PD-L1 Testing in Non-small Cell Lung Cancer: Past, Present, and Future
Article information
Abstract
Blockade of the programmed cell death-1 (PD-1) axis has already been established as an effective treatment of non-small cell lung cancer. Immunohistochemistry (IHC) for programmed death-ligand 1 (PD-L1) protein is the only available biomarker that can guide treatment with immune checkpoint inhibitors in non-small cell lung cancer. Because each PD-1/PD-L1 blockade was approved together with a specific PD-L1 IHC assay used in the clinical trials, pathologists have been challenged with performing various assays with a limited sample. To provide a more unified understanding of this, several cross-validation studies between platforms have been performed and showed consistent results. However, the interchangeability of assays may be limited in practice because of the risk of misclassification of patients for the treatment. Furthermore, several issues, including the temporal and spatial heterogeneity of PD-L1 expression in the tumor, and the potential for cytology specimens to be used as an alternative to tissue samples for PD-L1 testing, have still not been resolved. In the future, one of the main aims of immunotherapy research should be to find a novel predictive biomarker for PD-1 blockade therapy and a way to combine it with PD-L1 IHC and other tests.
The treatment of non-small cell lung cancer (NSCLC) has undergone a drastic paradigm shift since the introduction of immune checkpoint inhibitors (ICIs), primarily programmed cell death-1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors. Clinical trials have demonstrated that anti–PD-1/PD-L1 agents (such as nivolumab, pembrolizumab, and atezolizumab) have remarkable anti-tumor activity and that treatment with these factors resulted in prolonged overall survival in NSCLC patients [1-6]. Accordingly, these agents have been approved by the United States Food and Drug Administration (FDA) as second-line or first-line therapies for NSCLC, and clinical trials with novel agents have also shown promising results [7,8]. Subsequent studies have indicated that the cell-surface expression of PD-L1 protein is an effective biomarker for predicting the response to these drugs; PD-L1 immunohistochemistry (IHC) is to date the only testing method to guide the administration of anti–PD-1/PD-L1 agents in NSCLC patients [1,3-6]. Recently, anti–PD-1/PD-L1 agents have been integrated into the treatment option for advanced NSCLC patients. The National Comprehensive Cancer Network guidelines recommend that all advanced NSCLC samples be tested with PD-L1 IHC in a reflex manner [9].
In this review, we summarize the current status of PD-L1 testing in NSCLC and discuss the major issues that can arise when applying it to clinical practice.
PROGRAMMED DEATH-LIGAND 1 PROTEIN EXPRESSION AS A BIOMARKER IN LUNG CANCER: RATIONALE AND PERFORMANCE
The immune system is regulated through a number of receptor-ligand interactions to protect the host from external antigens and prevent autoimmune reactions [10]. The interaction of PD-1 expressed on cytotoxic T lymphocytes, and PD-L1 on antigenpresenting cells is one such example of an interaction (immune checkpoint) [11]. A tumor cell with variable neoantigens is recognized as non-self and is attacked by the immune system; however, to avoid elimination, the tumor cells may express PD-L1 protein on their surface [12]. Thus, blockade of this PD-1/PD-L1 interaction by monoclonal antibodies against either PD-1 (nivolumab and pembrolizumab) or PD-L1 (atezolizumab, durvalumab, and avelumab) seems a logical therapeutic approach, especially for a highly antigenic tumor like NSCLC [13].
However, the mechanisms of PD-1/PD-L1 blockade therapy and PD-L1 testing are completely different from those of EGFR, ALK, and ROS1 testing, which inhibit addictive driver oncogenes in lung cancer. ICIs block only the interaction, which is a part of the normal functioning of the immune system. Therefore, the clinical effect or duration of the PD-1/PD-L1 blockade response will be different from those of receptor tyrosine kinase inhibitors. PD-L1 is a protein that is expressed with biological continuity and shows profound intra-tumoral heterogeneity, unlike genetic variation, which is separated by a binary system. It is important to choose the correct cutoff levels to define biomarker-positive and -negative patient groups for PD-L1 testing to have a predictive value. In addition, IHC for detecting protein activity may be influenced by the choice of various factors including primary antibody clones, detection system, and platforms related to complex biochemistry.
PD-L1 expression assessment is now established as a routine practice but is not without challenges. Understanding these inherent characteristics of PD-L1 testing is an important basis for pathologists to correctly interpret PD-L1 IHC results and communicate with clinicians to recommend the most effective treatment options.
VARIOUS PROGRAMMED DEATH-LIGAND 1 ASSAY AND HARMONIZATION
The development of ICIs was led by high-profile clinical trials, and each pharmaceutical company designed a distinct PD-L1 IHC assay to support the clinical efficacy of their own drug. Therefore, four commercial antibodies are currently available to measure PD-L1 protein expression in formalin-fixed, paraffin-embedded (FFPE) lung tissue specimens. Each assay utilizes a different automated staining system, detection system, and even means of assessment and thresholds to determine positive PD-L1 protein expression (Table 1).
However, this situation has resulted in a dilemma for pathologists. It is impractical to perform several different assays to detect one protein in a pathology laboratory with limited resources. The number of PD-L1 assays that can be performed in conjunction with the required biomarker testing (EGFR, ALK, etc.) is also limited due to the limited availability of tumor tissue, especially for small biopsy specimens from patients with advanced lung cancer. In addition, selecting one assay from the several available ones is also challenging. While each of the four IHC assays successfully recognizes PD-L1 protein, each antibody has been developed to bind to a specific epitope, and each detection system is also applied differently. Thus, the performance of these assays may be different and each assay has a cutoff to predict drug response that was determined through a clinical trial, suggesting limited interchangeability of the assays in clinical practice.
As a consequence, a number of international projects have been launched to standardize the various PD-L1 assays. The most prominent result of the Blueprint project, led by the International Association for the Study of Lung Cancer, has been relatively promising. SP263, 22C3, and 28-8 showed a high concordance in the percentage of PD-L1 membrane staining of tumor cells at any intensity; conversely, a lower expression of PD-L1 in tumor cells was observed using the SP142 clone [14]. Following harmonization studies showed consistent results, and pathologists had hoped that several PD-L1 assays could be used interchangeably for the prescription of any of PD-1/PD-L1 blockade [15-18]. In addition, although 22C3 assay has been developed for use on the Dako platform, not every pathology laboratory has the Dako Autostainer, whereas the Ventana BenchMark platforms are more common in pathology laboratories. Several studies reported that the results of the 22C3 assay had shown a high correlation with those of the SP263 assay [19-22]. Based on these results, the SP263 assay gained Conformite Europeanne approval for nivolumab and pembrolizumab treatment as a complementary diagnostic test [23]. In Korea, the SP263 assay was also approved by the Korea FDA for nivolumab treatment. Although some studies have shown discordance between the SP263 and 22C3 assays [24,25], these discrepancies are recognized as due to the heterogeneity of PD-L1 expression or interobserver variability rather than due to the difference in assay performance. However, it is still burdensome that the misclassification of patients by using different PD-L1 assays interchangeably may lead to patients either not receiving the needed PD-1 blockade therapy, or receiving a treatment that is not beneficial. Strictly speaking, there is no gold standard assay that accurately measures PD-L1 expression and best predicts PD-1 blockade response.
INTERPRETATION AND PATHOLOGICAL REPORTING OF PROGRAMMED DEATH-LIGAND 1 IMMUNOHISTOCHEMISTRY
For the reasons mentioned above, several PD-L1 assays are generally performed simultaneously or sequentially in many pathology laboratories for the prescription of anti–PD-1/PD-L1 agents. The interpretation of PD-L1 IHC assays is a challenge for pathologists because of the different methods of interpreting positive results and in the different cutoff values for each assay (Table 1).
For the definition of positive PD-L1 staining, complete circumferential or partial linear membranous staining of tumor cells at any intensity is considered positive for the 22C3 and 28-8 assays, while any membranous and/or cytoplasmic expression of tumor cells is considered positive for the SP263 and SP142 assays (Fig. 1). In the SP142 assay, PD-L1–positive immune cells, as well as tumor cells, are considered in the criteria for positive PD-L1 staining. Interpretation issues relating to distinguishing tumor cell PD-L1 chromogenic signals from those of inflammatory cells, the mislocalized signal from the membrane to the cytoplasm (for 22C3 and 28-8), and the scoring of percentages of expression particularly around the thresholds of clinical significance are always a concern for pathologists, regardless of the assay type. Furthermore, each assay has a specific cutoff value for positive tumor cells, and the percentage may be different depending on whether it is the first line vs. second or further line of treatment, even for the same drug. In addition, when an assay is applied for two drug prescriptions, such as SP263, different cutoffs may be applied depending on the drug. The SP263 assay uses two different cutoffs; 25% for durvalumab and 10% cutoff for nivolumab, in Korea (Table 1). Because these inconsistent cutoffs are not proven by clinical trial, but a special situation in Korea related to national insurance, pathologists also have to pay close attention to the insurance policies related with PD-L1 testing.
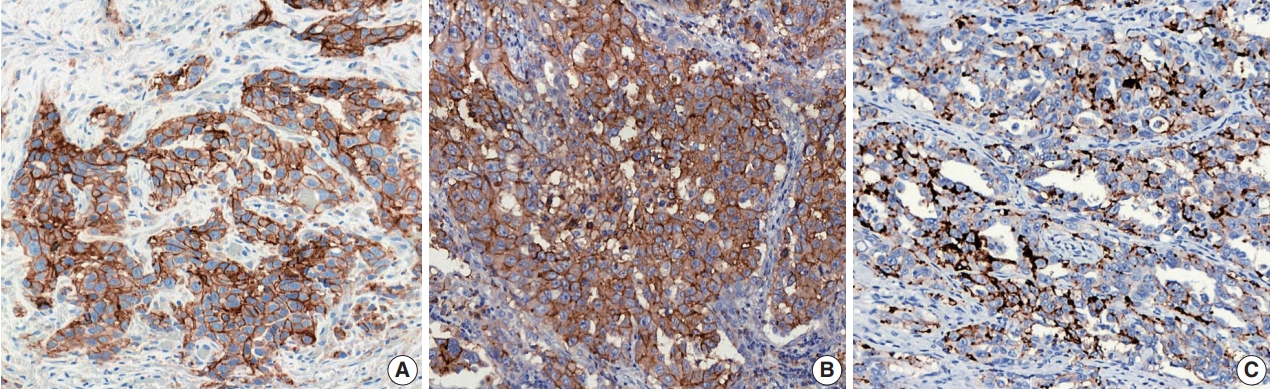
Representative images stained with the programmed death-ligand 1 immunohistochemical assays: 22C3 (A), SP263 (B), and SP142 (C).
Given these differences, pathological reports on PD-L1 IHC should be more comprehensive, giving more than a simple positive or negative result. The name of the diagnostic kit used, the assessment score, information on the required minimum number of cells that were assessed, and any comment on the meaning of the score with regard to the cutoff of that specific test should be included in the report. If the immunotherapeutic agent to be used is known at the time of testing, the results can be reported in terms of broader categories (e.g., < 1%, 1%–49%, and > 50% with 22C3 for pembrolizumab treatment), appropriate for recommending treatment with the drug of interest [26].
The interpretation and reporting for PD-L1 IHC assays in lung cancer samples differs from those for conventional IHC in that a wide variety of assays need to be interpreted according to the relevant criteria for each drug. Therefore, specialized training is important to maintain the consistency and quality of interpretation between pathologists.
ONGOING ISSUES
Use of archival tissue or need for re-biopsy
Concerns about performing PD-L1 testing using archival samples may be due to two reasons: (1) it is unclear whether antigenicity is preserved in the archival samples, and (2) treatment may alter the patterns of PD-L1 protein expression.
The answer to the first concern seems to have been resolved by a recently updated analysis of the KEYNOTE-010 trial [27]. They showed the overall survival benefit of pembrolizumab over docetaxel for both TPS ≥ 50% and ≥ 1%, regardless of whether PD-L1 was assessed in 456 archival or 578 newly collected tumor samples. Compared with newly collected tumor samples, archival samples were not associated with a loss of PD-L1 expression, suggesting that both newly acquired biopsy samples and aged archival specimens are suitable for PD-L1 testing.
Regarding the second issue, several reports have explored the changes in PD-L1 expression during the clinical course of NSCLC patients in relation to locoregional and/or systemic treatment. Omori et al. [28] demonstrated that major changes in PD-L1 expression were observed in 38% of a total of 76 NSCLC patients undergoing anticancer treatments, including systemic chemotherapy or targeted therapy, as well as only surgical resection. The effect of anticancer drugs on the expression of PD-L1 may be affected by the differences between agents as well as the characteristics of the tumor that affect the mechanisms of host immune system modulation. Recent results from several studies investigating the alterations in tumor PD-L1 expression in patients with NSCLC who received platinum-based neoadjuvant chemotherapy produced conflicting results, proving the importance of considering tumor-specific characteristics [29,30]. It has been reported that PD-L1 expression is increased by EGFR signaling conferred by the activation of EGFR mutations and that erlotinib could downregulate PD-L1 expression [31]. Conversely, several reports showed EGFR-TKI treatment appeared to increase PD-L1 expression in tumor cells with EGFR mutations [28,32]. Although the mechanism of these changes has not been elucidated, these discrepancies may be explained by differences in patient characteristics, such as tumor stage and anticancer treatments other than EGFR-TKI, which could lead to differences in PD-L1 expression. These dynamic properties of PD-L1 expression provide a possible explanation for the second- or further line treatment responses of PD-1/PD-L1 blockade therapies in treatment-naive samples with negative status. However, there is a lack of clinical data on the degree of accuracy with which the altered levels of PD-L1 expression after treatment predicts the response to immunotherapeutic agents. It is important to evaluate PD-L1 expression in serial samples throughout the treatment, and at least in the latest tumor specimen, especially for heavily treated NSCLC patients.
Heterogeneity
PD-L1 expression may show intratumoral or intertumoral heterogeneity; therefore, it is important to understand that the sampling method (surgical resection vs biopsy) and sites (primary vs. metastasis) may influence the PD-L1 expression status.
Several studies reported inconsistencies in the PD-L1 status of resected versus biopsied specimens. A comparison of PD-L1 expression by Ilie et al. [33] using an SP142 clone between preoperative biopsy specimens and their corresponding resected specimens in 160 NSCLC patients found a significant discordance (overall discordance rate = 48%; κ value = 0.218). The authors noted that most (75%) discordant cases were based on the assessment of PD-L1 staining in immune cells [33]. Gniadek et al. [34] compared four tissue microarray cores from 150 FFPE tissues of resected primary cancers using the SP142 clone. They found substantial inconsistencies in the percentages of PD-L1–positive cells in different tissue microarray cores in both 71 adenocarcinomas and 79 squamous cell carcinomas [34]. In our previous study, we used the 22C3 assay for comparison, as 22C3 showed the highest tumor proportion score and may reduce the effects of intratumoral heterogeneity in PD-L1 expression. However, seven of the 50 cases (14%) exhibited discordant PD-L1 expression between the tissue microarray cores and resected specimens [35].
Differences in PD-L1 expression between primary and metastatic lesions appear to be less important than the differences in sampling methods. Mansfield et al. [36] assessed the PD-L1 expression in 67 paired, resected multifocal lung cancers from thirty-two patients. They observed a strong consistency in PD-L1 expression in tumor cells among related, metastatic multifocal lung cancers; conversely, there was a low correlation of PD-L1 expression between multiple independent lesions [36]. Kim et al. [37] evaluated PD-L1 expression in 161 paired primary and metastatic adenocarcinoma tissues from 146 lung cancer patients using an E1L3N clone. Their study demonstrated that the concordance rate of PD-L1 expression between primary and metastatic tumors was 80.1% (k = 0.492) and 90.7% (k = 0.598) with a 1% cutoff, respectively [37].
This heterogeneity of PD-L1 expression is a major obstacle for PD-L1 testing; it may not be a perfect predictive biomarker for PD-1/PD-L1 blockade treatment, and this could be one of the reasons for the suboptimal correlation between PD-L1 expression and treatment responses. However, it is not practical to perform multiple biopsies at one or multiple sites to assess PD-L1 expression. Recently, novel techniques for testing PD-L1 expression using imaging [38] or peripheral blood [39,40] have been examined.
Cytological specimens
Currently, PD-L1 IHC is applicable to histologic samples only and is not recommended in cytologic samples, because cytologic materials were excluded for PD-L1 assessment in clinical trials. However, about one-third of patients with metastasis are still diagnosed by cytological materials only, which often is the only sample that can be used for PD-L1 testing. This has made some pathologists curious about the clinical use of cytology samples for PD-L1 testing.
Rebelatto et al. [41] showed that 95% alcohol, AFA, and Prefer are unsuitable fixatives for IHC with the SP263 clone. Evaluation of PD-L1–positive immune cells using the Ventana SP142 assay will likely be more challenging in cytological specimens, as the lack of tissue architecture precludes the ability to distinguish immune cells within the tumor area from those outside tumor boundaries that are considered irrelevant for PD-L1 scoring.
Using DAKO 28-8 and 22C3 clones, Skov and Skov [42] compared PD-L1–expression levels in 86 paired FFPE samples of cytologic cell blocks and histological materials from lung malignancies and observed a high degree of consistency between histologic and cytologic specimens within each assay. In cases showing discrepancies between the two sample types, the tumor tended to demonstrate heterogeneous PD-L1 staining in the histologic material, especially for PD-L1 expression ≥ 5% and ≥ 10% [42]. Additional studies have reported high conformity of PD-L1 expression between cell blocks and matched histological specimens and/or comparable PD-L1 expression among cell blocks, small biopsies, and resections in a prospective cohort using the 22C3 clone [43,44]. These data suggested that the assessment of PD-L1 expression in tumor cells can also be performed using cytologic materials that are processed to obtain cell blocks, and could be an alternative when histological samples are not available, at least when PD-L1 expression is detected.
However, before recommending the routine clinical use of cytological specimens, a standardized process should be established to account for the wide range of processing methods, including cell collection (e.g., aspiration, liquid-based, and cell block) and fixation (e.g., alcohol-based and formalin). Further large-scale validation studies are warranted to establish standardized PD-L1 IHC testing methods for cytology specimens.
BEYOND PROGRAMMED DEATH-LIGAND 1: IS THERE ANY PREDICTIVE BIOMARKER AS AN ALTERNATIVE TO PROGRAMMED DEATH-LIGAND 1?
PD-L1 IHC is the sole biomarker currently available for analysis; unfortunately, it is not an optimal biomarker owing to several major limitations, as discussed above. At present, there is a need to discover and validate additional predictive biomarkers other than PD-L1 IHC to improve patient selection and spare unnecessary toxicity and costs in non-responders. Various additional factors are under investigation, including the tumor mutation burden (TMB) [45,46], tumor-infiltrating lymphocytes [47,48], and immune gene signatures [5,6] that may identify tumors with preexisting immune activity and be correlated with the response to anti–PD-L1/PD-1 agents. Peripheral circulating immune cells and T-cell receptor diversity may be reflective of the tumor microenvironment, though this has yet to be validated in clinical practice [49,50]. Finally, although the gut microbiome is showing exciting promise as a marker for immune-checkpoint efficacy, its predictive value needs to be validated in larger clinical studies [51].
Of these, TMB defined as the total number of non-synonymous somatic mutations in the tumor genome is emerging as a predictive biomarker of response to ICIs in various cancers including NSCLC. Non-synonymous somatic mutations alter the amino acid sequence of proteins encoded by affected gene, forming neoantigens. It is hypothesized that neoantigen formation contributes to the intrinsic immunogenicity of a tumor [45,52]. In support of this premise, a higher TMB has also been shown to correlate with clinical benefit from ICI therapy in NSCLC [45,46], as well as small cell lung cancer [53], melanoma [52], and colorectal cancer [54]. While whole exome sequencing (WES) is widely considered the gold standard for measurement of TMB [45,55], performance of WES is currently impractical for several reasons including cost and turnaround time. Targeted panel sequencing has offered a practical estimate of TMB from the whole exome in the clinical setting [46,56]. Because the thresholds that define high TMB level vary, and reported values also depend on the different techniques used, it is important to harmonize and standardize TMB assay methods and reporting to ensure the successful implementation of clinical TMB testing [57]. Ongoing efforts to ensure reproducible assessment and reporting standards will facilitate the smooth implementation of TMB testing for cancer immunotherapy.
CONCLUSION
Much remains uncertain about the clinical response to PD-1/PD-L1 blockade therapy in NSCLC; however, it is very clear that one single test cannot be used as a reproducible surrogate to predict the benefit of immunotherapy. Rather, reflecting the clinical complexity of combination multi-modality therapies, the development of a predictive model that takes into account the complex components that affect tumor-host interactions is needed. Although pathologists need to face the practical reality that oncologists will regularly request the PD-L1 IHC results, it should also be considered that there may be room for improvement in terms of the biomarkers for immunotherapy response, and that PD-L1 expression alone is often insufficient for patient stratification for PD-1/PD-L1 blockade therapy.
Notes
Author Contributions
Conceptualization: HK, JHC.
Data curation: HK, JHC.
Formal analysis: HK.
Funding acquisition: JHC.
Investigation: HK, JHC.
Methodology: HK, JHC.
Supervision: JHC.
Writing—original draft: HK
Writing—review & editing: HK, JHC.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIT) (No. 2017R1A5A1015626).

