Hyalinizing Cholecystitis and Associated Carcinoma: A Case Report
Article information
Hyalinizing cholecystitis (HC) is a recently described rare variant of chronic cholecystitis that is characterized by replacement of the normal structures of the entire gallbladder wall with diffuse and dense hyaline sclerosis [1]. Some cases of cholecystitis are associated with variable degrees of calcification. Porcelain gallbladder (PG) is an extensively calcific example that is a vaguely and radiologically defined entity whose pathologic correlation has not been identified [2-4]. PG is subclassified into “complete porcelain,” showing extensive dystrophic calcifications that form an intramural continuous band involving more than 80% of the gallbladder wall, and “incomplete porcelain,” which lacks these findings [2,5-7]. Although HC is rare and found in only approximately 1.6% of cholecystectomy specimens, it is more commonly accompanied by carcinomas with more aggressive clinical behavior compared to usual gallbladder carcinomas [1]. However, preoperative diagnosis of HC-related carcinoma is challenging, because the wall is thinner than that of typical chronic cholecystitis or usual carcinomas, and discrete masses are not formed. There has been only one case report of HC, which was accompanied by immunoglobulin G4–related disease [8], since Patel et al. [1] first described the entity in a retrospective case series. We present a case of HC and associated carcinoma masquerading as primary biliary cancer.
CASE REPORT
A 54-year-old man visited the hospital due to fatigue, pruritus, and diarrhea. Laboratory findings revealed elevations in aspartate aminotransferase (98 IU/L; reference range, 3 to 45 IU/L), alanine transaminase (232 IU/L; reference range, 3 to 45 IU/L), alkaline phosphatase (320 IU/L; reference range, 30 to 120 IU/L), and gamma-glutamyl transpeptidase (619 IU/L; reference range, 9 to 64 IU/L). Biliary computed tomography scan and magnetic resonance cholangiography revealed enhancing wall thickening of the cystic duct and common hepatic duct and multiple calcified gallstones as well as sludge in the gallbladder with mild wall thickening (Fig. 1A). Radiologically, carcinoma of the primary bile duct or cystic duct and associated chronic calculous cholecystitis was suspected, and bile duct resection with cholecystectomy was performed.
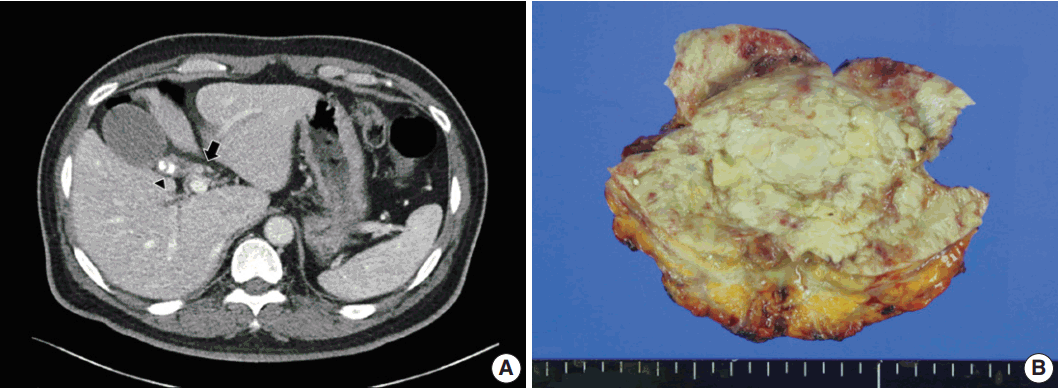
Computed tomography and gross findings of hyalinizing cholecystitis and associated carcinoma. (A) Biliary computed tomography scan reveals subtle enhancement of wall thickening at the confluence of the cystic duct and the common hepatic duct (arrow). There is mild wall thickening of the gallbladder with multiple calcified gallstones in the neck portion (arrowhead). (B) Grossly, the gallbladder wall shows diffuse fibrosis and is covered by yellowish necrotic materials, without mass-like lesions.
An 8.0 × 5.5 × 5.5 cm gallbladder was submitted for intraoperative frozen diagnosis. Grossly, the gallbladder wall was diffusely fibrotic, and the mucosal surface was covered by yellowish necrotic material with a gallstone (2.0 × 1.2 × 1.0 cm). There were no mass-like lesions (Fig. 1B). Two random frozen sections were examined microscopically, and an inflammatory lesion without malignancy was suggested. After the operation, we also received the segmentally resected common hepatic duct (1.7 cm in length, 0.6 cm in diameter) and the attached cystic duct (1.7 cm in length, 0.4 cm in diameter). The bile duct wall was diffusely thickened; however, the mucosa of the ducts was smooth and did not form mass-like lesions. The entire permanent sections of the gallbladder and bile ducts were examined microscopically. The gallbladder wall was nearly completely replaced by dense lamellated eosinophilic hyaline material, with multifocal neutrophilic and lymphoplasmacytic infiltration. Focal intramural calcifications were identified (Fig. 2A, B). Multifocal invasive glands with irregular borders were sparsely scattered in the hyalinized gallbladder wall in a longitudinal arrangement (Fig. 2C). The mucosal surface of the gallbladder showed extensive denudation of the epithelium, but some foci of high-grade biliary intraepithelial neoplasia were noted (Fig. 2D). There were focal areas composed of clusters of invasive glands in the gallbladder wall (Fig. 2E). The invasive glands were composed of stratified depolarized atypical cells with enlarged hyperchromatic nuclei containing coarse chromatin and occasionally prominent nucleoli. Some glands also showed perineural invasion (Fig. 2F). Diffuse involvement of the walls of the common hepatic duct and cystic duct by adenocarcinoma was observed (Fig. 3A, B); however, the mucosa was relatively spared, with no intraepithelial lesions (Fig. 3C). Considering all of these findings, we made the diagnosis of HC and associated adenocarcinoma, with direct invasion of the cystic duct and common hepatic duct.
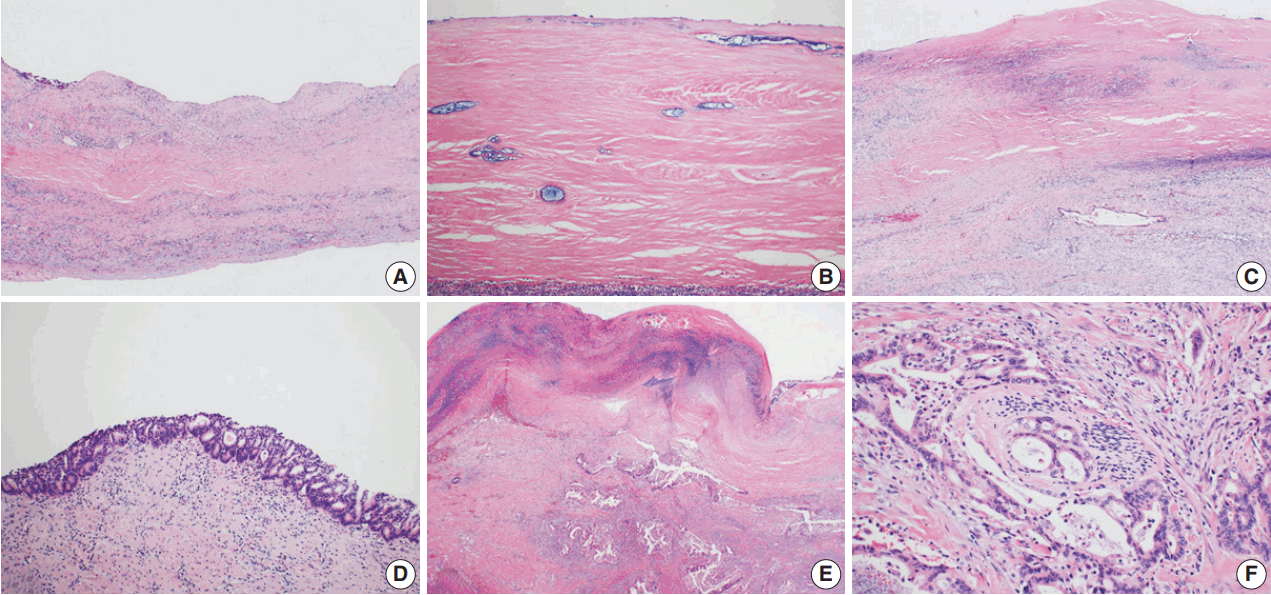
Microscopic findings of hyalinizing cholecystitis and associated carcinoma. (A, B) The gallbladder wall is replaced by dense lamellated eosinophilic hyaline material. Inflammatory cells are also seen. (C) A few invasive glands are longitudinally arranged in the hyalinized gallbladder wall with a denuded epithelium. (D) Multifocal carcinoma in situ lesions are found on the surface. (E) Focal clusters of invasive glands are identified in the hyalinized wall. (F) Invasive glands have irregular borders and cytologic atypia with perineural invasion.

Microscopic findings of the common hepatic and cystic ducts. (A, B) Invasive glands infiltrate the common hepatic and cystic ducts beneath the surface epithelium. (C) The surface epithelium is relatively spared, with no intraepithelial lesion.
This study was approved by the Institutional Review Board of Korea University Anam Hospital (ED17246), and informed consent was waived.
DISCUSSION
HC is a recently described, distinctive entity, comprising 1.6% of cholecystectomy specimens [1]. HC is characterized by diffuse and dense hyalinization of the gallbladder wall with complete effacement of normal histologic components. Focal to extensive calcification may also be seen. HC is more often associated with carcinoma; the frequency of carcinoma was 15% compared to 4% of all cholecystectomy specimens in a South American cohort [1]. However, to identify the carcinoma component is diagnostically challenging because it does not form distinct mass lesions or induce significant thickening of the gallbladder wall [1]. Furthermore, the malignant glands are not easily found due to the paucity and sparse distribution of tumor glands within an abundant hyalinizing sclerotic background. In our case, there were no mass-like lesions, and the entire gallbladder wall was diffusely fibrotic with no significant wall thickening. The carcinoma component was not found in two randomly submitted frozen sections; however, it was identified after extensive sampling of permanent sections. Therefore, careful examination with extensive or entire sampling of the gallbladder is crucial in HC to identify associated carcinoma.
Preoperative diagnosis of HC-related carcinoma is also highly challenging. Patel et al. [1] reported that about 70% of malignancies had been diagnosed as benign preoperatively. This might be because HC-associated carcinomas typically do not form a mass or result in thickening of the gallbladder. Subsequently, surgical resection can be delayed until progression of the malignancy. In our case, the malignancy was identified radiologically after the tumor directly extended to the extrahepatic bile duct, and the preoperative diagnosis was primary bile duct cancer with chronic calculous cholecystitis. The final pathologic stage was T3 (direct invasion of the extrahepatic bile ducts). The late discovery of carcinoma is associated with advanced stage at diagnosis and poor prognosis of HC-related carcinomas. The median survival for HC-associated carcinoma is shorter than that of usual carcinomas [1].
In conclusion, HC is a rare variant of chronic cholecystitis and is often associated with carcinoma with aggressive behavior. Suspicion of the possibility of HC and identification of HC as an unusual variant of chronic cholecystitis are important in gross examination of cholecystectomy specimens. After the identification of HC, extensive sampling and meticulous microscopic examination are essential to determine the possibility of associated carcinoma. In addition, close follow-up is recommended because carcinoma associated with HC can have more aggressive behavior than typical gallbladder cancer.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.