) spontaneous term labor and delivery (TIL) (n = 14); and (2) elective Cesarean section at term without labor (TNL) (n = 15). There were no significant differences among the study groups regarding demographic and clinical characteristics (Table 1). Patients with multiple pregnancies or neonates having congenital or chromosomal abnormalities were excluded. No women had any medical or obstetrical complications or clinical or histological signs of chorioamnionitis, and they delivered neonates of appropriate birth weight for their gestational age, according to the national birth weight distribution curve [74]. Labor was defined by the presence of regular uterine contractions at a frequency of at least two contractions every 10 minutes and cervical changes that resulted in delivery [74].
- Placental histopathological examinations
- Five-micrometer-thick sections of formalin-fixed, paraffin-embedded chorioamniotic membrane specimens (n = 29) were cut and mounted on Superfrost slides (Erie Scientific LLC, Portsmouth, NH, USA). After deparaffinization, the slides were rehydrated and stained with hematoxylin and eosin. Two pathologists, who were blinded to the clinical outcome, evaluated the slides according to published criteria [75,76].
- Sample preparation
- Fifteen-micrometer-thick sections of snap-frozen chorioamniotic membranes were embedded in Tissue-Tek O.C.T. Compound (Tissue-Tek 4538, Sakura Finetek USA, Inc., Torrance, CA, USA), sectioned with a Leica CM3050S Cryostat (Leica Micro-systems, Wetzlar, Germany), and then mounted on polyethylene membrane glass slides (Leica) and stored at –80°C until use. All further steps were carried out at room temperature. Slides were quickly treated with graded ethanol solutions (Val Tech Diagnostics, Inc., Pittsburgh, PA, USA), and then cleared by immersion in xylene (Dynamic Diagnostics Inc., Livonia, MI, USA). All steps were carried out under RNase-free conditions.
- Laser microdissection
- Decidual cell populations were collected from snap-frozen chorioamniotic membranes (n = 29, ~18 mm2/sample) using laser microdissection with an LMD 6000 laser microdissection microscope (Leica Microsystems). To compare decidual and non-decidual gene expression, chorioamniotic cell populations were also collected for three cases. Dissections were completed within 60–90 minutes after the slides were thawed to ensure minimal RNA degradation. Cell populations were cut and collected in extraction buffer (Arcturus Picopure RNA Isolation Kit, Applied Biosystems, Life Technologies Corporation, Foster City, CA, USA), and then incubated at 42°C for 30 minutes. Cell extracts were vortexed, centrifuged at 800 × g, and stored at –80°C until use.
- Total RNA isolation
- Total RNA was isolated from laser-microdissected and digested samples using the Arcturus Picopure RNA Isolation Kit (Applied Biosystems), according to the manufacturer’s protocol. The Ambion DNA-free Kit (Life Technologies) was used to remove genomic DNA. Total RNA concentrations were measured using the NanoDrop ND-1000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA) was used to determine RNA integrity and quality. RNA samples were then stored at –80°C until use.
- Complementary DNA preparation and quantitative real-time PCR
- The SuperScript III First-Strand Kit (Life Technologies) was used to generate cDNA. TaqMan gene expression assays (Applied Biosystems) (Table 2), the Biomark System (Fluidigm, San Francisco, CA, USA), and Fluidigm’s 96.96 Dynamic Array Chip were used for high-throughput quantitative real-time polymerase chain reaction (qRT-PCR). Pre-amplification procedures included combining cDNA with TaqMan PreAmp Master Mix (Applied Biosystems) and Pooled Assay Mix (Applied Biosystems), according to the manufacturer’s instructions. The reaction was performed with a thermal cycler for one cycle at 95°C for 10 minutes followed by another 14 cycles at 95°C for 15 seconds and at 60°C for 4 minutes. The Fluidigm 96.96 Dynamic Array Chip (Fluidigm) was used to perform the qRT-PCR assays. The 96.96 Array Chip was primed in an Integrated Fluidic Circuit Controller with control fluid. The TaqMan gene expression assays were mixed with 2 × assay loading reagent (Fluidigm) and loaded into the assay inlet on the chip after priming. The sample inlet was filled with a mixture of preamplified cDNA, TaqMan Universal PCR Master Mix (Applied Biosystems), and 20 × sample loading reagent (Fluidigm), and then the chip was loaded into the BioMark System. The cycle threshold (Ct) value of each reaction was obtained with the Fluidigm qRT-PCR analysis software.
- Immunohistochemistry
- Five-micrometer-thick sections of formalin-fixed, paraffin-embedded chorioamniotic membrane specimens were cut and placed on salinized TruBond 380 adhesive microscope slides (Tru Scientific, Bellingham, WA, USA). Immunostainings were performed using the Leica Bond-Max (Leica Microsystems) and the Ventana Discovery (Ventana Medical Systems, Inc., Tucson, AZ, USA) autostainer systems, as detailed in Supplementary Table S1. The Bond Polymer Refine Detection Kit (Leica Microsystems) and DAB-MAP HRP Kit (Ventana Medical Systems) were used to detect the chromogenic reaction of horseradish peroxidase. Negative control immunostainings were performed by omitting primary antibodies and using either rabbit IgG or mouse IgG isotype control antibodies (Supplementary Fig. S1).
- Semi-quantitative immunoscoring
- Semi-quantitative immunoscoring was carried out by scoring five random fields in each tissue section with an immunoreactive score modified from a previous publication [77]. The two examiners were blinded to the clinical information. Immunostaining intensity was graded as follows: 0, negative; 1, weak; 2, intermediate; and 3, strong.
- Immunoassays
- Maternal plasma concentrations of CCL2 (chemokine C-C motif ligand-2), CCL5, and IL-8 were determined by sensitive and specific immunoassays (Meso Scale Discovery, Rockville, MD, USA), according to the manufacturer’s protocol. Briefly, 1% (w/v) Blocker B Solution was dispensed into each well of the pre-coated plates and incubated with vigorous shaking for 1 hour at room temperature. Plates were then washed three times with phosphate buffered saline (PBS) and 0.05% Tween 20 (Sigma-Aldrich Corporation, St. Louis, MO, USA). Subsequently, samples or calibrators were dispensed into separate wells and incubated for 2 hours with vigorous shaking at room temperature. Plates were then washed three times with PBS and 0.05% Tween-20, followed by the addition of the 1 × Detection Antibody Solution (Meso Scale Discovery) into each well and incubation for 2 hours with vigorous shaking at room temperature. The plates were washed three times, and then 2 × Read Buffer T (Meso Scale Discovery) was added to each well; the signals were read by the SECTOR 2400 Imager (Meso Scale Discovery). Standard curves were generated and the assay values of the samples were interpolated from the curves. The sensitivity of the assays for CCL2, CCL5, and IL-8 was 0.769 pg/mL, 0.514 pg/mL, and 0.213 pg/mL, respectively.
- Statistical analysis
- Demographic and clinical data were analyzed using SPSS ver. 19.0 (SPSS Inc., Chicago, IL, USA). All other data analysis was conducted within the R statistical environment [78]. When analyzing qRT-PCR data, group comparisons were evaluated via a genewise linear model where gene expression was treated as a response and explained by the group variable while adjusting for gestational age. The derived p-values were further adjusted for false discovery rate (FDR) [79] to account for simultaneous testing on multiple genes. An FDR-adjusted p-value (pFDR value) of .1 and a 1.5-fold change were used as thresholds for significance.
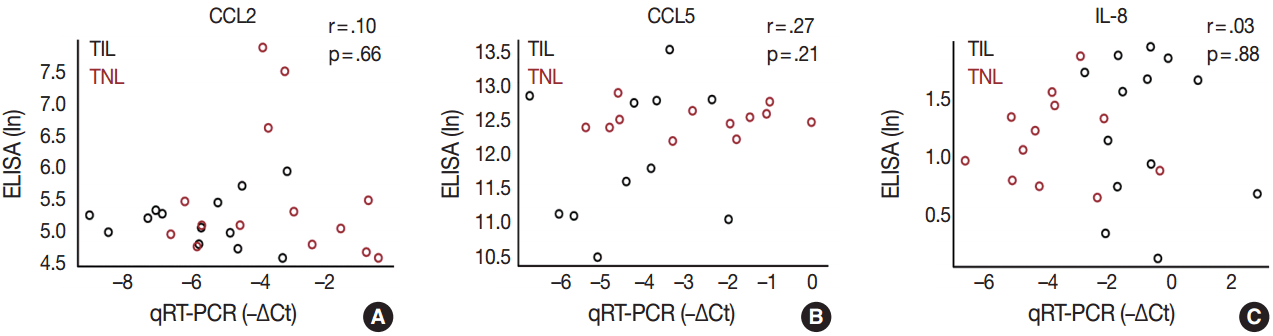
- Immunoassay data were log-transformed, matched with the qRT-PCR data for samples from the TIL and TNL groups, and represented by a scatter plot. Pearson correlations and their p-values were computed to assess the linear associations of measurements between enzyme-linked immunosorbent assay and qRT-PCR. To account for heterogeneous sample groups, an ANOVA comparison between the linear models was also conducted.
- Immunoreactive scores generated by two independent examiners were averaged to represent the quantity of a given protein in a given patient sample. The two patient groups were compared by using a t-test. A p-value of < .05 was considered statistically significant.
MATERIALS AND METHODS
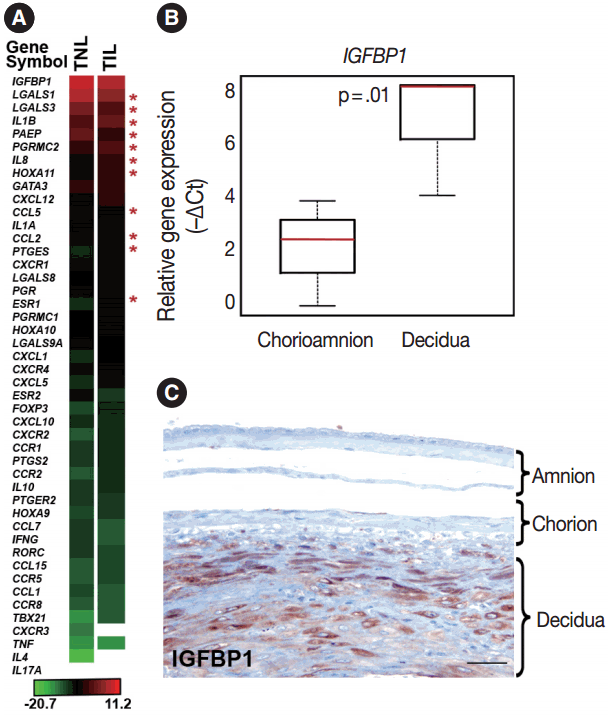
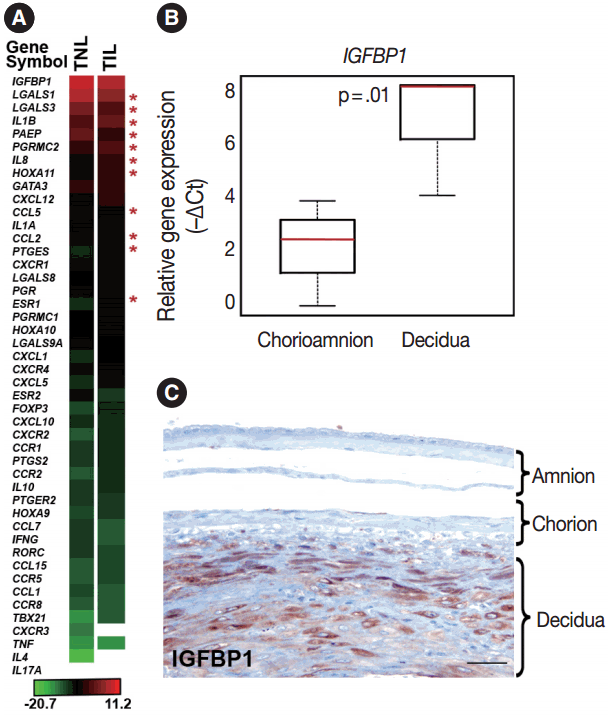
- Genes with the highest expression in the decidua at term gestation
- Genes included in this expression profiling study (Table 2) were selected according to their biological plausibility and/or their implication in decidual physiology. As a good concordance with published evidence, insulin-like growth factor-binding protein 1 (IGFBP1) and galectin-1 (LGALS1), genes with predominant endometrial expression among all human tissues [80,81], had their highest expression in the decidua at term gestation among the 46 tested genes (Fig. 1A). The expression of IGFBP1 was 28-fold higher (p = .01) in cells microdissected from the decidua than in those collected from the chorioamnion as an internal control in our study (Fig. 1B). This is in accord with the strong IGFBP-1 immunoreactivity in the decidual layer compared to the faint IGFBP-1 immunostaining in other layers of the membranes (Fig. 1C), confirming the accurate isolation of decidual cell populations by laser microdissection.
- Genes with differential expression in the decidua in term labor
- The expression of 11 genes was significantly different in TIL cases compared to TNL cases. These genes encode for sex steroid receptors, chemokines, cytokines, and galectins as well as an endometrial protein, a prostaglandin synthesis enzyme, and a transcription factor involved in uterine development.
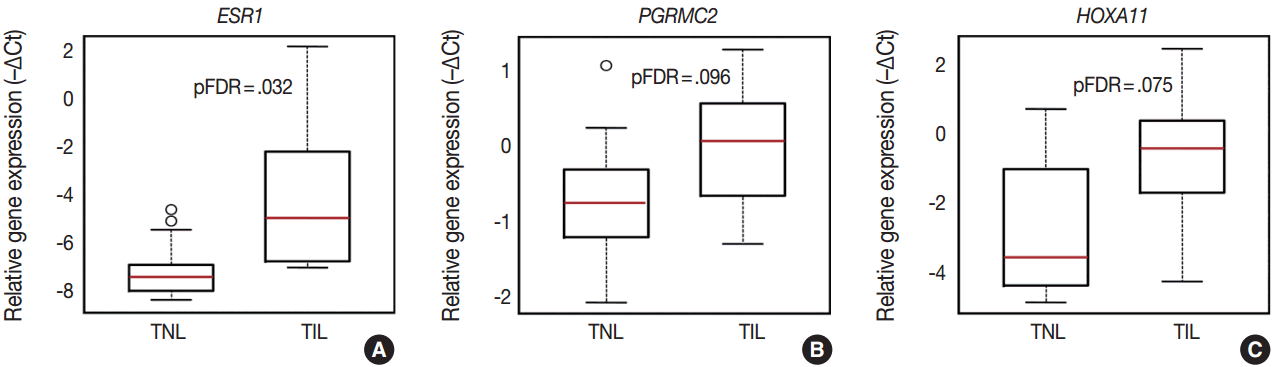
- Among the sex steroid receptor genes, estrogen receptor 1 (ESR1) and the progesterone receptor membrane component 2 (PGRMC2) had differential expression between the groups (Fig. 1A). ESR1 had an 8.1-fold (pFDR = .032) while PGRMC2 had a 1.7-fold (pFDR = .096) higher expression in TIL cases compared to TNL cases (Fig. 2A, B). Among the tested HOX genes involved in decidualization, HOXA11 had the highest expression in the decidua (Fig. 1A), and it had higher expression in TIL cases compared to TNL cases (4-fold, pFDR = .075) (Fig. 2C).
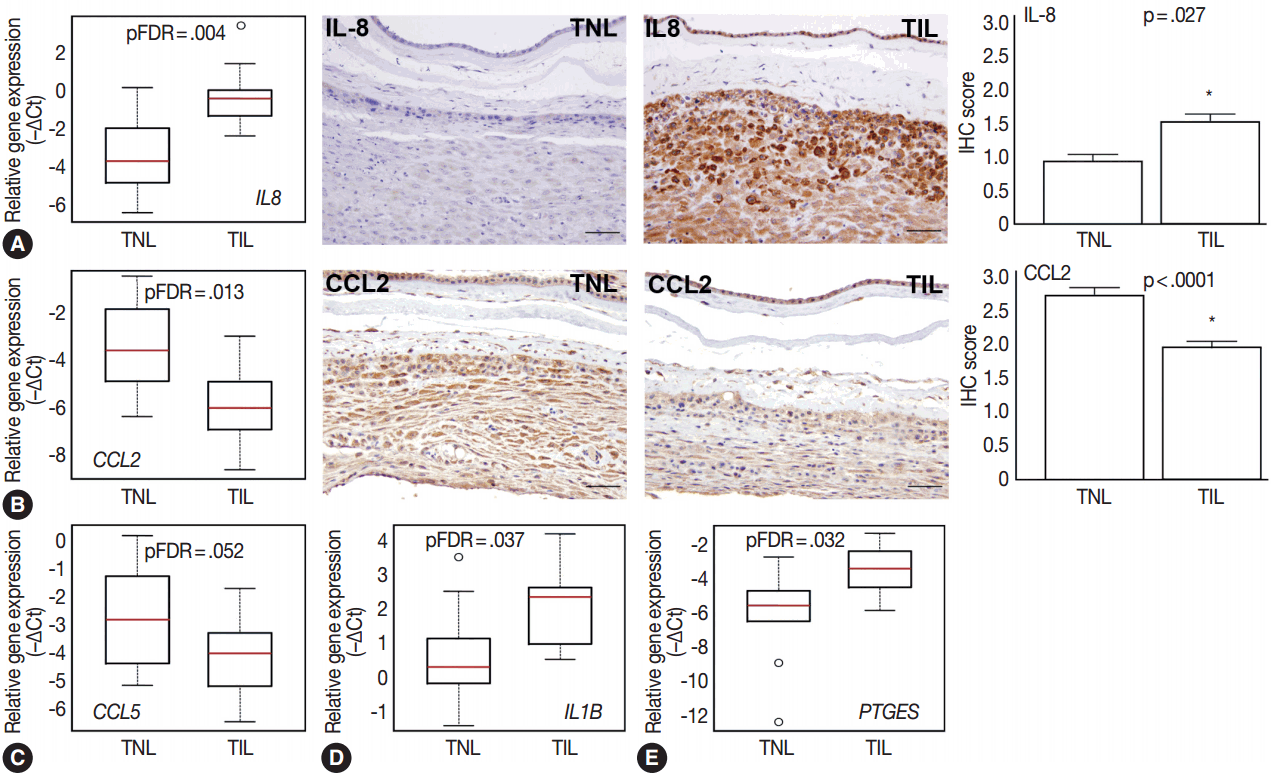
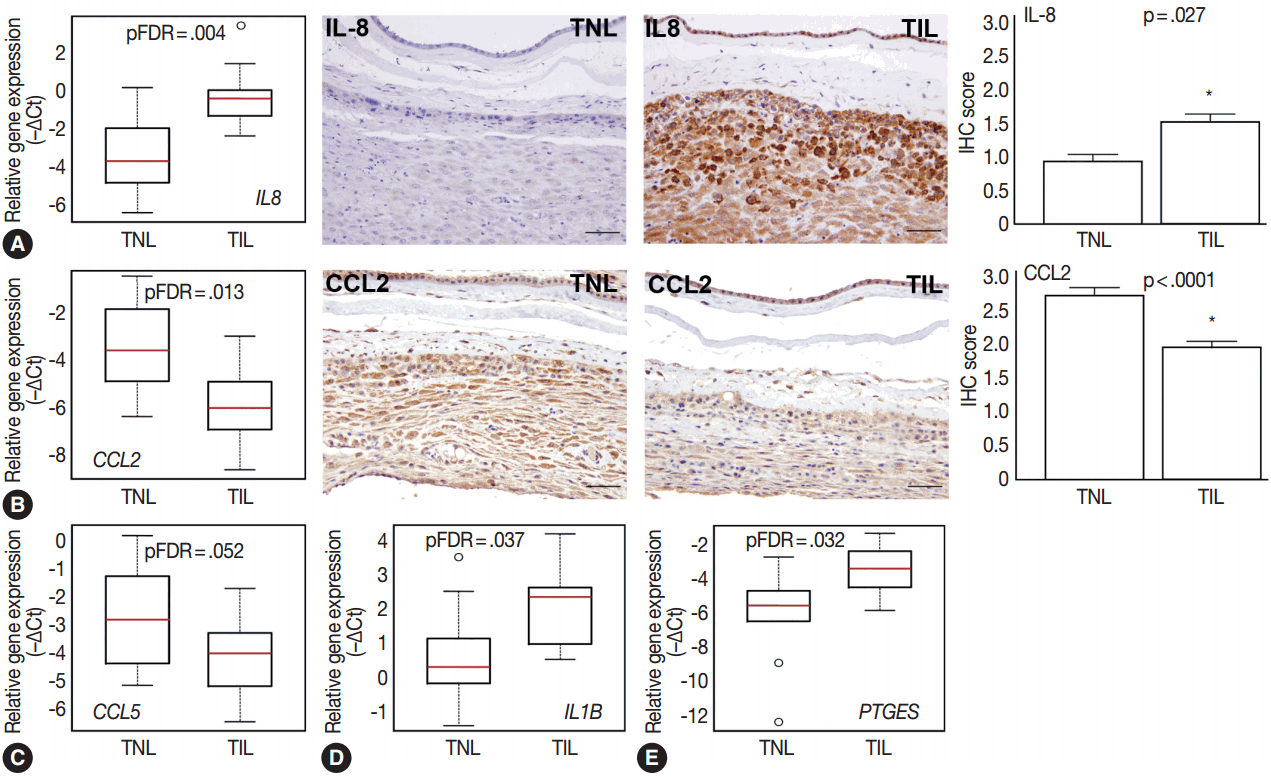
- Among the investigated chemokine genes, IL8, CCL5, and CCL2 were the most abundantly expressed genes in the decidua (Fig. 1A). Two of these genes had differential expression between TIL cases and TNL cases (Fig. 3A–C). IL8 expression was higher in TIL cases compared to TNL cases at the RNA (7.3-fold, pFDR = .004) and protein (immunohistochemistry [IHC] score, TIL 1.5 vs TNL 0.9; p = .027) levels (Fig. 3A). In contrast, there were low expressions of CCL2 in TIL cases compared to TNL cases at the mRNA (8.7-fold, pFDR = .013) and protein (IHC score, TIL 1.9 vs TNL 2.7; p < .0001) levels (Fig. 3B). Decidual CCL5 mRNA expression levels were lower in TIL cases compared to TNL cases (3.5-fold, pFDR = .052) (Fig. 3C).
- Compared to the tested cytokine genes, IL1B had the strongest expression in the decidua (Fig. 1A) and had an increased expression in TIL cases compared to TNL cases (2.8-fold, pFDR = .037) (Fig. 3D). Furthermore, among the prostaglandin signaling pathway genes, prostaglandin E synthase (PTGES) had higher expression levels in TIL cases compared to TNL cases (6.7-fold, pFDR = .032) (Fig. 3E).
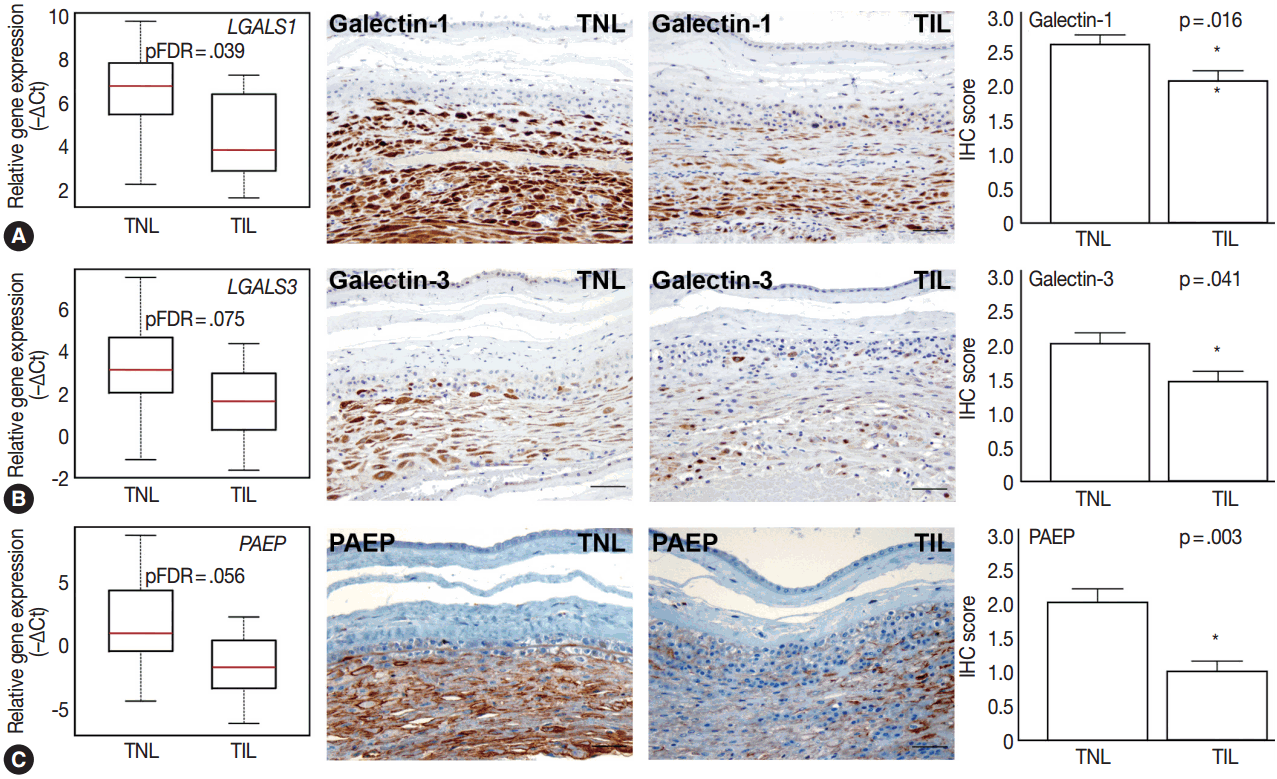
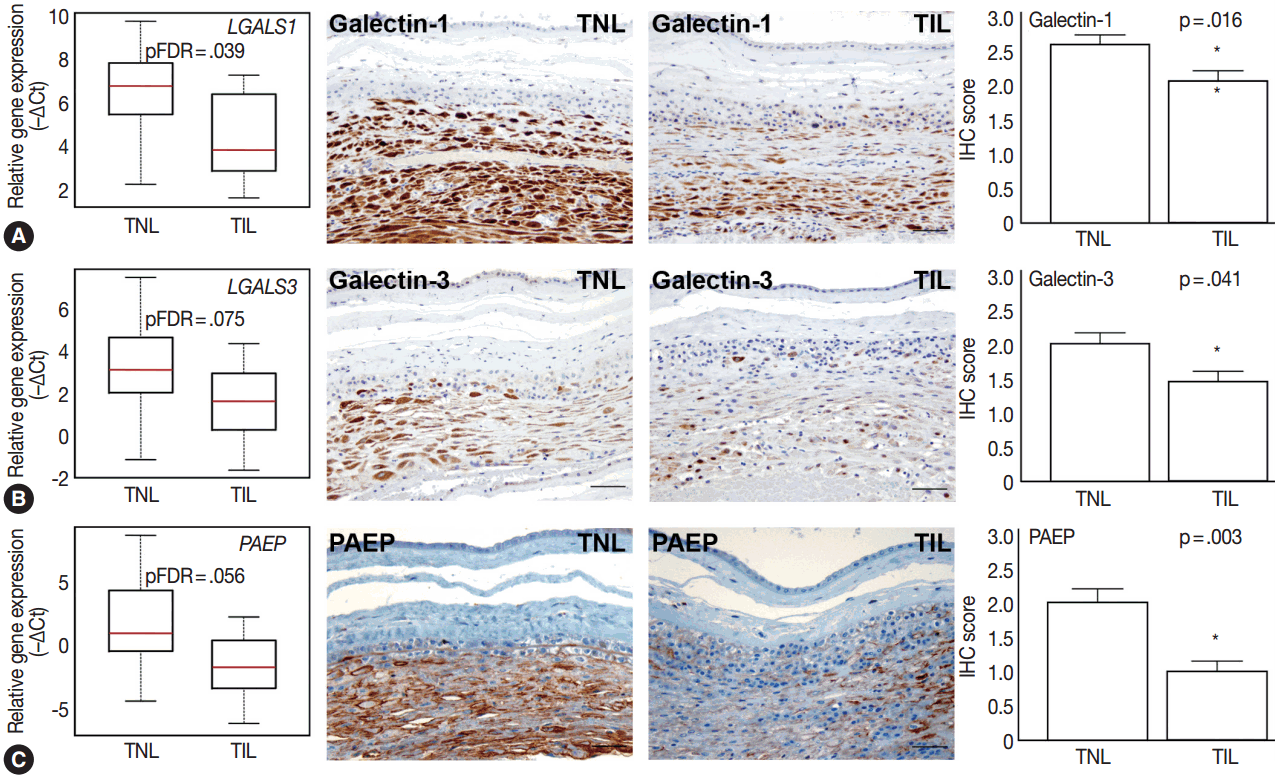
- Among the tested galectin genes, LGALS1 and LGALS3 had lower expression levels in TIL cases compared to TNL cases (LGALS1: 5.2-fold, pFDR = .039; LGALS3: 4.1-fold, pFDR = .075) (Fig. 4A, B). These findings were confirmed by immunostaining, as galectin-1 and galectin-3 proteins had decreased expression levels in TIL cases compared to TNL cases (galectin-1 IHC score, TIL 2.1 vs TNL 2.6, p = .016; galectin-3 IHC score, TIL 1.4 vs TNL 2.0, p = .041) (Fig. 4A, B).
- Among the genes encoding for endometrial proteins, PAEP, one of the most abundantly expressed genes in the decidua [53], had reduced expression levels in TIL cases compared to TNL cases (12.6-fold, pFDR = .056) (Fig. 4C). PAEP protein levels were also weaker in TIL cases compared to TNL cases (IHC score, TIL 1.0 vs TNL 2.0; p = .003) (Fig. 4C).
- Comparison of decidual gene expression and maternal plasma concentrations of chemokines
- In order to determine whether the local, decidual differential expression of chemokines is also reflected by changes in their systemic concentrations, maternal plasma samples collected at the time of delivery were analyzed with immunoassays for CCL2, CCL5, and IL-8. There was no difference in the concentrations of these chemokines between patient groups, and there was no correlation between the mRNA expression of these chemokines in the decidua and their protein concentrations in maternal plasma samples obtained from the same patients (Fig. 5).
RESULTS
Genes involved in sex steroid signaling and decidual development
Chemokine, cytokine, and prostaglandin signaling genes
Genes encoding anti-inflammatory proteins
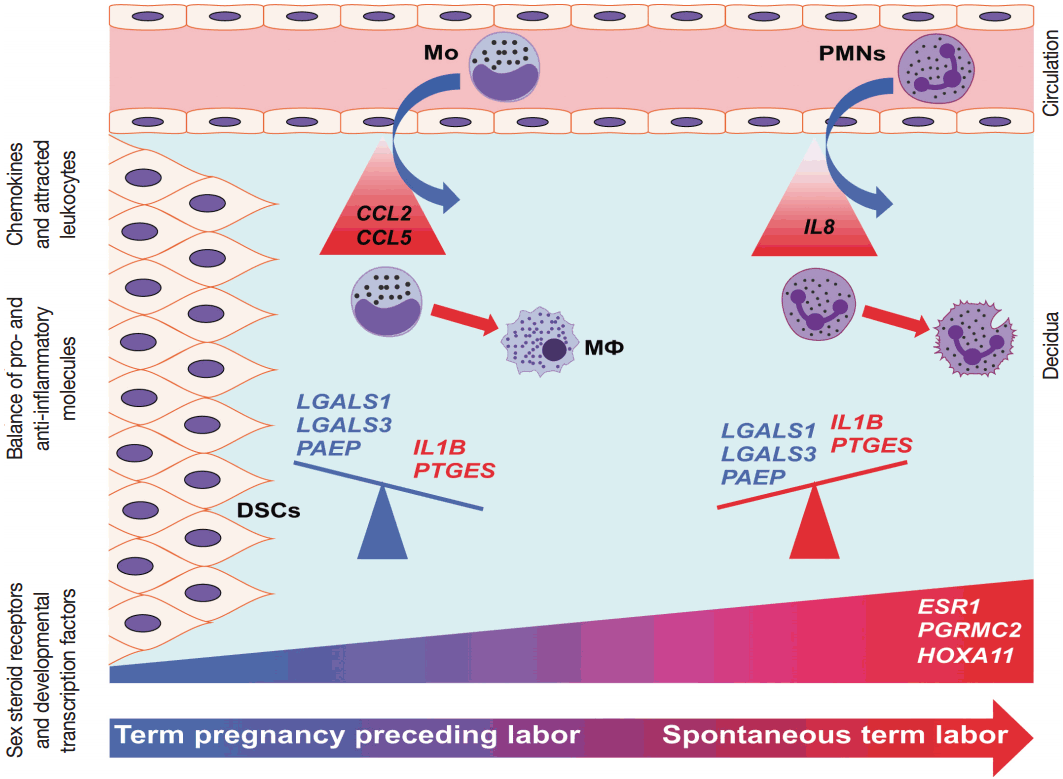
- Principal findings of the study
- (1) Transcriptomic analysis revealed differences in the expression of genes involved in developmental, signaling, and inflammatory pathways between decidual samples collected from women who delivered at TIL and TNL; (2) genes with the highest expression in the decidua at term included IGFBP1, LGALS1, and PAEP; (3) the expression of ESR1, HOXA11, IL1B, IL8, PGRMC2, and PTGES was higher in TIL cases compared to TNL cases; (4) the expression of CCL2, CCL5, LGALS1, LGALS3, and PAEP was lower in TIL cases compared to TNL cases; (5) immunostaining confirmed a high expression of IL-8 and a low expression of CCL2, galectin-1, and galectin-3 in TIL cases compared to TNL cases; and (6) no correlation between decidual gene expression and maternal plasma protein concentrations of CCL2, CCL5, and IL-8 was found.
- Significance of differential gene expression in the decidua
- Parturition is a result of pro-inflammatory processes in various gestational tissues, which interact with and orchestrate the terminal pathway of spontaneous labor [82]. This common pathway includes anatomical, physiological, biochemical, endocrinological, immunological, and clinical events that occur in the mother and her fetus. Based on targeted and high-dimensional studies, a growing body of evidence has shown that all uterine components of this terminal pathway—the increased myometrial contractility and cervical ripening as well as the activation of the decidua/fetal membranes—undergo pro-inflammatory changes during labor [82,83]. In spite of the similarities in gene expression signatures, the activation of certain biological pathways in various gestational tissues may be different. Unfortunately, the biological processes taking place in the decidua during spontaneous term parturition have been relatively underinvestigated. This is corroborated by a recent meta-analysis focusing on the transcriptomics of all human gestational tissues in the context of term and preterm birth [84]. This study found that the most commonly studied tissues are the placenta (61%), the fetal membranes (12%), and the myometrium (12%), while the decidua had been the focus of only less than 5% of all investigations [84]. Therefore, our study aimed to fill this gap in our understanding of the role of the decidua in the orchestration of pro-inflammatory pathways during spontaneous term parturition.
- Hormonal regulation of the decidua during labor
- The appropriate hormonal regulation and development of the decidua are fundamental in the establishment and maintenance of pregnancy. Among hormones, estrogen and progesterone play the most important roles in these processes mediated by estrogen and progesterone receptors [6], while HOX genes are essential for endometrial development, growth, and differentiation under the regulation of estrogen and progesterone [85,86]. Therefore, we studied the most relevant estrogen and progesterone receptors as well as HOX genes to gain insights into their decidual expression changes during term parturition. Members of all of these gene groups were found to have transcriptional changes, since ESR1, PGRMC2, and HOXA11 mRNA expression was increased in TIL cases compared to TNL cases.
- ERα (ESR1) is an estrogen receptor predominantly expressed in the uterus, and its expression elevates in the human myometrium during labor [87,88]. Interestingly, the increased relative abundance of ERα, but not ERβ (ESR2) mRNA, is associated with increased PR-A expression and PR-A/PR-B expression ratios in the human myometrium, which, in turn, is responsible for the inhibition of progesterone’s anti-inflammatory actions mediated by PR-B [7,8]. A similar increase in the PR-A/PR-B expression ratio was found in the decidua during term labor [8], which, in conjunction with our finding on the increased decidual ESR1 expression, may suggest that a similar regulatory process is effective in the decidua during term labor.
- The decidualization of the human endometrium depends on the actions of progesterone [89] promoted by the expression of nuclear PR isoforms in decidual cells [90], which are central to the genomic actions of progesterone. Moreover, the cellular responsiveness to this hormone also depends on the expression of the membrane progesterone receptors (mPRα, mPRβ, and mPRγ) and their adaptor proteins (PGRMC1 and PGRMC2), which act via a fast, “non-genomic pathway [91].” Several studies support the idea that membrane PRs actually suppress the actions of nuclear PRs and promote myometrial contractility [92] and that the PGRMC adaptor proteins are pivotal in enhancing the cell surface expression and receptor functions of membrane PRs [93]. A recent proteomics study on choriodecidual tissues revealed PGMRC2 to be up-regulated in samples from term and preterm labor samples compared to samples obtained from non-laboring women [94], implicating this protein in the hormonal regulation of parturition. Our finding of the higher decidual expression of PGRMC2 in laboring women, compared to non-laboring women, corroborates these earlier results and may suggest that this “non-genomic pathway” may be involved in the functional progesterone withdrawal in the decidua.
- Previous in vitro studies showed that estrogen and progesterone facilitated the expression of developmental HOX genes (HOXA9, HOXA10, and HOXA11) in human decidual cells at term, among which HOXA10 and HOXA11 are expressed in the decidua from early pregnancy [85,86] throughout gestation [95,96]. Furthermore, it has been shown that the interactions of HOXA11 with CCAAT/enhancer binding protein beta (CEBPB) and forkhead box O1 (FOXO1) are especially necessary for the regulation of decidual gene expression during the decidualization of endometrial fibroblasts [97,98]. In this context, it is of interest that FOXO1 can promote IL-1β, IL-6, IL-8, and cyclooxygenase-2 expression and the release of prostaglandins as well as matrix metalloproteinase 9 (MMP-9) expression and MMP activity as detected in pregnant human myometrial cells [99]. It is possible that the increased HOXA11 expression in the decidua of the TIL patients, compared to the TNL patients, who participated in our study may be related to the upstream regulatory events including ESR1 up-regulation and estrogen-mediated actions as well as downstream pro-inflammatory pathways involving the cooperation of HOXA11 with FOXO1 in the TIL cases.
- Chemokines, cytokines, and pro-inflammatory mediators in labor
- Among the mediators of pro-inflammatory pathways, chemokines are essential for the recruitment and activation of various immune cell populations into distinct tissue compartments at the maternal-fetal interface [100-102]. These infiltrating immune cells are involved in the orchestration of local inflammation, partly by releasing cytokines that regulate prostaglandin production [45]. Members of these gene groups of chemokines, cytokines, and receptors (CCL2, CCL5, IL8, IL1B, and PTGES) that we studied had transcriptional changes in TIL cases compared to TNL cases.
- CCL2 and CCL5 have been extensively studied in different gestational tissues as important inducers of immune cell infiltration. CCL2, a monocyte chemoattractant, has been studied in the context of pro-inflammatory changes and monocyte influx/activation in the myometrium, cervix, and fetal membranes during term and preterm parturition [103-105]. CCL5, another monocyte chemoattractant that has additional activity toward T cells, has been implicated in the regulation of inflammatory responses and in the recruitment of macrophages to the implantation site in early pregnancy [106-108]. The role of CCL5 in the mechanisms of human parturition is demonstrated by the increased concentration of this chemokine in the amniotic fluid of women who undergo term labor compared to those who deliver at term without labor [109]. The concentration of CCL5 is also higher in the amniotic fluid of women who undergo term or preterm labor with microbial invasion of the amniotic cavity than in those without this complication [107], supporting the role for CCL5 in the regulation of the host response against intrauterine infection. Of note, monocytes/macrophages are the second most abundant leukocyte population in the human decidua that contributes to parturition by expressing pro-inflammatory mediators (e.g., IL-1β, IL-6, TNFα, and prostaglandins) and that plays a key role in tissue remodeling [15]. Our findings demonstrate higher CCL2 and CCL5 mRNA expression in the decidua prior to labor, compared to TIL cases, which was validated for CCL2 expression at the protein level. These results are in agreement with rodent experiments showing pre-partum recruitment of macrophages into the uterine/decidual tissues in rats and mice [110-113] and with the increase in the proportion of human choriodecidual macrophages from preterm to term gestation [114]. Although the proportion of these macrophages among human choriodecidual leukocytes does not change before and after term labor [114], it was recently demonstrated that macrophages undergo a pro-inflammatory M1-like polarization in the decidua during spontaneous term labor [115]. Therefore, it is likely that macrophages infiltrate the human decidua prior to term gestation, and they acquire an M1 phenotype prior to parturition [115]. Concentrations of CCL2 and CCL5 in the maternal blood of women who undergo term labor are similar to those in women who undergo term delivery without labor [116]. In accord with this earlier finding, there was no correlation between decidual mRNA abundance and concentrations of CCL2 and CCL5 in maternal plasma, which further confirms their importance in the local inflammatory process. Overall, these data suggest that monocyte recruitment into the decidua and macrophage-mediated decidual inflammatory processes are early events during parturition and precede the CCL2- and CCL5-mediated recruitment of monocytes into other uterine tissue compartments.
- The local elevated expression of IL-8, a chief chemokine and activator of neutrophils [117,118], fulfills multifaceted roles, both in humans and other examined mammals during term and preterm parturition [119,120], and may determine the timing of neutrophil infiltration and activation in various compartments in the uterine cavity [121], where these cells release cytokines and MMPs to contribute to labor and post-partum wound sealing and healing [122]. Accordingly, the expression of IL-8 by a variety of cells—including monocytes/macrophages, fibroblasts, decidual stromal cells, cervical epithelial cells, and amnion cells [27,123]—have been reported to be elevated in gestational tissues and amniotic fluid in term and pretem parturition [124-133]. Our findings of the higher expression of IL-8 mRNA and protein in the decidua in TIL cases compared to TNL cases support these previous reports. Interestingly, studies that focus on IL-8 concentrations in maternal blood are controversial. One study found significantly higher IL-8 plasma concentrations in laboring women compared to non-laboring women who delivered at term or preterm [116], while another study showed no difference between these groups [134]. Our study agrees with the latter, since we found no difference between the TNL and TIL groups and no correlation between decidual mRNA expression and maternal plasma concentrations of IL-8.
- IL-1β and its receptor IL1R are widely expressed in human gestational tissues, where they are involved in the pro-inflammatory response during labor [135-137]; this is supported by the higher IL-1β concentrations in the amniotic, choriodecidual, and placental tissues [31] as well as in the amniotic fluid [138] of patients with spontaneous labor compared to those who delivered at term without labor. Our data suggests that the increased decidual IL1B expression in TIL cases compared to TNL cases is consistent with these earlier findings and further highlights the key role of IL-1β in parturition. As functional evidence, in vitro experiments with human decidual cells revealed that IL-1β treatment alters the expression of genes and microRNAs that function in pro-inflammatory signaling, including the induction of numerous cytokines and chemokines [139], such as IL-8 [140], as well as prostaglandin production [141].
- Prostaglandins have central roles in the separate but integrated physiological events of parturition: fetal membrane rupture, cervical dilatation, myometrial contractility, placental separation, and uterine involution [142-144]. There is abundant evidence showing that the decidua, among other gestational tissues, is a major source of prostaglandins in parturition [144]. PGF2α is the most abundant prostaglandin in the decidua, but decidual cells can also produce detectable amounts of prostaglandin E2 (PGF2) [145], which was increased in decidual cells obtained during spontaneous vaginal delivery at term compared to those obtained before the onset of labor [146].
- Among the prostaglandin-synthesizing enzymes, PTGS2, the rate-limiting enzyme of the synthesis of prostaglandins, was found to be up-regulated in the human myometrium [102], but not in the decidua [147], for TIL cases compared to TNL cases. Among other key enzymes of prostaglandin synthesis, PTGES, involved in PGF2 synthesis, was elevated in the amnion in TIL cases [148]. The same study found increased expression levels of AKR1C3 (aldo-keto reductase), involved in PGF2α synthesis, in the choriodecidua in TIL cases [148]. The expression of CBR1 (carbonyl reductase), responsible for the conversion of PGF2 to PGF2α and thereby favoring PGF2α elevation, was also detected in the decidua [148], but no difference was found in its expression between TIL and TNL cases. Our study only investigated PTGES from these three genes and found its increased expression in TIL cases compared to TNL cases. Since various pro-inflammatory cytokines [149] and estrogen can induce PTGES expression requiring nuclear factor κB and estrogen receptor signaling pathways, respectively [150], the elevated expression of PTGES during labor in the decidual inflammatory microenvironment is not surprising. All of these results may suggest that local inflammatory pathways induce PTGES, leading to heightened PGF2 production as well as increased production of PGF2α via enzymatic conversion.
- Transcription factors for T-cell polarization in labor
- The proper balance between innate and adaptive immune cells in gestational tissues is required to sustain pregnancy, and an alteration of this balance may lead to labor at term or preterm gestation [121]. Most studies investigated the roles of macrophage and neutrophil infiltration into gestational tissues during labor; however, the importance of adaptive immune responses in parturition has only recently been recognized [114]. In human pregnancies, almost one-half of the leukocytes in the decidua basalis are CD3+ T cells in term cases compared to the low decidual percentage (6%–30%) of these immune cells observed in the first trimester [151]. It has been proposed that the high proportion of T cells concentrated in the choriodecidua is a result of the expansion of the total number of CD4+ CD25+ regulatory T cells (Tregs) [114] induced by pregnancy [152,153]. It was suggested that these T cells are recruited during term gestation, particularly during parturition, to participate in the cascade of inflammatory mediators at the maternal-fetal interface [154]. Choriodecidual CD4+ T cells display a memory-like phenotype and express high levels of IL-1β, TNFα, and MMP-9 in spontaneous labor at term [114], suggesting that these immune cells have an effector-memory phenotype.
- The finding that different T helper (Th) lymphocyte populations (Th1, Th2, Treg, and Th17) could be identified in the early human decidua based on the expression of their lineage-specific differentiation transcription factors (TBX21, GATA3, FOXP3, and RORC) [155] gave us the idea to investigate various Th subsets in term decidua. The mRNAs for all of these transcription factors were found to be expressed, showing the presence of the four Th subsets in term decidua; however, no differences were found between the two groups. It is possible that certain differences could have been detected using phenotypic T-cell markers as previously reported [156] and also that the functional state of these cells may change during parturition.
- Anti-inflammatory molecules in labor
- While we detected an increased decidual expression among key pro-inflammatory mediator genes, the mRNA and protein expression levels of some anti-inflammatory molecules (galectin-1, galectin-3, and PAEP) were lower in TIL cases compared to TNL cases.
- Galectins are secreted proteins, multifunctional regulators of fundamental cellular processes. This is due to their high affinity for β-galactosides, which allow their binding to a broad range of glycosylated proteins critical for cell-cell and cell-extracellular matrix interactions [157] and the regulation of inflammation and immune responses [158]. Some galectins with strong expression at the maternal–fetal interface have been implicated in key functions of pregnancy maintenance in eutherian mammals [80,159,160]. Among these, galectin-1 and galectin-3 are expressed in the decidua under the regulation of sex steroids both in humans and mice [54,80,161,162] and are involved in decidual embryo implantation [163]. Of importance, decidual galectin-1 is one of the most potent mediators of maternal-fetal immune tolerance acting through multiple mechanisms, including the induction of tolerogenic dendritic cells, which, in turn, promote the expansion of IL-10–secreting regulatory T cells, as evidenced in mice [164]. Galectin-1 also has the ability to induce the apoptosis of Th1 cells, thereby causing a shift toward the Th2-type immune response, which is favorable during pregnancy [165]. Extracellular galectin-3 also has pro-apoptotic activity [166] and, thus, may have similar apoptotic functions compared to galectin-1 in the decidua. Our findings show decreased decidual LGALS1 and LGALS3 expression, which suggests that galectin-1 and galectin-3 may have important roles in the regulation of decidual immune cell populations and in the maintenance of a local microenvironment that favors progesterone production and pregnancy maintenance.
- PAEP, also known as glycodelin, placental protein 14, and progestagen-dependent endometrial protein, is a glycoprotein that has several isoforms according to its glycosylation pattern [167,168]. The most abundant glycodelin isoform, glycodelin-A, is highly expressed in the decidua [53] and has major anti-inflammatory properties [169]. Decidual PAEP expression is regulated by progesterone [170], and its immunoregulatory actions require estrogen priming [171]. This is in agreement with the role of PAEP as a potent regulator of predominant immune cell lineages at the maternal-fetal interface [172] and the capability of PAEP to induce the apoptosis of activated T cells [173], the inhibition of monocyte chemotaxis [174], and the induction of monocyte apoptosis [175]. Our results demonstrate decreased PAEP mRNA abundance and protein expression in TIL cases compared to TNL cases, which may suggest that glycodelin-A, similar to galectins, is involved in the maintenance of a local immune balance in the decidua.
- Our study also targeted the investigation of anti-inflammatory cytokines, but neither the decidual mRNA expression of IL4 nor IL10 changed with labor status at term. This may support other findings demonstrating no differences between IL-10 protein levels in decidual cells isolated from women who underwent labor compared to those who did not undergo labor at term [47]. However, the basal choriodecidual production rates of IL-10 have been found to be significantly decreased with labor [176], which relates to our findings on PAEP and galectins. This suggests that changes may also occur in IL-10 mRNA/protein expression at certain time-points during term labor, and that IL-10 may also be a part of the local molecular mechanisms that maintain decidual quiescence during pregnancy.
- Strengths and limitations
- The strengths of our study include the precise collection of decidual cells using laser microdissection, which allowed insights into decidual transcriptomic changes during spontaneous term parturition. High-throughput qRT-PCR enabled the parallel investigation of 46 genes involved in a large number of pathways. Immunostaining and semiquantitative immunoscoring of tissue sections from the same chorioamniotic membranes supported the decidual investigation of differentially expressed genes at the protein level. The comparisons between local decidual and maternal systemic expression of certain genes and their protein products in the same patients provided an additional strength of our study. This approach is unique since previous studies mainly focused on the investigation of the fetal membranes as a whole without illustrating which part of the membranes is responsible for the parturition-specific changes.
- Limitations of our study included the relatively small sample sizes due to the technical limitations (low yield, high labor intensity, and slow workpace) of laser microdissection. Many parturition-related molecules as well as alternative splicing variants of the examined genes have not been investigated since the tiny amount of RNA samples, which could be obtained with our methodology, only allowed the study of a maximum of 48 genes in our high-throughput qRT-PCR system. That is why we selected several key parturition-related genes with well-characterized decidual expression in spontaneous term labor as positive controls for our study as well as many other genes not yet characterized in the context of term labor. Since we examined gene expression signatures at two time-points, it was not possible to analyze in detail the exact temporal changes in the cascade of decidual gene expression changes as term parturition progressed.
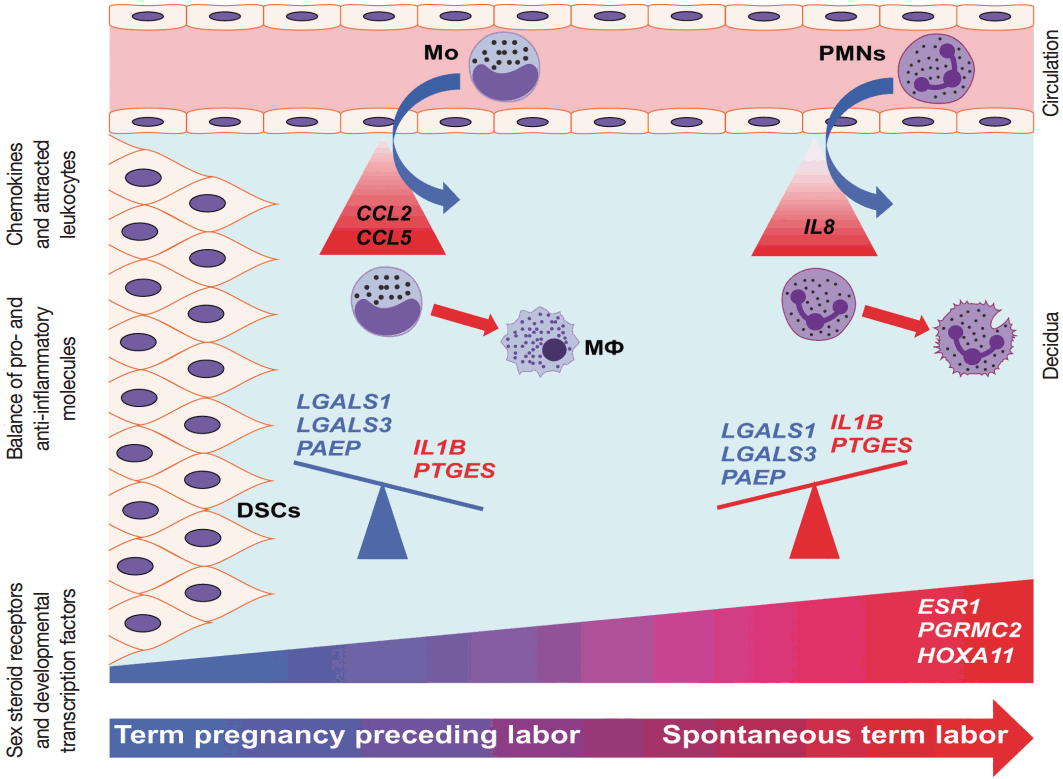
- Conclusions
- Our data proposes that, with the initiation of parturition, the decidual expression of anti-inflammatory mediators decreases while the expression of pro-inflammatory mediators and steroid receptors increases, affecting downstream signaling pathways that lead to chorioamniotic membrane weakening and myometrial contractions (Fig. 6). Our results agree with earlier findings that suggest that the decidua is one of the earliest gestational tissues that becomes primed during parturition. Nevertheless, functional studies are required to further explore and establish a causal link between the pathways that were found in our descriptive study during term labor.
DISCUSSION
Electronic Supplementary Material
Acknowledgments


- 1. Challis JR, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 2000; 21: 514-50. ArticlePubMed
- 2. Iliodromiti Z, Antonakopoulos N, Sifakis S, et al. Endocrine, paracrine, and autocrine placental mediators in labor. Hormones (Athens) 2012; 11: 397-409. ArticlePubMed
- 3. Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol 2007; 196: 289-96. ArticlePubMed
- 4. Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr Rev 1995; 16: 608-48. ArticlePubMed
- 5. Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab 2012; 97: E719-30. ArticlePubMedPMC
- 6. Csapo A. Progesterone block. Am J Anat 1956; 98: 273-91. ArticlePubMed
- 7. Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 2002; 87: 2924-30. ArticlePubMed
- 8. Oh SY, Kim CJ, Park I, et al. Progesterone receptor isoform (A/B) ratio of human fetal membranes increases during term parturition. Am J Obstet Gynecol 2005; 193(3 Pt 2):1156-60. ArticlePubMed
- 9. Mesiano S, Wang Y, Norwitz ER. Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reprod Sci 2011; 18: 6-19. ArticlePubMedPDF
- 10. Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol 2009; 200: 104.e1-11. ArticlePubMed
- 11. Hassan SS, Romero R, Haddad R, et al. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol 2006; 195: 778-86. ArticlePubMed
- 12. Stephen GL, Lui S, Hamilton SA, et al. Transcriptomic profiling of human choriodecidua during term labor: inflammation as a key driver of labor. Am J Reprod Immunol 2015; 73: 36-55. ArticlePubMedPDF
- 13. Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol Hum Reprod 2002; 8: 399-408. ArticlePubMed
- 14. Chaemsaithong P, Madan I, Romero R, et al. Characterization of the myometrial transcriptome in women with an arrest of dilatation during labor. J Perinat Med 2013; 41: 665-81. ArticlePubMedPMC
- 15. Thomson AJ, Telfer JF, Young A, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod 1999; 14: 229-36. ArticlePubMed
- 16. Gomez-Lopez N, Tanaka S, Zaeem Z, Metz GA, Olson DM. Maternal circulating leukocytes display early chemotactic responsiveness during late gestation. BMC Pregnancy Childbirth 2013; 13 Suppl 1: S8.ArticlePubMedPDF
- 17. Sakamoto Y, Moran P, Bulmer JN, Searle RF, Robson SC. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol 2005; 66: 161-73. ArticlePubMed
- 18. Haddad R, Tromp G, Kuivaniemi H, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol 2006; 195: 394.e1-24. ArticlePubMed
- 19. Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, VegaSanchez R, Vadillo-Ortega F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol 2009; 80: 122-31. ArticlePubMed
- 20. Gomez-Lopez N, Laresgoiti-Servitje E, Olson DM, Estrada-Gutiérrez G, Vadillo-Ortega F. The role of chemokines in term and premature rupture of the fetal membranes: a review. Biol Reprod 2010; 82: 809-14. PubMed
- 21. Gomez-Lopez N, Vadillo-Perez L, Nessim S, Olson DM, Vadillo-Ortega F. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am J Obstet Gynecol 2011; 204: 364.e9-16. ArticlePubMed
- 22. Osman I, Young A, Jordan F, Greer IA, Norman JE. Leukocyte density and proinflammatory mediator expression in regional human fetal membranes and decidua before and during labor at term. J Soc Gynecol Investig 2006; 13: 97-103. PubMed
- 23. Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM, Vadillo-Ortega F. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol 2011; 205: 235.e15-24. ArticlePubMed
- 24. Yellon SM, Mackler AM, Kirby MA. The role of leukocyte traffic and activation in parturition. J Soc Gynecol Investig 2003; 10: 323-38. ArticlePubMed
- 25. Hua R, Pease JE, Sooranna SR, et al. Stretch and inflammatory cytokines drive myometrial chemokine expression via NF-kappaB activation. Endocrinology 2012; 153: 481-91. PubMed
- 26. Kayisli UA, Mahutte NG, Arici A. Uterine chemokines in reproductive physiology and pathology. Am J Reprod Immunol 2002; 47: 213-21. ArticlePubMedPDF
- 27. Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod 2002; 66: 445-9. PubMed
- 28. Montes MJ, Tortosa CG, Borja C, et al. Constitutive secretion of interleukin-6 by human decidual stromal cells in culture: regulatory effect of progesterone. Am J Reprod Immunol 1995; 34: 188-94. ArticlePubMed
- 29. Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992; 27: 117-23. PubMed
- 30. Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol 1992; 166: 1576-87. ArticlePubMed
- 31. Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol 1999; 181: 1530-6. ArticlePubMed
- 32. Gomez-Lopez N, Hernandez-Santiago S, Lobb AP, Olson DM, Vadillo-Ortega F. Normal and premature rupture of fetal membranes at term delivery differ in regional chemotactic activity and related chemokine/cytokine production. Reprod Sci 2013; 20: 276-84. ArticlePubMedPDF
- 33. Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol 2008; 79: 50-7. ArticlePubMed
- 34. Winkler M, Rath W. The role of cytokines in the induction of labor, cervical ripening and rupture of the fetal membranes. Z Geburtshilfe Neonatol 1996; 200 Suppl 1: 1-12. PubMed
- 35. Bowen JM, Chamley L, Keelan JA, Mitchell MD. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta 2002; 23: 257-73. ArticlePubMed
- 36. Mitchell MD, Dudley DJ, Edwin SS, Schiller SL. Interleukin-6 stimulates prostaglandin production by human amnion and decidual cells. Eur J Pharmacol 1991; 192: 189-91. ArticlePubMed
- 37. Belt AR, Baldassare JJ, Molnár M, Romero R, Hertelendy F. The nuclear transcription factor NF-kappaB mediates interleukin-1beta-induced expression of cyclooxygenase-2 in human myometrial cells. Am J Obstet Gynecol 1999; 181: 359-66. PubMed
- 38. Kniss DA, Rovin B, Fertel RH, Zimmerman PD. Blockade NF-kappaB activation prohibits TNF-alpha-induced cyclooxygenase-2 gene expression in ED27 trophoblast-like cells. Placenta 2001; 22: 80-9. ArticlePubMed
- 39. Ackerman WE 4th, Summerfield TL, Vandre DD, Robinson JM, Kniss DA. Nuclear factor-kappa B regulates inducible prostaglandin E synthase expression in human amnion mesenchymal cells. Biol Reprod 2008; 78: 68-76. PubMed
- 40. Li R, Ackerman WE 4th, Summerfield TL, et al. Inflammatory gene regulatory networks in amnion cells following cytokine stimulation: translational systems approach to modeling human parturition. PLoS One 2011; 6: e20560. Article
- 41. Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol 2009; 297: R525-45. ArticlePubMed
- 42. Maymon E, Ghezzi F, Edwin SS, et al. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol 1999; 181(5 Pt 1):1142-8. PubMed
- 43. Romero R, Sepulveda W, Mazor M, et al. The natural interleukin-1 receptor antagonist in term and preterm parturition. Am J Obstet Gynecol 1992; 167(4 Pt 1):863-72. PubMed
- 44. Ammala M, Nyman T, Salmi A, Rutanen EM. The interleukin-1 system in gestational tissues at term: effect of labour. Placenta 1997; 18: 717-23. ArticlePubMed
- 45. Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition: a review. Placenta 2003; 24 Suppl A: S33-46. ArticlePubMed
- 46. Dudley DJ, Hunter C, Mitchell MD, Varner MW. Amniotic fluid interleukin-10 (IL-10) concentrations during pregnancy and with labor. J Reprod Immunol 1997; 33: 147-56. ArticlePubMed
- 47. Jones CA, Finlay-Jones JJ, Hart PH. Type-1 and type-2 cytokines in human late-gestation decidual tissue. Biol Reprod 1997; 57: 303-11. PubMed
- 48. Gotsch F, Romero R, Kusanovic JP, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med 2008; 21: 529-47. ArticlePubMedPMC
- 49. Dudley DJ, Hunter C, Varner MW, Mitchell MD. Elevation of amniotic fluid interleukin-4 concentrations in women with preterm labor and chorioamnionitis. Am J Perinatol 1996; 13: 443-7. ArticlePubMed
- 50. Than NG, Romero R, Goodman M, et al. A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc Natl Acad Sci U S A 2009; 106: 9731-6. ArticlePubMedPMC
- 51. Barrientos G, Freitag N, Tirado-Gonzalez I, et al. Involvement of galectin-1 in reproduction: past, present and future. Hum Reprod Update 2014; 20: 175-93. ArticlePubMed
- 52. Than NG, Romero R, Balogh A, et al. Galectins: Double-edged swords in the cross-roads of pregnancy complications and female reproductive tract inflammation and neoplasia. J Pathol Transl Med 2015; 49: 181-208. ArticlePubMedPMCPDF
- 53. Julkunen M, Rutanen EM, Koskimies A, Ranta T, Bohn H, Seppala M. Distribution of placental protein 14 in tissues and body fluids during pregnancy. Br J Obstet Gynaecol 1985; 92: 1145-51. ArticlePubMed
- 54. Phillips B, Knisley K, Weitlauf KD, Dorsett J, Lee V, Weitlauf H. Differential expression of two beta-galactoside-binding lectins in the reproductive tracts of pregnant mice. Biol Reprod 1996; 55: 548-58. PubMed
- 55. Lockwood CJ, Paidas M, Krikun G, et al. Inflammatory cytokine and thrombin regulation of interleukin-8 and intercellular adhesion molecule-1 expression in first trimester human decidua. J Clin Endocrinol Metab 2005; 90: 4710-5. ArticlePubMed
- 56. Huang SJ, Schatz F, Masch R, et al. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol 2006; 72: 60-73. ArticlePubMed
- 57. Sarno J, Schatz F, Huang SJ, Lockwood C, Taylor HS. Thrombin and interleukin-1beta decrease HOX gene expression in human first trimester decidual cells: implications for pregnancy loss. Mol Hum Reprod 2009; 15: 451-7. PubMedPMC
- 58. Segerer SE, Martignoni F, Bogdan A, et al. Thrombopoietin modulates the proliferation, migration and cytokine profile of decidual cell subsets during early gestation. Mol Hum Reprod 2013; 19: 361-8. ArticlePubMed
- 59. Kim JS, Romero R, Cushenberry E, et al. Distribution of CD14+ and CD68+ macrophages in the placental bed and basal plate of women with preeclampsia and preterm labor. Placenta 2007; 28: 571-6. ArticlePubMed
- 60. Kim SY, Romero R, Tarca AL, et al. Methylome of fetal and maternal monocytes and macrophages at the feto-maternal interface. Am J Reprod Immunol 2012; 68: 8-27. ArticlePubMedPMC
- 61. Paavola LG, Furth EE, Delgado V, et al. Striking changes in the structure and organization of rat fetal membranes precede parturition. Biol Reprod 1995; 53: 321-38. PubMed
- 62. Norwitz ER, Robinson JN, Challis JR. The control of labor. N Engl J Med 1999; 341: 660-6. ArticlePubMed
- 63. Mittal P, Romero R, Tarca AL, et al. A molecular signature of an arrest of descent in human parturition. Am J Obstet Gynecol 2011; 204: 177.e15-33. ArticlePubMed
- 64. Norwitz ER, Bonney EA, Snegovskikh VV, et al. Molecular regulation of parturition: the role of the decidual clock. Cold Spring Harb Perspect Med 2015; 5: a023143.ArticlePubMedPMC
- 65. Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update 2016; 22: 535-60. ArticlePubMedPMC
- 66. Daftary GS, Taylor HS. Implantation in the human: the role of HOX genes. Semin Reprod Med 2000; 18: 311-20. ArticlePubMed
- 67. Cermik D, Karaca M, Taylor HS. HOXA10 expression is repressed by progesterone in the myometrium: differential tissue-specific regulation of HOX gene expression in the reproductive tract. J Clin Endocrinol Metab 2001; 86: 3387-92. ArticlePubMed
- 68. Hsieh-Li HM, Witte DP, Weinstein M, et al. Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development 1995; 121: 1373-85. ArticlePubMedPDF
- 69. Taylor HS. The role of HOX genes in the development and function of the female reproductive tract. Semin Reprod Med 2000; 18: 81-9. ArticlePubMed
- 70. Wong KH, Wintch HD, Capecchi MR. HOXA11 regulates stromal cell death and proliferation during neonatal uterine development. Mol Endocrinol 2004; 18: 184-93. ArticlePubMed
- 71. Lu Z, Hardt J, Kim JJ. Global analysis of genes regulated by HOXA10 in decidualization reveals a role in cell proliferation. Mol Hum Reprod 2008; 14: 357-66. ArticlePubMedPMC
- 72. Lynch VJ, Tanzer A, Wang Y, et al. Adaptive changes in the transcription factor HoxA-11 are essential for the evolution of pregnancy in mammals. Proc Natl Acad Sci U S A 2008; 105: 14928-33. ArticlePubMedPMC
- 73. Xu B, Geerts D, Bu Z, et al. Regulation of endometrial receptivity by the highly expressed HOXA9, HOXA11 and HOXD10 HOX-class homeobox genes. Hum Reprod 2014; 29: 781-90. ArticlePubMed
- 74. Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol 1996; 87: 163-8. ArticlePubMed
- 75. Redline RW. Placental pathology: a systematic approach with clinical correlations. Placenta 2008; 29 Suppl A: S86-91. ArticlePubMed
- 76. Kim CJ, Romero R, Kusanovic JP, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol 2010; 23: 1000-11. ArticlePubMedPMCPDF
- 77. Than NG, Abdul Rahman O, Magenheim R, et al. Placental protein 13 (galectin-13) has decreased placental expression but increased shedding and maternal serum concentrations in patients presenting with preterm pre-eclampsia and HELLP syndrome. Virchows Arch 2008; 453: 387-400. ArticlePubMedPMCPDF
- 78. R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2011.
- 79. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 1995; 57: 289-300. ArticlePDF
- 80. Than NG, Romero R, Erez O, et al. Emergence of hormonal and redox regulation of galectin-1 in placental mammals: implication in maternal-fetal immune tolerance. Proc Natl Acad Sci U S A 2008; 105: 15819-24. ArticlePubMedPMC
- 81. Han VK, Bassett N, Walton J, Challis JR. The expression of insulinlike growth factor (IGF) and IGF-binding protein (IGFBP) genes in the human placenta and membranes: evidence for IGF-IGFBP interactions at the feto-maternal interface. J Clin Endocrinol Metab 1996; 81: 2680-93. ArticlePubMed
- 82. Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG 2006; 113 Suppl 3: 17-42. ArticlePubMed
- 83. Romero R, Espinoza J, Gotsch F, et al. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. BJOG 2006; 113 Suppl 3: 118-35. ArticlePubMed
- 84. Eidem HR, Ackerman WE 4th, McGary KL, Abbot P, Rokas A. Gestational tissue transcriptomics in term and preterm human pregnancies: a systematic review and meta-analysis. BMC Med Genomics 2015; 8: 27.ArticlePubMedPMCPDF
- 85. Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest 1998; 101: 1379-84. Article
- 86. Taylor HS, Igarashi P, Olive DL, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab 1999; 84: 1129-35. ArticlePubMed
- 87. Bisits AM, Smith R, Mesiano S, et al. Inflammatory aetiology of human myometrial activation tested using directed graphs. PLoS Comput Biol 2005; 1: 132-6. ArticlePubMed
- 88. Welsh T, Johnson M, Yi L, et al. Estrogen receptor (ER) expression and function in the pregnant human myometrium: estradiol via ERalpha activates ERK1/2 signaling in term myometrium. J Endocrinol 2012; 212: 227-38. PubMed
- 89. Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology 1999; 140: 4809-20. ArticlePubMed
- 90. Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology 1999; 140: 5310-21. ArticlePubMedPMC
- 91. Gellersen B, Fernandes MS, Brosens JJ. Non-genomic progesterone actions in female reproduction. Hum Reprod Update 2009; 15: 119-38. ArticlePubMed
- 92. Karteris E, Zervou S, Pang Y, et al. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol 2006; 20: 1519-34. ArticlePubMedPDF
- 93. Thomas P, Pang Y, Dong J. Enhancement of cell surface expression and receptor functions of membrane progestin receptor alpha (mPRalpha) by progesterone receptor membrane component 1 (PGRMC1): evidence for a role of PGRMC1 as an adaptor protein for steroid receptors. Endocrinology 2014; 155: 1107-19. ArticlePubMedPMC
- 94. Shankar R, Johnson MP, Williamson NA, et al. Molecular markers of preterm labor in the choriodecidua. Reprod Sci 2010; 17: 297-310. ArticlePubMedPDF
- 95. Salih SM, Taylor HS. HOXA10 gene expression in human fallopian tube and ectopic pregnancy. Am J Obstet Gynecol 2004; 190: 1404-6. ArticlePubMed
- 96. Evans KN, Bulmer JN, Kilby MD, Hewison M. Vitamin D and placental-decidual function. J Soc Gynecol Investig 2004; 11: 263-71. ArticlePubMedPDF
- 97. Lynch VJ, Brayer K, Gellersen B, Wagner GP. HoxA-11 and FOXO1A cooperate to regulate decidual prolactin expression: towards inferring the core transcriptional regulators of decidual genes. PLoS One 2009; 4: e6845. Article
- 98. Wagner GP, Kin K, Muglia L, Pavlicev M. Evolution of mammalian pregnancy and the origin of the decidual stromal cell. Int J Dev Biol 2014; 58: 117-26. ArticlePubMed
- 99. Lappas M. Forkhead box O1 (FOXO1) in pregnant human myometrial cells: a role as a pro-inflammatory mediator in human parturition. J Reprod Immunol 2013; 99: 24-32. ArticlePubMed
- 100. Dudley DJ, Edwin SS, Dangerfield A, Van Waggoner J, Mitchell MD. Regulation of cultured human chorion cell chemokine production by group B streptococci and purified bacterial products. Am J Reprod Immunol 1996; 36: 264-8. ArticlePubMed
- 101. Dudley DJ, Edwin SS, Van Wagoner J, Augustine NH, Hill HR, Mitchell MD. Regulation of decidual cell chemokine production by group B streptococci and purified bacterial cell wall components. Am J Obstet Gynecol 1997; 177: 666-72. ArticlePubMed
- 102. Mittal P, Romero R, Tarca AL, et al. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med 2010; 38: 617-43. ArticlePubMedPMC
- 103. Tornblom SA, Klimaviciute A, Byström B, Chromek M, Brauner A, Ekman-Ordeberg G. Non-infected preterm parturition is related to increased concentrations of IL-6, IL-8 and MCP-1 in human cervix. Reprod Biol Endocrinol 2005; 3: 39.PubMedPMC
- 104. Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol 2008; 181: 1470-9. ArticlePubMedPDF
- 105. Esplin MS, Peltier MR, Hamblin S, et al. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta 2005; 26: 661-71. ArticlePubMed
- 106. Jones RL, Hannan NJ, Kaitu’u TJ, Zhang J, Salamonsen LA. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab 2004; 89: 6155-67. ArticlePubMed
- 107. Engert S, Rieger L, Kapp M, Becker JC, Dietl J, Kämmerer U. Profiling chemokines, cytokines and growth factors in human early pregnancy decidua by protein array. Am J Reprod Immunol 2007; 58: 129-37. ArticlePubMed
- 108. Li M, Wu ZM, Yang H, Huang SJ. NFkappaB and JNK/MAPK activation mediates the production of major macrophage- or dendritic cell-recruiting chemokine in human first trimester decidual cells in response to proinflammatory stimuli. J Clin Endocrinol Metab 2011; 96: 2502-11. PubMedPMC
- 109. Athayde N, Romero R, Maymon E, et al. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol 1999; 181: 989-94. ArticlePubMed
- 110. Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod 1999; 61: 879-83. ArticlePubMed
- 111. Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, Nguyen T, Lye SJ. Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. J Cell Mol Med 2013; 17: 311-24. ArticlePubMedPMCPDF
- 112. Hamilton S, Oomomian Y, Stephen G, et al. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod 2012; 86: 39.PubMed
- 113. Srikhajon K, Shynlova O, Preechapornprasert A, Chanrachakul B, Lye S. A new role for monocytes in modulating myometrial inflammation during human labor. Biol Reprod 2014; 91: 10.PubMed
- 114. Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol 2013; 69: 212-30. ArticlePubMedPMC
- 115. Xu Y, Romero R, Miller D, et al. An M1-like macrophage polarization in decidual tissue during spontaneous preterm labor that is attenuated by rosiglitazone treatment. J Immunol 2016; 196: 2476-91. ArticlePubMedPMCPDF
- 116. Hamilton SA, Tower CL, Jones RL. Identification of chemokines associated with the recruitment of decidual leukocytes in human labour: potential novel targets for preterm labour. PLoS One 2013; 8: e56946. Article
- 117. Peveri P, Walz A, Dewald B, Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med 1988; 167: 1547-59. ArticlePubMedPMCPDF
- 118. Kelly RW, Illingworth P, Baldie G, Leask R, Brouwer S, Calder AA. Progesterone control of interleukin-8 production in endometrium and chorio-decidual cells underlines the role of the neutrophil in menstruation and parturition. Hum Reprod 1994; 9: 253-8. ArticlePubMed
- 119. Gervasi MT, Chaiworapongsa T, Pacora P, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol 2001; 185: 792-7. ArticlePubMed
- 120. Yuan M, Jordan F, McInnes IB, Harnett MM, Norman JE. Leukocytes are primed in peripheral blood for activation during term and preterm labour. Mol Hum Reprod 2009; 15: 713-24. ArticlePubMedPMC
- 121. Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell Mol Immunol 2014; 11: 571-81. ArticlePubMedPMCPDF
- 122. Giaglis S, Stoikou M, Grimolizzi F, et al. Neutrophil migration into the placenta: good, bad or deadly? Cell Adh Migr 2016; 10: 208-25. ArticlePubMedPMCPDF
- 123. Castillo-Castrejon M, Meraz-Cruz N, Gomez-Lopez N, et al. Choriodecidual cells from term human pregnancies show distinctive functional properties related to the induction of labor. Am J Reprod Immunol 2014; 71: 86-93. ArticlePubMed
- 124. Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol 1991; 165(4 Pt 1):813-20. ArticlePubMed
- 125. Tashima LS, Millar LK, Bryant-Greenwood GD. Genes upregulated in human fetal membranes by infection or labor. Obstet Gynecol 1999; 94: 441-9. ArticlePubMed
- 126. Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 2003; 9: 41-5. ArticlePubMed
- 127. Osmers RG, Bläser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol 1995; 86: 223-9. ArticlePubMed
- 128. Sennström MK, Brauner A, Lu Y, Granström LM, Malmström AL, Ekman GE. Interleukin-8 is a mediator of the final cervical ripening in humans. Eur J Obstet Gynecol Reprod Biol 1997; 74: 89-92. ArticlePubMed
- 129. Sennström MB, Ekman G, Westergren-Thorsson G, et al. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod 2000; 6: 375-81. ArticlePubMed
- 130. Charpigny G, Leroy MJ, Breuiller-Fouché M, et al. A functional genomic study to identify differential gene expression in the preterm and term human myometrium. Biol Reprod 2003; 68: 2289-96. PubMed
- 131. Bethin KE, Nagai Y, Sladek R, et al. Microarray analysis of uterine gene expression in mouse and human pregnancy. Mol Endocrinol 2003; 17: 1454-69. ArticlePubMedPDF
- 132. Rehman KS, Yin S, Mayhew BA, Word RA, Rainey WE. Human myometrial adaptation to pregnancy: cDNA microarray gene expression profiling of myometrium from non-pregnant and pregnant women. Mol Hum Reprod 2003; 9: 681-700. ArticlePubMed
- 133. Axemo P, Brauner A, Pettersson M, Eriksson L, Rwamushaija E, Bergstrom S. Amniotic fluid interleukins in Swedish and Mozambican pregnant women. Gynecol Obstet Invest 1996; 41: 113-7. ArticlePubMed
- 134. Laham N, Rice GE, Bishop GJ, Ransome C, Brennecke SP. Interleukin 8 concentrations in amniotic fluid and peripheral venous plasma during human pregnancy and parturition. Acta Endocrinol (Copenh) 1993; 129: 220-4. ArticlePubMed
- 135. Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989; 160(5 Pt 1):1117-23. ArticlePubMedPDF
- 136. Romero R, Wu YK, Brody DT, Oyarzun E, Duff GW, Durum SK. Human decidua: a source of interleukin-1. Obstet Gynecol 1989; 73: 31-4. PubMed
- 137. Steinborn A, Geisse M, Kaufmann M. Expression of cytokine receptors in the placenta in term and preterm labour. Placenta 1998; 19: 165-70. ArticlePubMed
- 138. Romero R, Parvizi ST, Oyarzun E, et al. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med 1990; 35: 235-8. PubMed
- 139. Ibrahim SA, Ackerman WE 4th, Summerfield TL, Lockwood CJ, Schatz F, Kniss DA. Inflammatory gene networks in term human decidual cells define a potential signature for cytokine-mediated parturition. Am J Obstet Gynecol 2016; 214: 284.e1-47. ArticlePubMed
- 140. Dudley DJ, Trautman MS, Mitchell MD. Inflammatory mediators regulate interleukin-8 production by cultured gestational tissues: evidence for a cytokine network at the chorio-decidual interface. J Clin Endocrinol Metab 1993; 76: 404-10. ArticlePubMed
- 141. Alnaif B, Benzie RJ, Gibb W. Studies on the action of interleukin-1 on term human fetal membranes and decidua. Can J Physiol Pharmacol 1994; 72: 133-9. ArticlePubMed
- 142. Challis JR, Lye SJ. Parturition. Oxf Rev Reprod Biol 1986; 8: 61-129. PubMed
- 143. Challis JR, Lye SJ, Gibb W. Prostaglandins and parturition. Ann N Y Acad Sci 1997; 828: 254-67. PubMed
- 144. Olson DM. The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol 2003; 17: 717-30. ArticlePubMed
- 145. Mitchell MD, Edwin S, Romero RJ. Prostaglandin biosynthesis by human decidual cells: effects of inflammatory mediators. Prostaglandins Leukot Essent Fatty Acids 1990; 41: 35-8. ArticlePubMed
- 146. Skinner KA, Challis JR. Changes in the synthesis and metabolism of prostaglandins by human fetal membranes and decidua at labor. Am J Obstet Gynecol 1985; 151: 519-23. ArticlePubMed
- 147. Fuentes A, Spaziani EP, O’Brien WF. The expression of cyclooxygenase-2 (COX-2) in amnion and decidua following spontaneous labor. Prostaglandins 1996; 52: 261-7. ArticlePubMed
- 148. Phillips RJ, Fortier MA, López Bernal A. Prostaglandin pathway gene expression in human placenta, amnion and choriodecidua is differentially affected by preterm and term labour and by uterine inflammation. BMC Pregnancy Childbirth 2014; 14: 241.ArticlePubMedPMCPDF
- 149. Phillips RJ, Al-Zamil H, Hunt LP, Fortier MA, López Bernal A. Genes for prostaglandin synthesis, transport and inactivation are differentially expressed in human uterine tissues, and the prostaglandin F synthase AKR1B1 is induced in myometrial cells by inflammatory cytokines. Mol Hum Reprod 2011; 17: 1-13. ArticlePubMed
- 150. Frasor J, Weaver AE, Pradhan M, Mehta K. Synergistic up-regulation of prostaglandin E synthase expression in breast cancer cells by 17beta-estradiol and proinflammatory cytokines. Endocrinology 2008; 149: 6272-9. PubMedPMC
- 151. Vargas ML, Sántos JL, Ruiz C, et al. Comparison of the proportions of leukocytes in early and term human decidua. Am J Reprod Immunol 1993; 29: 135-40. ArticlePubMed
- 152. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 2004; 5: 266-71. ArticlePubMedPDF
- 153. Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 2004; 112: 38-43. ArticlePubMedPMC
- 154. Gomez-Lopez N, Guilbert LJ, Olson DM. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol 2010; 88: 625-33. ArticlePubMedPDF
- 155. Mjösberg J, Berg G, Jenmalm MC, Ernerudh J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol Reprod 2010; 82: 698-705. PubMed
- 156. Sindram-Trujillo AP, Scherjon SA, van Hulst-van Miert PP, Kanhai HH, Roelen DL, Claas FH. Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J Reprod Immunol 2004; 62: 125-37. ArticlePubMed
- 157. Hughes RC. Galectins as modulators of cell adhesion. Biochimie 2001; 83: 667-76. ArticlePubMed
- 158. Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol 2009; 9: 338-52. ArticlePubMedPDF
- 159. Hirota Y, Burnum KE, Acar N, Rabinovich GA, Daikoku T, Dey SK. Galectin-1 markedly reduces the incidence of resorptions in mice missing immunophilin FKBP52. Endocrinology 2012; 153: 2486-93. ArticlePubMedPMCPDF
- 160. Than NG, Romero R, Kim CJ, McGowen MR, Papp Z, Wildman DE. Galectins: guardians of eutherian pregnancy at the maternalfetal interface. Trends Endocrinol Metab 2012; 23: 23-31. ArticlePubMed
- 161. Szekeres-Bartho J, Halasz M, Palkovics T. Progesterone in pregnancy; receptor-ligand interaction and signaling pathways. J Reprod Immunol 2009; 83: 60-4. ArticlePubMed
- 162. von Wolff M, Wang X, Gabius HJ, Strowitzki T. Galectin fingerprinting in human endometrium and decidua during the menstrual cycle and in early gestation. Mol Hum Reprod 2005; 11: 189-94. ArticlePubMed
- 163. Tirado-González I, Freitag N, Barrientos G, et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol Hum Reprod 2013; 19: 43-53. ArticlePubMed
- 164. Blois SM, Ilarregui JM, Tometten M, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med 2007; 13: 1450-7. ArticlePubMedPDF
- 165. Than NG, Kim SS, Abbas A, et al. Chorioamnionitis and increased galectin-1 expression in PPROM: an anti-inflammatory response in the fetal membranes? Am J Reprod Immunol 2008; 60: 298-311. PubMedPMC
- 166. Dumic J, Dabelic S, Flögel M. Galectin-3: an open-ended story. Biochim Biophys Acta 2006; 1760: 616-35. ArticlePubMed
- 167. Seppälä M, Taylor RN, Koistinen H, Koistinen R, Milgrom E. Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev 2002; 23: 401-30. ArticlePubMed
- 168. Than GN, Bohn H, Szabo DG. Advances in pregnancy-related protein research: functional and clinical applications. Boca Raton: CRC Press Inc., 1993.
- 169. Alok A, Karande AA. The role of glycodelin as an immune-modulating agent at the feto-maternal interface. J Reprod Immunol 2009; 83: 124-7. ArticlePubMed
- 170. Taylor RN, Savouret JF, Vaisse C, et al. Promegestone (R5020) and mifepristone (RU486) both function as progestational agonists of human glycodelin gene expression in isolated human epithelial cells. J Clin Endocrinol Metab 1998; 83: 4006-12. ArticlePubMed
- 171. Li TC, Dalton C, Bolton AE, Ling E, Warren A, Cooke ID. An analysis of the variation of plasma concentrations of placental protein 14 in artificial cycles. Fertil Steril 1992; 57: 776-82. ArticlePubMed
- 172. Bolton AE, Pockley AG, Clough KJ, et al. Identification of placental protein 14 as an immunosuppressive factor in human reproduction. Lancet 1987; 1: 593-5. ArticlePubMed
- 173. Mukhopadhyay D, Sundereshan S, Rao C, Karande AA. Placental protein 14 induces apoptosis in T cells but not in monocytes. J Biol Chem 2001; 276: 28268-73. ArticlePubMed
- 174. Vigne JL, Hornung D, Mueller MD, Taylor RN. Purification and characterization of an immunomodulatory endometrial protein, glycodelin. J Biol Chem 2001; 276: 17101-5. ArticlePubMed
- 175. Alok A, Mukhopadhyay D, Karande AA. Glycodelin A, an immunomodulatory protein in the endometrium, inhibits proliferation and induces apoptosis in monocytic cells. Int J Biochem Cell Biol 2009; 41: 1138-47. ArticlePubMed
- 176. Simpson KL, Keelan JA, Mitchell MD. Labor-associated changes in interleukin-10 production and its regulation by immunomodulators in human choriodecidua. J Clin Endocrinol Metab 1998; 83: 4332-7. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- The sweet and the bitter sides of galectin-1 in immunity: its role in immune cell functions, apoptosis, and immunotherapies for cancer with a focus on T cells
Julianna Novák, Tamás Takács, Álmos Tilajka, Loretta László, Orsolya Oravecz, Emese Farkas, Nándor Gábor Than, László Buday, Andrea Balogh, Virág Vas
Seminars in Immunopathology.2025;[Epub] CrossRef - Exosomal Delivery of Interleukin‐10 Reduces Infection‐Associated Inflammation in a 3D‐Printed Model of a Humanized Feto–Maternal Interface
Leah M. Saylor, Rahul Cherukuri, Ananth K. Kammala, Lauren Richardson, Marc Ferrer, Cristina Antich, Shayne Frebert, Arum Han, Ramkumar Menon
The FASEB Journal.2025;[Epub] CrossRef - Progesterone inactivation in decidual stromal cells: A mechanism for inflammation-induced parturition
Angela DeTomaso, Hyeyon Kim, Jacqueline Shauh, Anika Adulla, Sarah Zigo, Maya Ghoul, Pietro Presicce, Suhas G. Kallapur, Wendy Goodman, Tamara Tilburgs, Sing-Sing Way, David Hackney, John Moore, Sam Mesiano
Proceedings of the National Academy of Sciences.2024;[Epub] CrossRef - The endoplasmic reticulum protein HSPA5/BiP is essential for decidual transformation of human endometrial stromal cells
Laura Fernández, Chow-Seng Kong, Majd Alkhoury, Maria Tryfonos, Paul J. Brighton, Thomas M. Rawlings, Joanne Muter, Maria Soledad Gori, Claudia Pérez Leirós, Emma S. Lucas, Jan J. Brosens, Rosanna Ramhorst
Scientific Reports.2024;[Epub] CrossRef - Galectin‐1 Elicits a Tissue‐Specific Anti‐Inflammatory and Anti‐Degradative Effect Upon LPS‐Induced Response in an Ex Vivo Model of Human Fetal Membranes Modeling an Intraamniotic Inflammation
Jazmin Hernández‐Rodríguez, Jesús Pérez‐Hernández, Pilar Flores‐Espinosa, Andrea Olmos‐Ortiz, Pilar Velazquez, Rodrigo Zamora‐Escudero, Marcela Islas‐López, Addy Cecilia Helguera‐Repetto, Karla Hernández‐Bones, Samara Rodríguez‐Flores, Rodrigo Jiménez‐Esc
American Journal of Reproductive Immunology.2024;[Epub] CrossRef - Is human labor at term an inflammatory condition?
Chandrashekara Kyathanahalli, Madeline Snedden, Emmet Hirsch
Biology of Reproduction.2023; 108(1): 23. CrossRef - CCL2: An important cytokine in normal and pathological pregnancies: A review
Zhi Lin, Jia-Lu Shi, Min Chen, Zi-Meng Zheng, Ming-Qing Li, Jun Shao
Frontiers in Immunology.2023;[Epub] CrossRef - Proteomic profile of extracellular vesicles in maternal plasma of women with fetal death
Dahiana M. Gallo, Wendy Fitzgerald, Roberto Romero, Nardhy Gomez-Lopez, Dereje W. Gudicha, Nándor Gábor Than, Mariachiara Bosco, Tinnakorn Chaiworapongsa, Eunjung Jung, Arun Meyyazhagan, Manaphat Suksai, Francesca Gotsch, Offer Erez, Adi L. Tarca, Leonid
The Journal of Maternal-Fetal & Neonatal Medicine.2023;[Epub] CrossRef - Downregulated INHBB in endometrial tissue of recurrent implantation failure patients impeded decidualization through the ADCY1/cAMP signalling pathway
Hui Zhang, Zhilong Wang, Quan Zhou, Zhiwen Cao, Yue Jiang, Manlin Xu, Jingyu Liu, Jidong Zhou, Guijun Yan, Haixiang Sun
Journal of Assisted Reproduction and Genetics.2023; 40(5): 1135. CrossRef - Regulation of inflammatory genes in decidual cells: Involvement of the bromodomain and extra-terminal family proteins
Sandeep Ajgaonkar, Jonathan J. Hirst, Mary Norris, Tamas Zakar, Giovanni Camussi
PLOS ONE.2023; 18(3): e0280645. CrossRef - Decidual cells and decidualization in the carnivoran endotheliochorial placenta
Mónica Elizabeth Diessler, Rocío Hernández, Gimena Gomez Castro, Claudio Gustavo Barbeito
Frontiers in Cell and Developmental Biology.2023;[Epub] CrossRef - Further Evidence that an Episode of Premature Labor Is a Pathologic State: Involvement of the Insulin-Like Growth Factor System
Priya Prasad, Roberto Romero, Tinnakorn Chaiworapongsa, Nardhy Gomez-Lopez, Anderson Lo, Jose Galaz, Andreea B. Taran, Eunjung Jung, Francesca Gotsch, Nandor Gabor Than, Adi L. Tarca
Fetal Diagnosis and Therapy.2023; 50(4): 236. CrossRef - Galectins-1, -3 and -9 Are Present in Breast Milk and Have a Role in Early Life Development
Karla Rio-Aige, Marina Girbal, Marta Selma-Royo, Anna Parra-Llorca, Sonia González, Cecilia Martínez-Costa, Margarida Castell, María Carmen Collado, Francisco J. Pérez-Cano, María J. Rodríguez-Lagunas
Nutrients.2022; 14(20): 4338. CrossRef - The Expression of IL-1β Correlates with the Expression of Galectin-3 in the Tissue at the Maternal–Fetal Interface during the Term and Preterm Labor
Nikola Jovic, Marija Milovanovic, Jovana Joksimovic Jovic, Marija Bicanin Ilic, Dejana Rakic, Vladimir Milenkovic, Bojana Stojanovic, Jelena Milovanovic, Aleksandar Arsenijevic, Nebojsa Arsenijevic, Mirjana Varjacic
Journal of Clinical Medicine.2022; 11(21): 6521. CrossRef - Galectin-1 and Galectin-9 Concentration in Maternal Serum: Implications in Pregnancies Complicated with Preterm Prelabor Rupture of Membranes
Dorota Grażyna Boroń, Aleksy Świetlicki, Michał Potograbski, Grażyna Kurzawińska, Przemysław Wirstlein, Daniel Boroń, Krzysztof Drews, Agnieszka Seremak-Mrozikiewicz
Journal of Clinical Medicine.2022; 11(21): 6330. CrossRef - Placental galectins regulate innate and adaptive immune responses in pregnancy
Orsolya Oravecz, Roberto Romero, Eszter Tóth, Judit Kapitány, Máté Posta, Dahiana M. Gallo, Simona W. Rossi, Adi L. Tarca, Offer Erez, Zoltán Papp, János Matkó, Nándor Gábor Than, Andrea Balogh
Frontiers in Immunology.2022;[Epub] CrossRef - Galectin-Levels Are Elevated in Infants Born Preterm Due to Amniotic Infection and Rapidly Decline in the Neonatal Period
Kirstin Faust, Nancy Freitag, Gabriela Barrientos, Christoph Hartel, Sandra M. Blois
Frontiers in Immunology.2021;[Epub] CrossRef - Inflammatory Amplification: A Central Tenet of Uterine Transition for Labor
Kelycia B. Leimert, Wendy Xu, Magdalena M. Princ, Sylvain Chemtob, David M. Olson
Frontiers in Cellular and Infection Microbiology.2021;[Epub] CrossRef - Medawar’s PostEra: Galectins Emerged as Key Players During Fetal-Maternal Glycoimmune Adaptation
Ellen Menkhorst, Nandor Gabor Than, Udo Jeschke, Gabriela Barrientos, Laszlo Szereday, Gabriela Dveksler, Sandra M. Blois
Frontiers in Immunology.2021;[Epub] CrossRef - The Role of Decidual Subpopulations in Implantation, Menstruation and Miscarriage
Joanne Muter, Chow-Seng Kong, Jan J. Brosens
Frontiers in Reproductive Health.2021;[Epub] CrossRef - Multiomic immune clockworks of pregnancy
Laura S. Peterson, Ina A. Stelzer, Amy S. Tsai, Mohammad S. Ghaemi, Xiaoyuan Han, Kazuo Ando, Virginia D. Winn, Nadine R. Martinez, Kevin Contrepois, Mira N. Moufarrej, Stephen Quake, David A. Relman, Michael P. Snyder, Gary M. Shaw, David K. Stevenson, R
Seminars in Immunopathology.2020; 42(4): 397. CrossRef - Role of galectin-glycan circuits in reproduction: from healthy pregnancy to preterm birth (PTB)
Sandra M. Blois, Stefan Verlohren, Gang Wu, Gary Clark, Anne Dell, Stuart M. Haslam, Gabriela Barrientos
Seminars in Immunopathology.2020; 42(4): 469. CrossRef - Functional changes in decidual mesenchymal stem/stromal cells are associated with spontaneous onset of labour
Joan C Wijaya, Ramin Khanabdali, Harry M Georgiou, Maria I Kokkinos, Patrick F James, Shaun P Brennecke, Bill Kalionis
Molecular Human Reproduction.2020; 26(8): 636. CrossRef - Ageing in human parturition: impetus of the gestation clock in the decidua†
Joan C Wijaya, Ramin Khanabdali, Harry M Georgiou, Bill Kalionis
Biology of Reproduction.2020; 103(4): 695. CrossRef - Cytotrophoblast extracellular vesicles enhance decidual cell secretion of immune modulators via TNF-alpha
Sara K. Taylor, Sahar Houshdaran, Joshua F. Robinson, Matthew J. Gormley, Elaine Y. Kwan, Mirhan Kapidzic, Birgit Schilling, Linda C. Giudice, Susan J. Fisher
Development.2020;[Epub] CrossRef - A trypsin-based method for isolating leukocytes from human choriodecidua suitable for immunophenotyping and transcriptome studies
Karla MacDonald-Ramos, Marcia Arenas-Hernandez, Ismael Mancilla-Herrera, Claudia Rangel-Escareño, Rodrigo Vega-Sanchez
Immunobiology.2019; 224(1): 177. CrossRef - Transcriptomic analysis of fetal membranes reveals pathways involved in preterm birth
Silvana Pereyra, Claudio Sosa, Bernardo Bertoni, Rossana Sapiro
BMC Medical Genomics.2019;[Epub] CrossRef - Placental Galectins Are Key Players in Regulating the Maternal Adaptive Immune Response
Andrea Balogh, Eszter Toth, Roberto Romero, Katalin Parej, Diana Csala, Nikolett L. Szenasi, Istvan Hajdu, Kata Juhasz, Arpad F. Kovacs, Hamutal Meiri, Petronella Hupuczi, Adi L. Tarca, Sonia S. Hassan, Offer Erez, Peter Zavodszky, Janos Matko, Zoltan Pap
Frontiers in Immunology.2019;[Epub] CrossRef - Amnion epithelial cell–derived exosomes induce inflammatory changes in uterine cells
Emily E. Hadley, Samantha Sheller-Miller, George Saade, Carlos Salomon, Sam Mesiano, Robert N. Taylor, Brandie D. Taylor, Ramkumar Menon
American Journal of Obstetrics and Gynecology.2018; 219(5): 478.e1. CrossRef - Selective immuno-modulatory effect of prolactin upon pro-inflammatory response in human fetal membranes
Pilar Flores-Espinosa, Eduardo Preciado-Martínez, Araceli Mejía-Salvador, Gabriela Sedano-González, Luisa Bermejo-Martínez, Adalberto Parra-Covarruvias, Guadalupe Estrada-Gutiérrez, Rodrigo Vega-Sánchez, Isabel Méndez, Braulio Quesada-Reyna, Andrea Olmos-
Journal of Reproductive Immunology.2017; 123: 58. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure






Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
| Variable | Spontaneous term labor (n = 14) | Term no labor (n = 15) | p-value |
|---|---|---|---|
| Maternal age (yr) | 25 (20–29) | 27 (23–34) | .13 |
| Race | .60 | ||
| African American | 12 (85.7) | 11 (73.3) | - |
| Caucasian | 1 (7.1) | 3 (20.0) | - |
| Other | 1 (7.1) | 1 (6.7) | - |
| Maternal weight (kg) | 64 (62–85) | 75 (68–82) | .13 |
| Body mass index (kg/m2) | 26.7 (24.6–32.9) | 28.3 (27.4–30.9) | .49 |
| Gravidity | 3 (2.5–5) | 3 (2–4) | .76 |
| Gestational age at delivery (wk) | 39.1 (38.7–39.3) | 39.0 (38.9–39.1) | .31 |
| Birth weight (g) | 3,230 (3,083–3,602) | 3,490 (3,185–3,560) | .66 |
| Group | Gene symbol | Gene name | Assay ID |
|---|---|---|---|
| Chemokines | CCL1 | Chemokine (C-C motif) ligand 1 | Hs00171072_m1 |
| CCL2 | Chemokine (C-C motif) ligand 2 | Hs00234140_m1 | |
| CCL5 | Chemokine (C-C motif) ligand 5 | Hs00174575_m1 | |
| CCL7 | Chemokine (C-C motif) ligand 7 | Hs00171147_m1 | |
| CCL15 | Chemokine (C-C motif) ligand 15 | Hs00361122_m1 | |
| CXCL1 | Chemokine (C-X-C motif) ligand 1 | Hs00236937_m1 | |
| CXCL5 | Chemokine (C-X-C motif) ligand 5 | Hs00171085_m1 | |
| CXCL10 | Chemokine (C-X-C motif) ligand 10 | Hs00171042_m1 | |
| CXCL12 | Chemokine (C-X-C motif) ligand 12 | Hs00171022_m1 | |
| IL8 | Interleukin-8/Chemokine (C-X-C motif) ligand 8 | Hs00174103_m1 | |
| Chemokine receptors | CCR1 | Chemokine (C-C motif) receptor 1 | Hs00174298_m1 |
| CCR2 | Chemokine (C-C motif) receptor 2 | Hs00356601_m1 | |
| CCR5 | Chemokine (C-C motif) receptor 5 | Hs00152917_m1 | |
| CCR8 | Chemokine (C-C motif) receptor 8 | Hs00174764_m1 | |
| CXCR1 | Chemokine (C-X-C motif) receptor 1 | Hs01921207_s1 | |
| CXCR2 | Chemokine (C-X-C motif) receptor 2 | Hs01011557_m1 | |
| CXCR3 | Chemokine (C-X-C motif) receptor 3 | Hs00171041_m1 | |
| CXCR4 | Chemokine (C-X-C motif) receptor 4 | Hs00976734_m1 | |
| Cytokines | IL1A | Interleukin 1, alpha | Hs99999028_m1 |
| IL1B | Interleukin 1, beta | Hs00174097_m1 | |
| IL4 | Interleukin 4 | Hs00174122_m1 | |
| IL10 | Interleukin 10 | Hs00174086_m1 | |
| IL17A | Interleukin 17A | Hs00174383_m1 | |
| IFNG | Interferon, gamma | Hs99999041_m1 | |
| TNF | Tumor necrosis factor alpha | Hs00174128_m1 | |
| Galectins | LGALS1 | Lectin, galactoside-binding, soluble, 1 | Hs00169327_m1 |
| LGALS3 | Lectin, galactoside-binding, soluble, 3 | Hs00173587_m1 | |
| LGALS8 | Lectin, galactoside-binding, soluble, 8 | Hs00374634_m1 | |
| LGALS9A | Lectin, galactoside-binding, soluble, 9 | Hs00371321_m1 | |
| Endometrial proteins | IGFBP1 | Insulin-like growth factor-binding protein 1 | Hs00236877_m1 |
| PAEP | Progestagen-associated endometrial protein | Hs01046125_m1 | |
| HOX transcription factors | HOXA9 | Homeobox A9 | Hs00266821_m1 |
| HOXA10 | Homeobox A10 | Hs00172012_m1 | |
| HOXA11 | Homeobox A11 | Hs00194149_m1 | |
| Prostaglandin signaling molecules | PTGER2 | Prostaglandin E receptor 2 | Hs00168754_m1 |
| PTGES | Prostaglandin E synthase | Hs00610420_m1 | |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 | Hs01573471_m1 | |
| Sex steroid receptors | ESR1 | Estrogen receptor 1 | Hs00174860_m1 |
| ESR2 | Estrogen receptor 2 (beta) | Hs01100356_m1 | |
| PGRMC1 | Progesterone receptor membrane component 1 | Hs00198499_m1 | |
| PGRMC2 | Progesterone receptor membrane component 2 | Hs01128672_m1 | |
| PGR | Progesterone receptor | Hs01556702_m1 | |
| T-cell transcription factors | FOXP3 | Forkhead box P3 | Hs01085835_m1 |
| GATA3 | GATA binding protein 3 | Hs00231122_m1 | |
| RORC | RAR-related orphan receptor gamma | Hs01076122_m1 | |
| TBX21 | T-box 21 | Hs00203436_m1 | |
| Housekeeping genes | GAPDH | Glyceraldehyde 3-phosphate dehydrogenase | Hs99999905_m1 |
| RPLPO | Ribosomal protein, large, P0 | Hs99999902_m1 |
Values are presented as median (interquartile range) or number (%).
qRT-PCR, quantitative real-time polymerase chain reaction; RAR, retinoic acid-receptor.

 E-submission
E-submission