Cytologic Diagnosis of Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features and Its Impact on the Risk of Malignancy in the Bethesda System for Reporting Thyroid Cytopathology: An Institutional Experience
Article information
Abstract
Background
This study was performed to analyze cytologic diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) and its impact on the risk of malignancy (ROM) in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC).
Methods
Five thousand five hundred and forty-nine cases of thyroid fine-needle aspiration cytology (FNAC) diagnosed between 2012 and 2014 were included in this study. Diagnostic categories based on TBSRTC were compared with final surgical diagnoses, and the ROM in each category was calculated both when NIFTP was included in malignant lesions and when excluded from malignant lesions.
Results
Of the 5,549 thyroid FNAC cases, 1,891 cases underwent surgical resection. In final diagnosis, 1,700 cases were revealed as papillary thyroid carcinoma (PTC), and 25 cases were reclassified as NIFTP. The cytologic diagnoses of NIFTP were non-diagnostic in one, benign in five, atypia of undetermined significance (AUS) in 14, follicular neoplasm in two, and suspicious for malignancy in three cases. Collectively, NIFTP/encapsulated follicular variant of PTC (EFVPTC) were more frequently classified as benign, AUS, or follicular neoplasm and less frequently categorized as malignant compared to conventional PTCs. Exclusion of NIFTP from malignant diagnoses resulted in a slight decrease in malignancy rates in non-diagnostic, benign, AUS, follicular neoplasm, and suspicious for malignancy categories without any statistical significance.
Conclusions
The decrease in the ROM was not significant when NIFTP was excluded from malignant lesions. In thyroid FNACs, NIFTP/EFVPTCs were mostly classified into indeterminate categories. Therefore, it might be feasible to separate NIFTP/EFVPTC from conventional PTC on FNAC to guide clinicians to conservative management for patients with NIFTP/EFVPTC.
Fine-needle aspiration cytology (FNAC) with ultrasonography is the most commonly used method for preoperative testing of thyroid nodules. FNAC is a diagnostic test for the majority of benign nodules, most papillary thyroid carcinomas (PTCs), and other carcinomas, while it is considered a screening test for follicular-patterned lesions [1]. Most thyroid FNAC specimens are currently classified according to the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC). This scheme consists of six major diagnostic categories: non-diagnostic/unsatisfactory, benign, atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), follicular neoplasm/suspicious for a follicular neoplasm, suspicious for malignancy, and malignant [2]. Each diagnostic category possesses a different risk of malignancy (ROM) and thus, offers a guide to optimal clinical management [2,3].
Recently, a new diagnostic term “noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP)” was introduced for thyroid neoplasms previously classified as noninvasive encapsulated follicular variant of PTC (noninvasive EFVPTC) noting its indolent clinical behavior [4]. The principal intent was to avoid overtreatment for patients with these tumors. Several outcomes are expected from revision of the nomenclature. In addition to alleviating the psychological impact of cancer diagnosis, it would also reduce complications of total thyroidectomy, risk of secondary tumors following radioactive iodine therapy, and the overall cost of health care [5,6]. Since NIFTP denotes an indolent behavior, one may regard surgical resection as unnecessary. However, surgical resection is essential for diagnosis of NIFTP. Moreover, it is important to note that the advent of the term NIFTP is not a discovery but rather an introduction of a new terminology for the clarification of a borderline concept from the former dichotomous era.
The introduction of the nomenclature NIFTP and its exclusion from malignant diagnoses raised some issues in thyroid FNAC. Retrospective analyses have demonstrated that it has affected the ROM in each of the TBSRTC diagnostic categories accordingly [1,7-10]. Some studies, in particular, have shown that, if NIFTP were no longer termed carcinoma, there would be a marked decrease in the ROM for the indeterminate categories of TBSRTC such as AUS/FLUS, follicular neoplasm/suspicious for a follicular neoplasm, and suspicious for malignancy [1,8,11].
Western series have reported that NIFTP comprise 7%–28% of all PTCs [8,11-14]. However, a recent report from Asian countries showed a very low incidence of NIFTP, comprising 0% to 4.7% of PTCs [15,16]. Alteration in the risk of malignancies after the introduction of NIFTP is closely related to its incidence [9], and thus, the impact of NIFTP diagnoses on the ROM in diagnostic categories of TBSRTC would be low in Asian countries. Thus, we aimed to analyze cytologic diagnosis of NIFTP and its impact on the ROM in each diagnostic category of TBSRTC.
MATERIALS AND METHODS
Case selection
We retrospectively reviewed a total of 5,624 thyroid FNAC specimens from 5,127 patients, diagnosed at Seoul National University Bundang Hospital from January 2012 to December 2014. Of the 5,624 FNACs, 1,784 (31.7%) were consult cases from outside hospitals. Of the 5,127 patients, 174 patients had multiple thyroid nodules for which FNAC was performed separately and each counted as an individual case in this study. In the 71 patients who had repeated FNACs for the same nodule, the diagnostic category with the highest ROM was selected. Finally, 5,549 thyroid FNACs were included in this study. All thyroid FNAC slides were prepared with conventional smear with Papanicolaou staining and were diagnosed according to the diagnostic categories of TBSRTC [2]. In our institution, category III (AUS) is subcategorized into four subgroups: (1) AUS-NA, AUS having focal nuclear atypia (NA) suggestive of papillary carcinoma, but not diagnostic for category V (suspicious for malignancy); (2) AUS-MF, AUS showing a predominant population of microfollicles (MFs), but not sufficient for diagnosis of category IV (follicular neoplasm); (3) AUS-HC, AUS showing predominance of Hurthle cells (HC); and (4) AUS-others, AUS not otherwise specified. This study was approved by the Institutional Review Board (IRB) of Seoul National University Bundang Hospital (IRB No. B-1803-456-102), and the requirement for informed consent was waived.
Pathologic review of surgical resection specimens
Of the 5,549 thyroid FNACs, cases with subsequent surgical resection were extracted from the electronic medical records. Histologic diagnoses for surgical resection specimens were reviewed and matched with the results of FNACs for the corresponding nodules. For PTC, we re-evaluated histologic variants such as conventional type, encapsulated follicular variant, infiltrative follicular variant, tall cell variant and etc. We carefully reviewed all the slides of EFVPTC and identified NIFTP according to the recently proposed diagnostic criteria [4]. For a histological diagnosis of NIFTP, a thyroid tumor had to fulfill the following criteria: (1) encapsulation or clear demarcation, (2) follicular growth pattern, (3) nuclear features of PTC (nuclear score 2–3), (4) no capsular or vascular invasion, and (5) no aggressive histology (no tumor necrosis, no high mitotic activity). Regarding the follicular growth pattern, specific conditions were set as follows: (1) < 1% papillae, (2) no psammoma bodies, and (3) < 30% solid/trabecular/insular pattern. The ROM in each category of TBSRTC was calculated using cases with subsequent surgical resection or all FNAC cases.
Statistical analyses
Statistical analyses were performed using SPSS statistics ver. 22.0 (IBM Corp., Armonk, NY, USA). The Pearson chi-square test or Fisher exact test was used to compare the frequencies of categorical variables between two groups. A p-value of less than .05 was considered statistically significant. All p-values reported were two-sided.
RESULTS
Diagnostic categories of TBSRTC
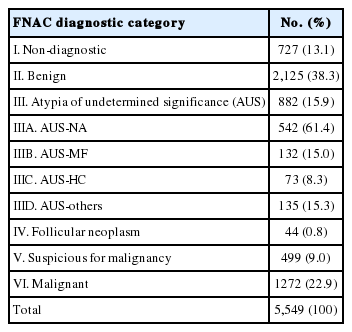
Diagnostic categories of the 5,549 thyroid FNACs and their frequencies are as follows (Table 1): 727 cases (13.1%) of category I (non-diagnostic), 2,125 cases (38.3%) of category II (benign), 882 cases (15.9%) of category III (AUS), 44 cases (0.8%) of category IV (follicular neoplasm), 499 cases (9.0%) of category V (suspicious for malignancy), and 1,272 cases (22.9) of category VI (malignant). The 882 cases of AUS were composed of 542 AUS-NA (61.4%), 132 AUS-MF (15.0%), 73 AUS-HC (8.3%), and 135 AUS-others (15.3%).
Comparison of diagnostic categories of TBSRTC with final surgical diagnoses
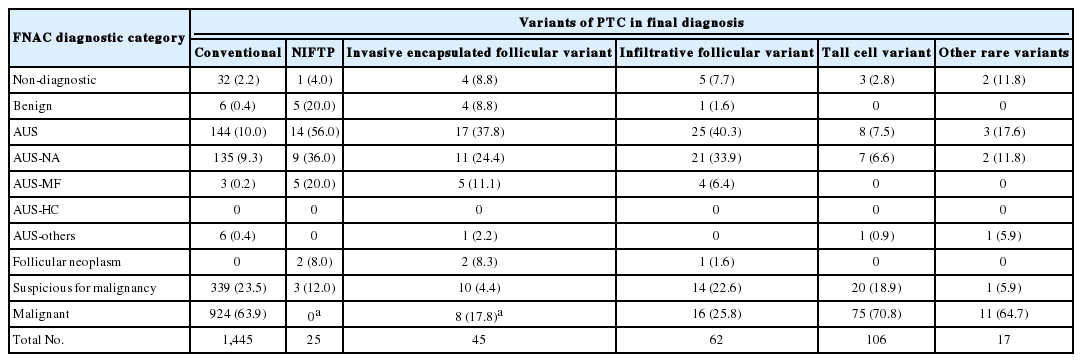
Of the 5,549 thyroid FNAC cases, 1,891 cases underwent surgical resection. Final surgical diagnoses are presented in Table 2. In surgical resection specimens, 1,700 cases were revealed as PTC including 1,445 conventional PTCs, 70 EFVTPTCs, 62 infiltrative follicular variant of PTC (infiltrative FVPTCs), 106 tall cell variants, and 17 other rare variants (Table 3). Of the 70 EFVPTCs in surgical resection specimens, 25 cases were finally reclassified as NIFTP after reviewing of the slides. The cytologic diagnoses of NIFTP were non-diagnostic in one (4.0%), benign in five (20.0%), AUS in 14 (56.0%) (Fig. 1), follicular neoplasm in two (8.0%), and suspicious for malignancy in three (12.0%). Of the 14 cases with AUS diagnoses, nine showed NA and five revealed MF pattern. The non-diagnostic case was diagnosed as follicular neoplasm in a repeated core needle biopsy. Diagnostic categories of FNAC for invasive EFVPTC were as follows: non-diagnostic in four (8.8%), benign in four (8.8%), AUS in 17 (37.8%), follicular neoplasm in two (8.3%), suspicious for malignancy in 10 (8.3%), and malignant in eight (17.8%) (Fig. 2). When comparing the cytologic diagnoses of NIFTP and EFVPTC with capsular or vascular invasion, malignant cytologic diagnoses were found in invasive EFVPTC only with a statistical difference (p = .044). However, there were no statistically significant differences in the frequencies of other diagnostic categories.
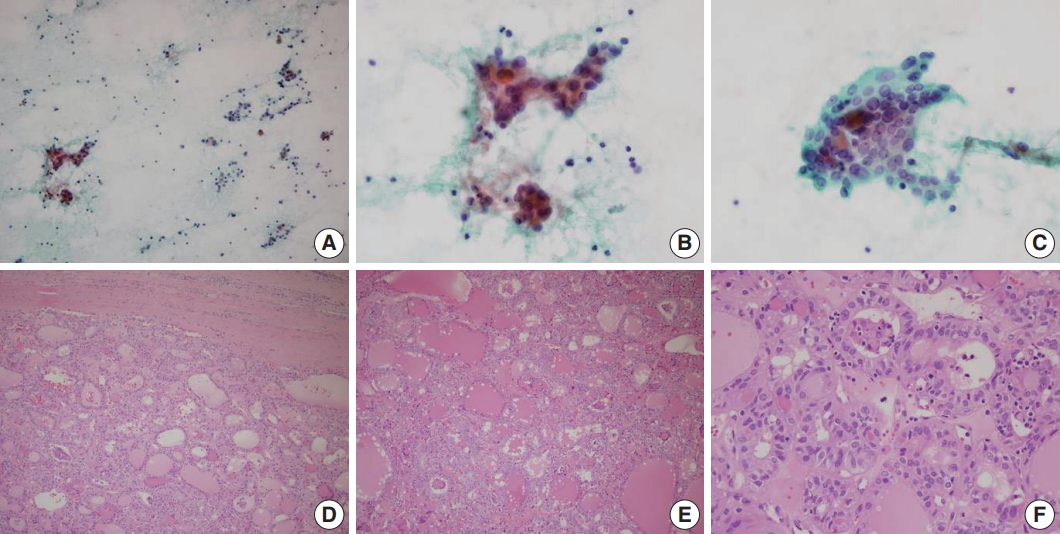
A representative case of noninvasive follicular thyroid neoplasm with papillary-like nuclear features diagnosed as atypia of undetermined significance in fine-needle aspiration cytology. (A) Low power examination of cytology smear reveals small clusters and scattered single cells with low cellularity. (B) Small cell clusters showing nuclear enlargement and overlapping. (C) A cell cluster with microfollicles. Tumor cell nuclei show nuclear clearing, nuclear elongation and peripherally located micronucleoli. (D) In final histology, the tumor has a thick fibrous capsule with smooth border and shows a follicular growth pattern. (E) Follicles have densely eosinophilic colloid with peripheral scalloping and mutinucleated giant cells. (F) Tumor cells show papillary thyroid carcinoma–like nuclear features.
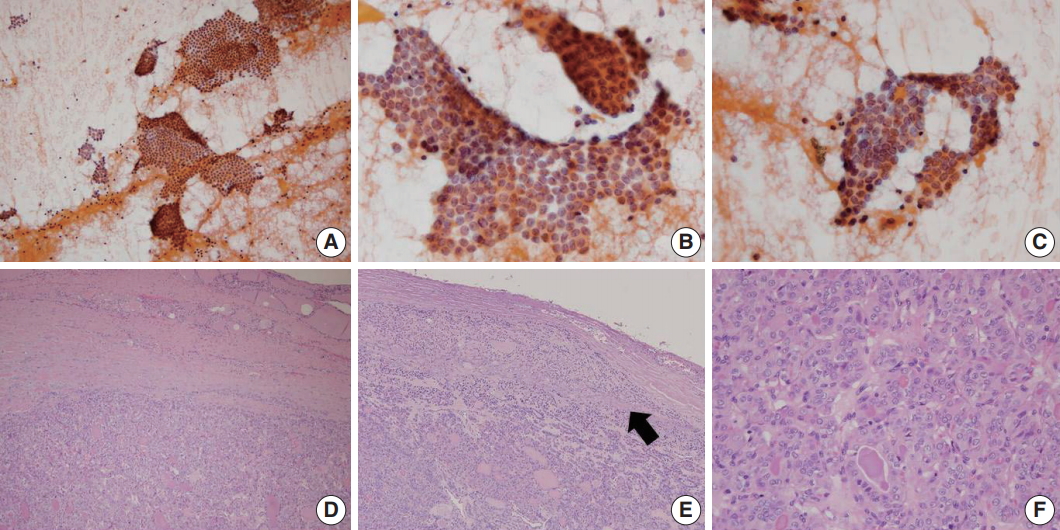
A representative example of encapsulated follicular variant of papillary thyroid carcinoma with invasion classified as papillary carcinoma in fine-needle aspiration cytology. (A) Low power examination reveals large tissue fragments and small clusters in cytologic smear. (B, C) Monolayered sheets or syncytial clusters showing nuclear elongation, overlapping, loss of polarity and grooves. (D) In the final histology of surgical excision specimen, the tumor has a thick fibrous capsule with irregular border. (E) A focus of capsular invasion (arrow) is evident. (F) Tumor cells are arranged in microfollicular or trabecular pattern, showing typical papillary thyroid carcinoma–like nuclear features.
Comparison of diagnostic categories of TBSRTC between conventional PTC, NIFTP/EFVPTC, and infiltrative FVPTC
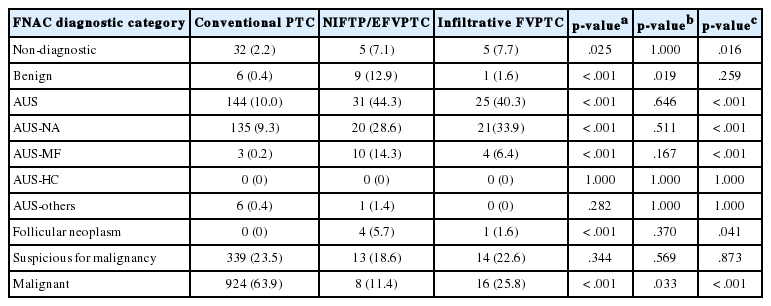
When comparing diagnostic categories between conventional PTC and NIFTP/EFVPTCs (Table 4), 924 of 1,445 conventional PTCs (63.9%) were diagnosed as category VI, whereas eight of the 70 NIFTP/EFVPTCs (11.4%) were diagnosed as malignancy (p < .001). Four NIFTP/EFVPTCs (5.7%) and none of the conventional PTCs were categorized as follicular neoplasm on FNAC (p < .001). While 144 conventional PTCs (10.0%) were classified as AUS, 31 NIFTP/EFVPTCs (44.3%) were classified as AUS (p<.001). In particular, only three of 1,445 conventional PTCs (0.2%) were classified as AUS-MF, whereas 10 of 70 NIFTP/EFVPTCs (14.3%) revealed AUS-MF pattern (p < .001). Six conventional PTCs (0.4%) and nine NIFTP/EFVPTCs (12.9%) were classified as benign on FNAC (p < .001). Collectively, NIFTP/EFVPTCs were more frequently classified as benign, AUS, or follicular neoplasm diagnostic categories and less frequently diagnosed as malignant compared with conventional PTCs. Similarly, infiltrative FVPTC was more frequently diagnosed as AUS (p < .001) and follicular neoplasm (p = .041) and less frequently diagnosed as malignant (p < .001), compared with conventional PTCs (Table 4). When comparing cytologic diagnoses between infiltrative FVPTCs and NIFTP/EFVPTCs (Table 4), infiltrative FVPTCs were less frequently classified as benign and more frequently diagnosed as malignant, compared with NIFTP/EFVPTCs (p = .019 and p = .033, respectively).
Impact of NIFTP on ROM in each diagnostic category of TBSRTC
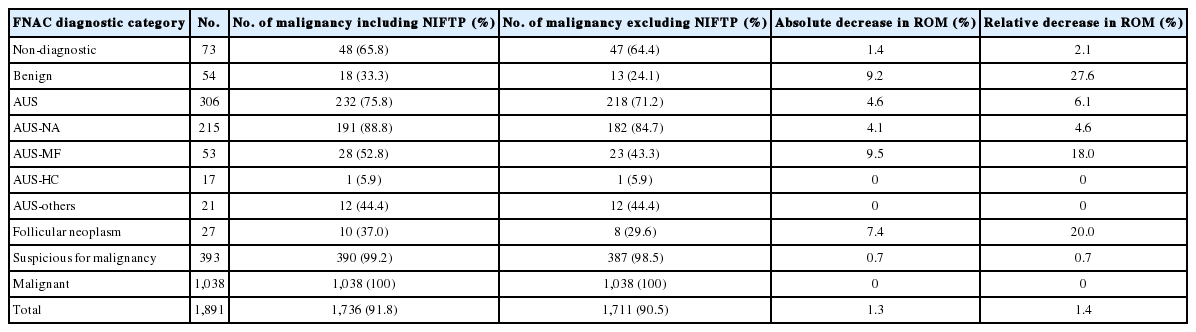
When NIFTPs were regarded as malignant tumors, the ROM in each diagnostic category of FNAC was as follows in histology-proven cases: non-diagnostic, 65.8%; benign, 33.3%; AUS, 75.8%; follicular neoplasm, 37.0%; suspicious for malignancy, 99.2%; malignant, 100% (Table 5). Next, we assessed the impact of NIFTP on the ROM in each diagnostic category. When excluding NIFTP from malignant diagnoses, an absolute decrease ranged from 0% to 9.2% and a relative decrease ranged from 0% to 27.6%. The ROM seemed to change largely in benign (33.3% to 24.1%) and AUS-MF categories (52.8% to 43.3%), but there were no statistical differences in ROMs in all diagnostic categories whether NIFTP was included in malignancy diagnoses or not.
When the ROMs were calculated in total FNAC cases, the ROM including NIFTP in each diagnostic category was as follows: non-diagnostic, 6.6%; benign, 0.8%; AUS, 26.3%; follicular neoplasm, 22.7%; suspicious for malignancy, 78.2%; malignant, 81.6% (Table 6). Exclusion of NIFTP from malignant diagnoses resulted in an absolute decrease of 0% to 4.5% and a relative decrease of 0% to 25.0% without any statistical difference.
DISCUSSION
Introduction of NIFTP has created some challenges for thyroid FNAC: among them, its impact on the ROM in the diagnostic categories of TBSRTC and the resulting need for modification of clinical management guidelines may be critical. Previous studies from Western countries have revealed decreases in the ROM across all TBSRTC categories, and significant reductions in the ROM were detected in the three indeterminate TBSRTC categories upon reclassification of noninvasive EFVPTC as NIFTP [8,9,11]. However, in this study, introduction of the term NIFTP resulted in an insignificant decrease in the ROM in the diagnostic categories of non-diagnostic, benign, AUS, follicular neoplasm, and suspicious for malignancy.
The decrease in the risk of malignancies in each diagnostic category after the introduction of NIFTP will vary according to the institutional frequency and preceding FNAC diagnoses of NIFTP [9,17]. While NIFTP comprises 7%–28% of PTCs in previous studies from Western countries [8,11-14], its proportion is quite low in Asian countries, ranging from 0% to 4.7% of PTCs [15,16]. In this study, NIFTP represented 1.5% of all PTCs. Thus, exclusion of NIFTP from malignant diagnoses and the resultant decrease in the ROM in diagnostic categories of TBSRTC was not significant. The incidence of NIFTP may be directly linked to the incidence of EFVPTC. In this study, EFVPTC comprised 4.1% of all PTCs and it was reported to account for 0.7% to 5.5% of PTCs in Asian counties [15,16]. However, EFVPTC represented about 24% of all PTC cases in Western countries with its incidence increasing [4,18].
Previous studies have shown that NIFTP is usually diagnosed into indeterminate categories, that is, AUS/FLUS, follicular neoplasm/suspicious for a follicular neoplasm, and suspicious for malignancy. Comparative studies on cytomorphologic features of NIFTP and conventional PTC revealed that cytologic features of PTC such as nuclear pseudoinclusions, crowding, irregularities, and clearing are less frequent in NIFTP compared to conventional PTC [19,20]. Especially, among the various nuclear features of PTC, nuclear pseudoinclusions are known to be almost absent in NIFTP [20,21]. Moreover, by definition, NIFTP should not have papillae and psammomatous calcification which can be found in cytologic smear of conventional PTC. Thus, the borderline nuclear features, lack of papillae and predominance of MFs in NIFTP lead to cytologic diagnoses of indeterminate categories, and rarely to malignant category. Accordingly, it is reasonable that changes in ROM are significant in indeterminate categories but insignificant in malignant category after the introduction of NIFTP. Similar to the previous studies [11,13,19], our study showed that NIFTP was most frequently diagnosed as AUS. Five cases were even diagnosed as benign: however, this can be explained by the frequent focal PTC-like nuclear changes in NIFTP.
In this study, FNAC diagnoses of NIFTP and EFVPTC with invasion did not show a significant difference in other diagnostic categories except for malignant category. As the distinction between invasive EFVPTC and NIFTP is basically based on the demonstration of capsular and/or vascular invasion, their cytologic features can be overlapping. However, 17.8% of invasive EFVPTC but none of the NIFTC was categorized as malignant in FNAC. It is thought that NIFTP progresses to invasive EFVPTC and thus, invasive EFVPTC may show more typical nuclear features of PTC compared to NIFTP. Recently, Chandler et al. [21] reported that predominantly MF pattern, absence of pseudoinclusions, and less frequent nuclear elongation and grooves are more likely to be associated with NIFTP in comparison with invasive EFVPTC. However, other previous studies did not demonstrate differences in FNAC diagnostic categories between them [15,22]. Maletta et al. [22] reported that EFVPTC with invasion were typically diagnosed as follicular neoplasm or suspicious for malignancy and nuclear features including size, contour irregularities, and chromatin clearing did not differ between NIFTP and invasive EFVPTC.
There are a few limitations in this study. As a tertiary medical center, malignant cytologic diagnoses comprised as much as 22.9% of all FNAC diagnoses in our institution. That is, our study population had selection bias, and thus, the incidence of NIFTP in this study may not reflect its normal distribution. Moreover, we reviewed only EFVPTC cases to find NIFTP. Some cases which were previously diagnosed as follicular adenoma or even nodular hyperplasia may have incomplete PTC-like nuclei and may have been missed. For these reasons, there is a possibility that NIFTP was underestimated in this study.
To conclude, in this study, the decrease in the ROM was not significant when NIFTP was excluded from malignant lesions due to the low frequency of NIFTP. NIFTP/EFVPTCs were more frequently classified as benign, AUS, or follicular neoplasm categories and less frequently diagnosed as malignant, when compared with conventional PTCs. Though there are no drastic changes in the 2017 revision of TBSRTC, it emphasizes that malignant diagnoses should be limited to cases with classic features of PTC including true papillae, psammoma bodies, and nuclear pseudoinclusions to avoid false-positives due to NIFTP [3]. It appears that in thyroid FNACs, NIFTP/EFVPTCs are mostly classified into indeterminate diagnostic categories. Therefore, it might be feasible to separate NIFTP/EFVPTC from conventional PTC on FNAC to guide clinicians to conservative management for patients with NIFTP/EFVPTC.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.