Utility of BRAF VE1 Immunohistochemistry as a Screening Tool for Colorectal Cancer Harboring BRAF V600E Mutation
Article information
Abstract
Background
BRAF mutation has been recognized as an important biomarker of colorectal cancer (CRC) for targeted therapy and prognosis prediction. However, sequencing for every CRC case is not cost-effective. An antibody specific for BRAF V600E mutant protein has been introduced, and we thus examined the utility of BRAF VE1 immunohistochemistry for evaluating BRAF mutations in CRC.
Methods
Fifty-one BRAF-mutated CRCs and 100 age and sexmatched BRAF wild-type CRCs between 2005 and 2015 were selected from the archives of Asan Medical Center. Tissue microarrays were constructed and stained with BRAF VE1 antibody.
Results
Forty-nine of the 51 BRAF-mutant CRCs (96.1%) showed more than moderate cytoplasmic staining, except for two weakly stained cases. Six of 100 BRAF wild-type cases also stained positive with BRAF VE1 antibody; four stained weakly and two stained moderately. Normal colonic crypts showed nonspecific weak staining, and a few CRC cases exhibited moderate nuclear reactivity (3 BRAF-mutant and 10 BRAF wild-type cases). BRAF-mutated CRC patients had higher pathologic stages and worse survival than BRAF wild-type patients.
Conclusions
BRAF VE1 immunohistochemistry showed high sensitivity and specificity, but occasional nonspecific staining in tumor cell nuclei and normal colonic crypts may limit their routine clinical use. Thus, BRAF VE1 immunohistochemistry may be a useful screening tool for BRAF V600E mutation in CRCs, provided that additional sequencing studies can be done to confirm the mutation in BRAF VE1 antibody-positive cases.
Colorectal cancer (CRC) is one of most common forms of malignancy worldwide and deadliest cancer-related diseases [1]. It has been described to result from sequential activation of oncogenes and concomitant inactivation of tumor suppressor genes [1]. Among these various oncogenic events, approximately 10%–15% of CRC patients are characterized by a mutation in v-Raf murine sarcoma viral oncogene homolog B (BRAF) [1,2]. BRAF oncogene encoding BRAF protein, which is localized in the downstream of RAS, leads to the stimulation of mitogen-activated protein kinase pathway. It contains a typical hot spot oncogenic mutation, typically V600E (change from valine to glutamic acid at codon 600), which accounts for up to 80% of all BRAF mutations [3]. BRAF mutation has also been reported to be an independent predictor of poor prognosis in CRC [1,4]. Typically, BRAF mutations in CRC can be detected by Sanger sequencing or allele-specific polymerase chain reaction (PCR), but these methods are time-consuming and costly. Recently, immunohistochemistry (IHC) using an antibody specific for BRAF V600E mutant protein (BRAF VE1 antibody) has been proposed as a useful diagnostic tool for BRAF V600E mutation detection in CRC [5,6], but its clinical utility is controversial. For instance, the staining quality of BRAF VE1 antibody in CRC has been reported to be inferior to that in melanoma or thyroid cancer [7,8]. Thus, it is unclear whether BRAF VE1 antibody can be used in the clinic to detect BRAF V600E mutation in CRC in place of sequencing analyses. In this study, we evaluated the usefulness of BRAF VE1 IHC for detecting BRAF V600E mutations in CRC and analyzed the clinicopathologic characteristics of BRAF-mutant CRCs compared to those in BRAF wild-type controls.
MATERIALS AND METHODS
Patients and samples
The study group consisted of 51 surgically resected primary or metastatic CRC cases harboring BRAF V600E mutation (colonoscopic biopsy [n = 17], primary tumor resection [n = 31], and metastasectomy [n = 3]) and 100 age and sex-matched BRAF wild-type CRCs (colonoscopic biopsy [n = 14], primary tumor resection [n = 81], and metastasectomy [n = 5]). They were selected from the surgical pathology files between 2005 and 2015 at the Department of Pathology, Asan Medical Center, University of Ulsan Collage of Medicine, Seoul, Korea. The BRAF V600E mutation status was confirmed by Sanger sequencing (n = 75), quantitative allele-specific PCR (n = 16), and mass spectrometry-based genotyping (n = 60). All cases were KRAS wild-type. Histopathological features of the 151 CRCs were reviewed by two pathologists (J.H.K. and J.K.) and clinical information including age, gender, tumor location, histology, lymphovascular invasion, perineural invasion, serosal involvement, nodal status, and follow-up results was obtained from the medical records. This study was approved by the Institutional Review Board (IRB) (2015-1393) of Asan Medical Center, and patient innformed consent was waived by the IRB.
Tissue microarray construction and IHC
Tissue microarrays (TMAs) were constructed from 34 surgically resected BRAF-mutated samples and 86 BRAF wild-type samples.
The TMA was constructed using a hollow needle to remove a tissue core (0.2 cm in diameter) from tumors on paraffin-embedded tissue blocks. These cores were then inserted into recipient blocks. Sections of the TMA blocks were cut using a microtome, mounted on a microscope slide, and then stained. TMA and biopsy samples were subjected to IHC analysis using anti-BRAF antibody (mouse monoclonal, clone VE1, catalog number: 790-4855, Ventana Medical Systems, Tucson, AZ, USA) and a BenchMark XT automatic immunostaining device (Ventana Medical Systems) with an OptiView DAB IHC Detection Kit (Ventana Medical Systems) according to the manufacturer’s instructions with slight modifications: we diluted primary antibody with recommended dilution buffer to 1:4 and increased primary antibody incubation time from 16 to 32 minutes, in order to prevent nonspecific background signals.
IHC staining results were graded using a 4-tier grading system according to the staining intensity as follows: 0 (no staining), 1+ (faint), 2+ (moderate), and 3+ (strong) (Fig. 1A–C). Only cytoplasmic staining was considered positive. As a positive control, we selected a case of papillary thyroid carcinoma harboring BRAF V600E mutation and strong BRAF VE1 staining. As a negative control, we used normal tonsil tissue stained in the same manner with and without primary antibody. When the results of BRAF VE1 IHC differed from those of BRAF sequencing, we repeated BRAF VE1 IHC using whole tumor sections that were cut from the same paraffin block from which DNA had been extracted for BRAF sequencing.
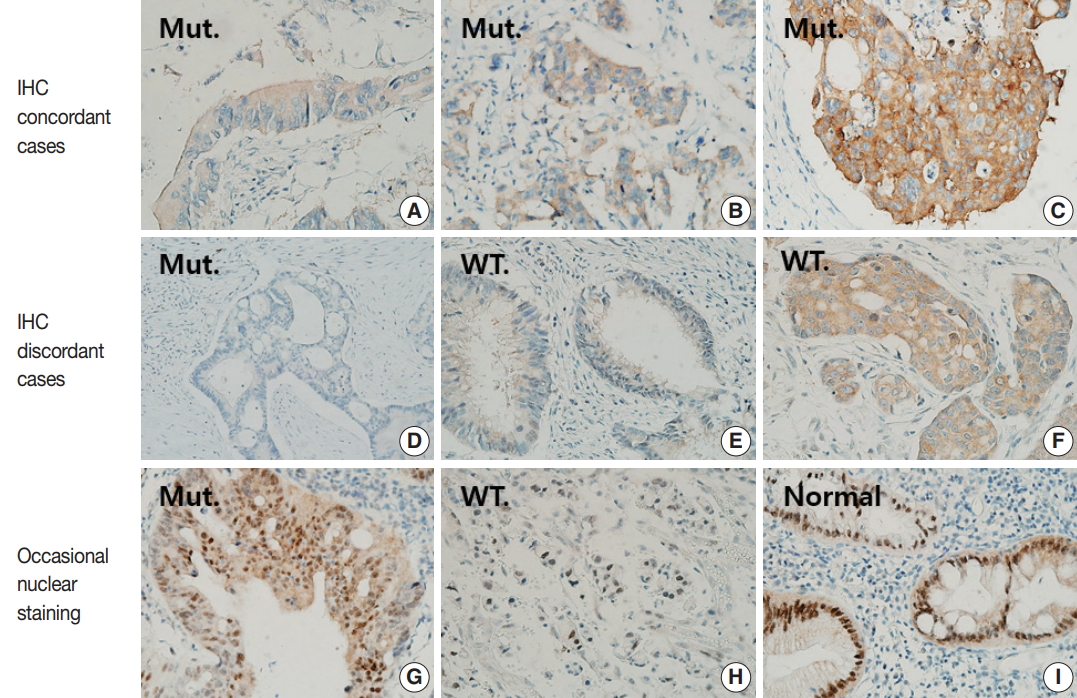
Various staining patterns for BRAF VE1 immunohistochemistry (IHC). (A–C) BRAF VE1 is stained in cytoplasm with variable intensities in BRAF-mutated colorectal cancers (CRCs). 1+, faint (A); 2+, moderate (B); and 3+, strong (C). (D–F) Representative figures for cases with discrepancies between BRAF VE1 IHC and BRAF sequencing results. Negative staining in a BRAF-mutated CRC (D); 1+, faint cytoplasmic staining in a BRAF wild-type CRC (E); and 2+, moderate cytoplasmic staining in a BRAF wild-type CRC (F). (G–I) Representative figures for cases showing nuclear BRAF VE1 staining. (G) A BRAF-mutated CRC showing nuclear staining as well as moderate cytoplasmic staining. (H) A BRAF wild-type CRC showing only nuclear staining. (I) Non-neoplastic colonic crypts showing strong nuclear and faint cytoplasmic staining. Mut., BRAF-mutated CRCs; WT, BRAF wild-type CRCs.
Determination of BRAF mutation status
BRAF V600E mutation status was confirmed by Sanger sequencing (n = 75), quantitative allele-specific PCR (n = 16), or mass spectrometry-based genotyping (n = 60) as described previously [9-11]. All tumor tissue sections were macrodissected to increase tumor purity. When tumor purity in the macrodissected area was low (< 40%) and Sanger sequencing did not detect BRAF mutations, the BRAF mutation status was confirmed by a more sensitive method such as quantitative allele-specific PCR or mass spectrometric genotyping.
Statistical analysis
To compare clinicopathologic variables, statistical analyses were performed using SPSS ver. 20.0 statistical software (IBM Corp., Armonk, NY, USA) and differences between the two groups were compared by either chi-square test or Fisher’s exact test. The Kaplan-Meier method with log-rank test and multivariate Cox proportional hazards regression models were applied for survival analyses. Two-sided p-values of < .05 were considered statistically significant.
RESULTS
Diagnostic performance of BRAF VE1 IHC
Forty-nine of 51 CRCs (96.1%) harboring BRAF V600E mutation showed cytoplasmic staining for BRAF VE1 antibody with variable intensities (Table 1, Fig. 1A–D): 3+ in 23 cases (45.1%), 2+ in 24 cases (47.1%), and 1+ in two cases (3.9%). In two BRAF-mutant cases (3.9%), no signal was detected by BRAF VE1 IHC. In 100 BRAF wild-type controls, 94 (94%) cases showed no staining, while six cases (6%) showed cytoplasmic staining with moderate (2 cases, 2%) or weak (4 cases, 4%) intensities (Table 1, Fig. 1E, F). Thus, the sensitivity, specificity, positive predictive value, and negative predictive value of BRAF VE1 IHC were 96.1%, 94%, 89.1%, and 97.9%, respectively. The cutoff for a positive staining was set to 1+ or bigger score because the area under curve was maximal at this cutoff in receiver operating characteristic (ROC) curve analysis (Supplementary Fig. S1). BRAF V600E mutant tumors with negative BRAF VE1 staining or BRAF wild-type tumors with positive BRAF VE1 staining did not exhibit any distinct clinicopathologic features (Supplementary Tables S1, S2).
Analysis of cases with discrepant results between BRAF mutation status and BRAF VE1 IHC results
For cases with discrepant results between BRAF sequencing and BRAF VE1 IHC, we repeated BRAF VE1 IHC on the same paraffin block from which DNA had been extracted for sequencing analyses. However, BRAF VE1 IHC on the whole tumor section showed the same results as those on TMA. As for BRAF-mutant cases that showed negative BRAF VE1 IHC results, IHC was repeated using matched biopsy tissues to exclude the possibility of false negative results due to poor fixation. However, the matched biopsy tissues showed the same results. Conversely, for BRAF wild-type cases that showed positive IHC results, we first investigated whether the discrepancies were due to false negative sequencing results associated with low tumor purity. All BRAF wild-type cases with positive immunostaining were examined for tumor purity on hematoxylin and eosin–stained slides; in most cases, tumor purity was more than 30%, and BRAF wild-type status of those cases were confirmed by allele-specific PCR study. One BRAF wild-type CRC with BRAF IHC staining intensity of 2+ had tumor purity of about 5%, but repeated allele-specific PCR study failed to reveal BRAF V600E mutation.
Atypical patterns of BRAF VE1 IHC and nonspecific staining in normal colonic mucosa
Three BRAF V600E mutated cases (5.9%) showed moderate nuclear staining together with moderate cytoplasmic staining, and one BRAF V600E wild type case showed only moderate nuclear staining (Fig. 1G, H, Supplementary Table S3). In addition, normal colonic mucosa was also stained, especially along the crypt surface (Fig. 1I).
Clinicopathologic characteristics of BRAF mutant CRC
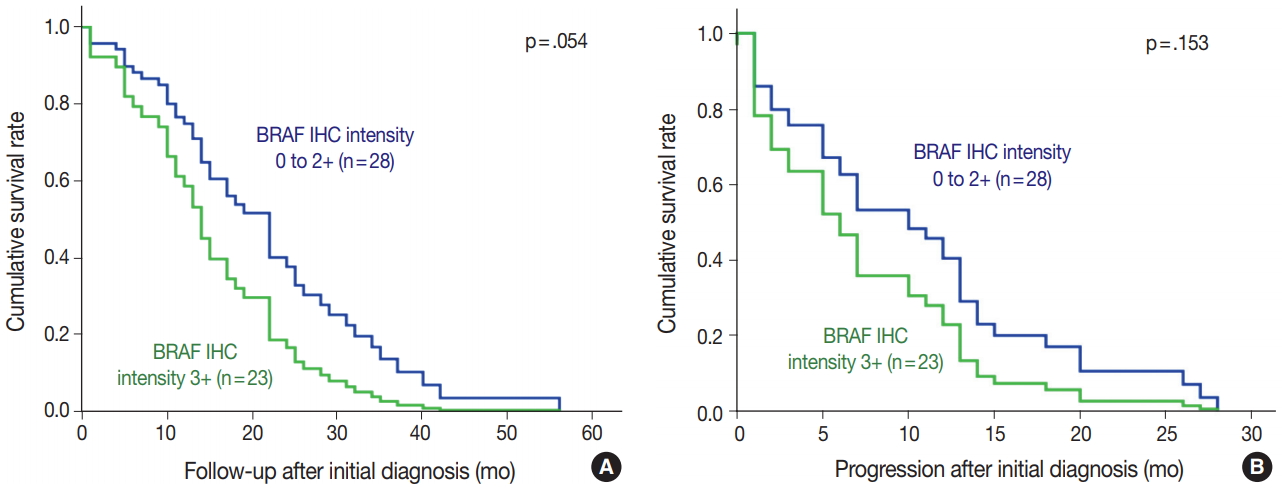
BRAF V600E mutated CRC cases showed significantly worse overall and progression-free survival (Fig. 2). Patients with BRAF V600E mutant CRC more frequently showed right-sided location, lymphovascular invasion, larger tumor size, and higher TNM stage at diagnosis than did patients with BRAF wildtype CRC. Particularly, BRAF V600E mutant CRCs showed more frequent serosal penetration and peritoneal seeding (Table 2). Because the intensities of BRAF VE1 immunostaining varied within the BRAF V600E mutant CRC group, we speculated that BRAF mutant CRCs with higher mutant BRAF protein expression might show worse prognosis if mutant BRAF protein actually plays a role in the aggressive biologic behavior. Indeed, BRAF mutant CRC cases with higher mutant BRAF protein expression tended to show shorter overall and progression-free survival than those with lower mutant BRAF protein expression, although the differences were not statistically significant (Table 3, Fig. 3).
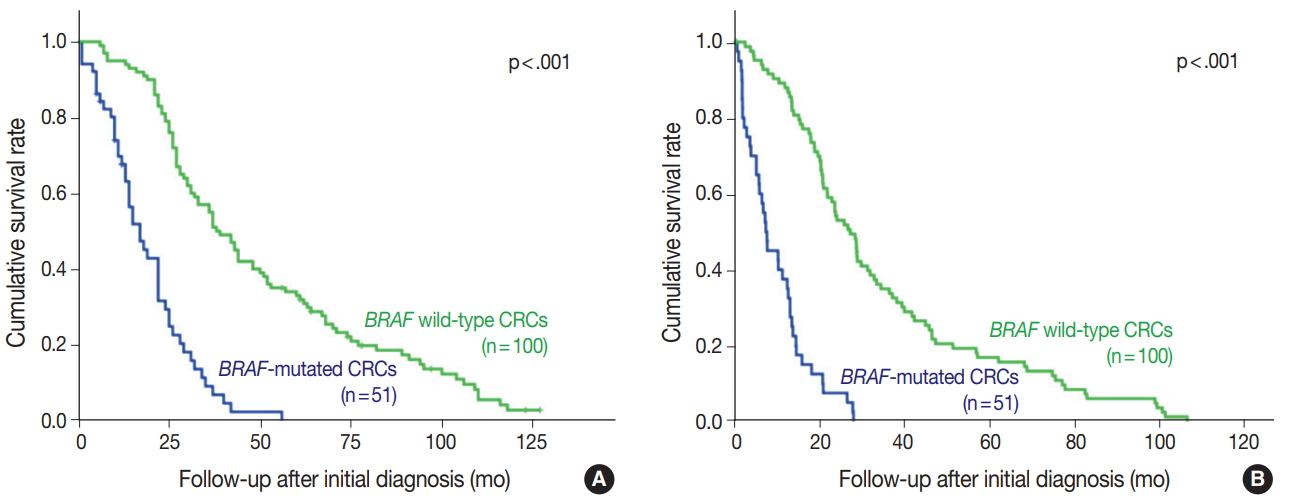
BRAF-mutated colorectal cancer (CRC) patients have shorter overall (A) and progression-free survival (B) periods (p<.001).
DISCUSSION
In the present study, we showed that the diagnostic performance of BRAF VE1 antibody was relatively good (sensitivity, specificity, and positive predictive values of 96.1%, 94%, and 89.1%, respectively). However, several BRAF V600E mutant CRCs showed no or weak BRAF VE1 staining (n = 4) or BRAF wild-type CRCs showed unequivocal cytoplasmic BRAF VE1 staining (n = 6). In addition, four CRC cases showed nonspecific nuclear BRAF VE1 staining as did normal colonic mucosa. Thus, the usefulness of BRAF VE1 IHC may be limited; it may be difficult to use BRAF VE1 IHC as a routine clinical test, although it may be useful as a screening tool when used in conjunction with subsequent confirmatory sequencing.
BRAF-mutant CRCs, which were all microsatellite stable, were in advanced stages at diagnosis (p < .001) and showed worse overall and recurrence-free survival than BRAF wild-type CRCs. These results are consistent with those of most previous studies [12-14]. Moreover, BRAF-mutant CRCs were associated with the right colon, larger primary tumor size, and presence of lymphovascular invasion, all of which are consistent with the results of most previous studies [2].
Recently, BRAF VE1 antibody has been used as a biomarker of CRC in IHC studies of BRAF. The clinical usefulness of BRAF VE1 antibody in colon cancer is controversial, but most studies showed that BRAF VE1 IHC has an excellent sensitivity [5,6]. Interpretation of BRAF VE1 IHC may be difficult due to technical problems such as poor fixation or staining failure [15,16]. Thus, we compared BRAF VE1 IHC and fixation quality between surgically resected tissues and matched colonoscopic biopsy tissues of two BRAF mutant CRC cases that showed negative staining results. There was no difference between the biopsied tissue and surgically resected tissue.
For BRAF wild-type CRCs showing positive immunostaining results, the discrepancies might have resulted from false-negative sequencing results if the tumor purity is very low. For example, tumors with signet rings or tumors with high mucin content have low tumor purity [17]. Therefore, we examined the tumor purity of all BRAF wild-type CRCs that stained positive in IHC. In most cases, false-negative sequencing results were excluded by repeating BRAF mutation analyses using more sensitive methods, but in one BRAF wild-type CRC with a tumor purity of approximately 5% and BRAF VE1 2+, we could not conduct more sensitive mutation analysis because tissue material was unavailable. Therefore, in this case, the possibility of a falsenegative sequencing result could not be excluded.
Interestingly, CRC cases with more intense BRAF VE1 immunostaining had a tendency to the shorter overall and progression-free survival. Although this result is difficult to interpret, the expression of BRAF V600E mutant protein may play a biological role in tumor aggressiveness rather than being a simple surrogate marker for prognosis. However, our study has a few limitations. Since we performed BRAF VE1 IHC in CRCs with known BRAF mutational status in a retrospective manner, the strength of evidence may be limited compared to that of a prospective design. In addition, a relatively small number of BRAF V600E mutant CRCs may limit the statistical power. Finally, the evaluation of prognostic value of BRAF mutations might be limited because the study population had a selection bias; it had not been selected in a consecutive manner.
Based on our results, the diagnostic performance of BRAF VE1 IHC showed relatively good but sometimes ambiguous staining, which may limit its routine clinical use; thus, BRAF VE1 IHC cannot replace BRAF sequencing studies. Despite these limitations, BRAF VE1 IHC may be carefully used as a screening tool for BRAF V600E mutation detection in a research basis, as BRAF VE1 IHC is more cost-effective and less time-consuming than BRAF sequencing studies.
Electronic Supplementary Material
Supplementary materials are available at Journal of Pathology and Translational Medicine (http://jpatholtm.org).
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.