Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 53(4); 2019 > Article
-
Review
PD-L1 Testing in Non-small Cell Lung Cancer: Past, Present, and Future -
Hyojin Kim1
 , Jin-Haeng Chung1,2
, Jin-Haeng Chung1,2
-
Journal of Pathology and Translational Medicine 2019;53(4):199-206.
DOI: https://doi.org/10.4132/jptm.2019.04.24
Published online: May 2, 2019
1Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea
2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
- Corresponding Author Jin-Haeng Chung, MD, PhD Department of Pathology, Seoul National University Bundang Hospital, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea Tel: +82-31-787-3379 Fax: +82-31-787-4412 E-mail: chungjh@snu.ac.kr
© 2019 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Abstract
- PROGRAMMED DEATH-LIGAND 1 PROTEIN EXPRESSION AS A BIOMARKER IN LUNG CANCER: RATIONALE AND PERFORMANCE
- VARIOUS PROGRAMMED DEATH-LIGAND 1 ASSAY AND HARMONIZATION
- INTERPRETATION AND PATHOLOGICAL REPORTING OF PROGRAMMED DEATH-LIGAND 1 IMMUNOHISTOCHEMISTRY
- ONGOING ISSUES
- BEYOND PROGRAMMED DEATH-LIGAND 1: IS THERE ANY PREDICTIVE BIOMARKER AS AN ALTERNATIVE TO PROGRAMMED DEATH-LIGAND 1?
- CONCLUSION
- NOTES
- Acknowledgments
- REFERENCES
Abstract
- Blockade of the programmed cell death-1 (PD-1) axis has already been established as an effective treatment of non-small cell lung cancer. Immunohistochemistry (IHC) for programmed death-ligand 1 (PD-L1) protein is the only available biomarker that can guide treatment with immune checkpoint inhibitors in non-small cell lung cancer. Because each PD-1/PD-L1 blockade was approved together with a specific PD-L1 IHC assay used in the clinical trials, pathologists have been challenged with performing various assays with a limited sample. To provide a more unified understanding of this, several cross-validation studies between platforms have been performed and showed consistent results. However, the interchangeability of assays may be limited in practice because of the risk of misclassification of patients for the treatment. Furthermore, several issues, including the temporal and spatial heterogeneity of PD-L1 expression in the tumor, and the potential for cytology specimens to be used as an alternative to tissue samples for PD-L1 testing, have still not been resolved. In the future, one of the main aims of immunotherapy research should be to find a novel predictive biomarker for PD-1 blockade therapy and a way to combine it with PD-L1 IHC and other tests.
- The immune system is regulated through a number of receptor-ligand interactions to protect the host from external antigens and prevent autoimmune reactions [10]. The interaction of PD-1 expressed on cytotoxic T lymphocytes, and PD-L1 on antigenpresenting cells is one such example of an interaction (immune checkpoint) [11]. A tumor cell with variable neoantigens is recognized as non-self and is attacked by the immune system; however, to avoid elimination, the tumor cells may express PD-L1 protein on their surface [12]. Thus, blockade of this PD-1/PD-L1 interaction by monoclonal antibodies against either PD-1 (nivolumab and pembrolizumab) or PD-L1 (atezolizumab, durvalumab, and avelumab) seems a logical therapeutic approach, especially for a highly antigenic tumor like NSCLC [13].
- However, the mechanisms of PD-1/PD-L1 blockade therapy and PD-L1 testing are completely different from those of EGFR, ALK, and ROS1 testing, which inhibit addictive driver oncogenes in lung cancer. ICIs block only the interaction, which is a part of the normal functioning of the immune system. Therefore, the clinical effect or duration of the PD-1/PD-L1 blockade response will be different from those of receptor tyrosine kinase inhibitors. PD-L1 is a protein that is expressed with biological continuity and shows profound intra-tumoral heterogeneity, unlike genetic variation, which is separated by a binary system. It is important to choose the correct cutoff levels to define biomarker-positive and -negative patient groups for PD-L1 testing to have a predictive value. In addition, IHC for detecting protein activity may be influenced by the choice of various factors including primary antibody clones, detection system, and platforms related to complex biochemistry.
- PD-L1 expression assessment is now established as a routine practice but is not without challenges. Understanding these inherent characteristics of PD-L1 testing is an important basis for pathologists to correctly interpret PD-L1 IHC results and communicate with clinicians to recommend the most effective treatment options.
PROGRAMMED DEATH-LIGAND 1 PROTEIN EXPRESSION AS A BIOMARKER IN LUNG CANCER: RATIONALE AND PERFORMANCE
- The development of ICIs was led by high-profile clinical trials, and each pharmaceutical company designed a distinct PD-L1 IHC assay to support the clinical efficacy of their own drug. Therefore, four commercial antibodies are currently available to measure PD-L1 protein expression in formalin-fixed, paraffin-embedded (FFPE) lung tissue specimens. Each assay utilizes a different automated staining system, detection system, and even means of assessment and thresholds to determine positive PD-L1 protein expression (Table 1).
- However, this situation has resulted in a dilemma for pathologists. It is impractical to perform several different assays to detect one protein in a pathology laboratory with limited resources. The number of PD-L1 assays that can be performed in conjunction with the required biomarker testing (EGFR, ALK, etc.) is also limited due to the limited availability of tumor tissue, especially for small biopsy specimens from patients with advanced lung cancer. In addition, selecting one assay from the several available ones is also challenging. While each of the four IHC assays successfully recognizes PD-L1 protein, each antibody has been developed to bind to a specific epitope, and each detection system is also applied differently. Thus, the performance of these assays may be different and each assay has a cutoff to predict drug response that was determined through a clinical trial, suggesting limited interchangeability of the assays in clinical practice.
- As a consequence, a number of international projects have been launched to standardize the various PD-L1 assays. The most prominent result of the Blueprint project, led by the International Association for the Study of Lung Cancer, has been relatively promising. SP263, 22C3, and 28-8 showed a high concordance in the percentage of PD-L1 membrane staining of tumor cells at any intensity; conversely, a lower expression of PD-L1 in tumor cells was observed using the SP142 clone [14]. Following harmonization studies showed consistent results, and pathologists had hoped that several PD-L1 assays could be used interchangeably for the prescription of any of PD-1/PD-L1 blockade [15-18]. In addition, although 22C3 assay has been developed for use on the Dako platform, not every pathology laboratory has the Dako Autostainer, whereas the Ventana BenchMark platforms are more common in pathology laboratories. Several studies reported that the results of the 22C3 assay had shown a high correlation with those of the SP263 assay [19-22]. Based on these results, the SP263 assay gained Conformite Europeanne approval for nivolumab and pembrolizumab treatment as a complementary diagnostic test [23]. In Korea, the SP263 assay was also approved by the Korea FDA for nivolumab treatment. Although some studies have shown discordance between the SP263 and 22C3 assays [24,25], these discrepancies are recognized as due to the heterogeneity of PD-L1 expression or interobserver variability rather than due to the difference in assay performance. However, it is still burdensome that the misclassification of patients by using different PD-L1 assays interchangeably may lead to patients either not receiving the needed PD-1 blockade therapy, or receiving a treatment that is not beneficial. Strictly speaking, there is no gold standard assay that accurately measures PD-L1 expression and best predicts PD-1 blockade response.
VARIOUS PROGRAMMED DEATH-LIGAND 1 ASSAY AND HARMONIZATION
- For the reasons mentioned above, several PD-L1 assays are generally performed simultaneously or sequentially in many pathology laboratories for the prescription of anti–PD-1/PD-L1 agents. The interpretation of PD-L1 IHC assays is a challenge for pathologists because of the different methods of interpreting positive results and in the different cutoff values for each assay (Table 1).
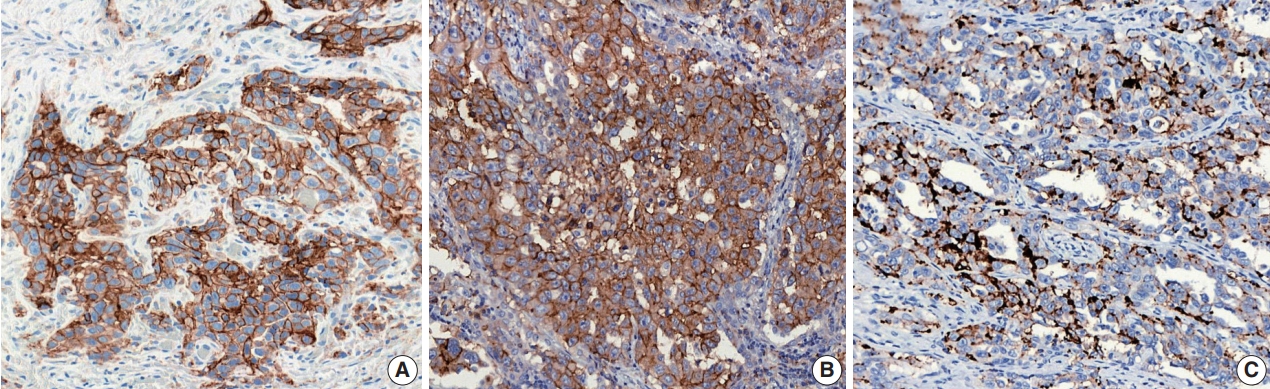
- For the definition of positive PD-L1 staining, complete circumferential or partial linear membranous staining of tumor cells at any intensity is considered positive for the 22C3 and 28-8 assays, while any membranous and/or cytoplasmic expression of tumor cells is considered positive for the SP263 and SP142 assays (Fig. 1). In the SP142 assay, PD-L1–positive immune cells, as well as tumor cells, are considered in the criteria for positive PD-L1 staining. Interpretation issues relating to distinguishing tumor cell PD-L1 chromogenic signals from those of inflammatory cells, the mislocalized signal from the membrane to the cytoplasm (for 22C3 and 28-8), and the scoring of percentages of expression particularly around the thresholds of clinical significance are always a concern for pathologists, regardless of the assay type. Furthermore, each assay has a specific cutoff value for positive tumor cells, and the percentage may be different depending on whether it is the first line vs. second or further line of treatment, even for the same drug. In addition, when an assay is applied for two drug prescriptions, such as SP263, different cutoffs may be applied depending on the drug. The SP263 assay uses two different cutoffs; 25% for durvalumab and 10% cutoff for nivolumab, in Korea (Table 1). Because these inconsistent cutoffs are not proven by clinical trial, but a special situation in Korea related to national insurance, pathologists also have to pay close attention to the insurance policies related with PD-L1 testing.
- Given these differences, pathological reports on PD-L1 IHC should be more comprehensive, giving more than a simple positive or negative result. The name of the diagnostic kit used, the assessment score, information on the required minimum number of cells that were assessed, and any comment on the meaning of the score with regard to the cutoff of that specific test should be included in the report. If the immunotherapeutic agent to be used is known at the time of testing, the results can be reported in terms of broader categories (e.g., < 1%, 1%–49%, and > 50% with 22C3 for pembrolizumab treatment), appropriate for recommending treatment with the drug of interest [26].
- The interpretation and reporting for PD-L1 IHC assays in lung cancer samples differs from those for conventional IHC in that a wide variety of assays need to be interpreted according to the relevant criteria for each drug. Therefore, specialized training is important to maintain the consistency and quality of interpretation between pathologists.
INTERPRETATION AND PATHOLOGICAL REPORTING OF PROGRAMMED DEATH-LIGAND 1 IMMUNOHISTOCHEMISTRY
- Use of archival tissue or need for re-biopsy
- Concerns about performing PD-L1 testing using archival samples may be due to two reasons: (1) it is unclear whether antigenicity is preserved in the archival samples, and (2) treatment may alter the patterns of PD-L1 protein expression.
- The answer to the first concern seems to have been resolved by a recently updated analysis of the KEYNOTE-010 trial [27]. They showed the overall survival benefit of pembrolizumab over docetaxel for both TPS ≥ 50% and ≥ 1%, regardless of whether PD-L1 was assessed in 456 archival or 578 newly collected tumor samples. Compared with newly collected tumor samples, archival samples were not associated with a loss of PD-L1 expression, suggesting that both newly acquired biopsy samples and aged archival specimens are suitable for PD-L1 testing.
- Regarding the second issue, several reports have explored the changes in PD-L1 expression during the clinical course of NSCLC patients in relation to locoregional and/or systemic treatment. Omori et al. [28] demonstrated that major changes in PD-L1 expression were observed in 38% of a total of 76 NSCLC patients undergoing anticancer treatments, including systemic chemotherapy or targeted therapy, as well as only surgical resection. The effect of anticancer drugs on the expression of PD-L1 may be affected by the differences between agents as well as the characteristics of the tumor that affect the mechanisms of host immune system modulation. Recent results from several studies investigating the alterations in tumor PD-L1 expression in patients with NSCLC who received platinum-based neoadjuvant chemotherapy produced conflicting results, proving the importance of considering tumor-specific characteristics [29,30]. It has been reported that PD-L1 expression is increased by EGFR signaling conferred by the activation of EGFR mutations and that erlotinib could downregulate PD-L1 expression [31]. Conversely, several reports showed EGFR-TKI treatment appeared to increase PD-L1 expression in tumor cells with EGFR mutations [28,32]. Although the mechanism of these changes has not been elucidated, these discrepancies may be explained by differences in patient characteristics, such as tumor stage and anticancer treatments other than EGFR-TKI, which could lead to differences in PD-L1 expression. These dynamic properties of PD-L1 expression provide a possible explanation for the second- or further line treatment responses of PD-1/PD-L1 blockade therapies in treatment-naive samples with negative status. However, there is a lack of clinical data on the degree of accuracy with which the altered levels of PD-L1 expression after treatment predicts the response to immunotherapeutic agents. It is important to evaluate PD-L1 expression in serial samples throughout the treatment, and at least in the latest tumor specimen, especially for heavily treated NSCLC patients.
- Heterogeneity
- PD-L1 expression may show intratumoral or intertumoral heterogeneity; therefore, it is important to understand that the sampling method (surgical resection vs biopsy) and sites (primary vs. metastasis) may influence the PD-L1 expression status.
- Several studies reported inconsistencies in the PD-L1 status of resected versus biopsied specimens. A comparison of PD-L1 expression by Ilie et al. [33] using an SP142 clone between preoperative biopsy specimens and their corresponding resected specimens in 160 NSCLC patients found a significant discordance (overall discordance rate = 48%; κ value = 0.218). The authors noted that most (75%) discordant cases were based on the assessment of PD-L1 staining in immune cells [33]. Gniadek et al. [34] compared four tissue microarray cores from 150 FFPE tissues of resected primary cancers using the SP142 clone. They found substantial inconsistencies in the percentages of PD-L1–positive cells in different tissue microarray cores in both 71 adenocarcinomas and 79 squamous cell carcinomas [34]. In our previous study, we used the 22C3 assay for comparison, as 22C3 showed the highest tumor proportion score and may reduce the effects of intratumoral heterogeneity in PD-L1 expression. However, seven of the 50 cases (14%) exhibited discordant PD-L1 expression between the tissue microarray cores and resected specimens [35].
- Differences in PD-L1 expression between primary and metastatic lesions appear to be less important than the differences in sampling methods. Mansfield et al. [36] assessed the PD-L1 expression in 67 paired, resected multifocal lung cancers from thirty-two patients. They observed a strong consistency in PD-L1 expression in tumor cells among related, metastatic multifocal lung cancers; conversely, there was a low correlation of PD-L1 expression between multiple independent lesions [36]. Kim et al. [37] evaluated PD-L1 expression in 161 paired primary and metastatic adenocarcinoma tissues from 146 lung cancer patients using an E1L3N clone. Their study demonstrated that the concordance rate of PD-L1 expression between primary and metastatic tumors was 80.1% (k = 0.492) and 90.7% (k = 0.598) with a 1% cutoff, respectively [37].
- This heterogeneity of PD-L1 expression is a major obstacle for PD-L1 testing; it may not be a perfect predictive biomarker for PD-1/PD-L1 blockade treatment, and this could be one of the reasons for the suboptimal correlation between PD-L1 expression and treatment responses. However, it is not practical to perform multiple biopsies at one or multiple sites to assess PD-L1 expression. Recently, novel techniques for testing PD-L1 expression using imaging [38] or peripheral blood [39,40] have been examined.
- Cytological specimens
- Currently, PD-L1 IHC is applicable to histologic samples only and is not recommended in cytologic samples, because cytologic materials were excluded for PD-L1 assessment in clinical trials. However, about one-third of patients with metastasis are still diagnosed by cytological materials only, which often is the only sample that can be used for PD-L1 testing. This has made some pathologists curious about the clinical use of cytology samples for PD-L1 testing.
- Rebelatto et al. [41] showed that 95% alcohol, AFA, and Prefer are unsuitable fixatives for IHC with the SP263 clone. Evaluation of PD-L1–positive immune cells using the Ventana SP142 assay will likely be more challenging in cytological specimens, as the lack of tissue architecture precludes the ability to distinguish immune cells within the tumor area from those outside tumor boundaries that are considered irrelevant for PD-L1 scoring.
- Using DAKO 28-8 and 22C3 clones, Skov and Skov [42] compared PD-L1–expression levels in 86 paired FFPE samples of cytologic cell blocks and histological materials from lung malignancies and observed a high degree of consistency between histologic and cytologic specimens within each assay. In cases showing discrepancies between the two sample types, the tumor tended to demonstrate heterogeneous PD-L1 staining in the histologic material, especially for PD-L1 expression ≥ 5% and ≥ 10% [42]. Additional studies have reported high conformity of PD-L1 expression between cell blocks and matched histological specimens and/or comparable PD-L1 expression among cell blocks, small biopsies, and resections in a prospective cohort using the 22C3 clone [43,44]. These data suggested that the assessment of PD-L1 expression in tumor cells can also be performed using cytologic materials that are processed to obtain cell blocks, and could be an alternative when histological samples are not available, at least when PD-L1 expression is detected.
- However, before recommending the routine clinical use of cytological specimens, a standardized process should be established to account for the wide range of processing methods, including cell collection (e.g., aspiration, liquid-based, and cell block) and fixation (e.g., alcohol-based and formalin). Further large-scale validation studies are warranted to establish standardized PD-L1 IHC testing methods for cytology specimens.
ONGOING ISSUES
- PD-L1 IHC is the sole biomarker currently available for analysis; unfortunately, it is not an optimal biomarker owing to several major limitations, as discussed above. At present, there is a need to discover and validate additional predictive biomarkers other than PD-L1 IHC to improve patient selection and spare unnecessary toxicity and costs in non-responders. Various additional factors are under investigation, including the tumor mutation burden (TMB) [45,46], tumor-infiltrating lymphocytes [47,48], and immune gene signatures [5,6] that may identify tumors with preexisting immune activity and be correlated with the response to anti–PD-L1/PD-1 agents. Peripheral circulating immune cells and T-cell receptor diversity may be reflective of the tumor microenvironment, though this has yet to be validated in clinical practice [49,50]. Finally, although the gut microbiome is showing exciting promise as a marker for immune-checkpoint efficacy, its predictive value needs to be validated in larger clinical studies [51].
- Of these, TMB defined as the total number of non-synonymous somatic mutations in the tumor genome is emerging as a predictive biomarker of response to ICIs in various cancers including NSCLC. Non-synonymous somatic mutations alter the amino acid sequence of proteins encoded by affected gene, forming neoantigens. It is hypothesized that neoantigen formation contributes to the intrinsic immunogenicity of a tumor [45,52]. In support of this premise, a higher TMB has also been shown to correlate with clinical benefit from ICI therapy in NSCLC [45,46], as well as small cell lung cancer [53], melanoma [52], and colorectal cancer [54]. While whole exome sequencing (WES) is widely considered the gold standard for measurement of TMB [45,55], performance of WES is currently impractical for several reasons including cost and turnaround time. Targeted panel sequencing has offered a practical estimate of TMB from the whole exome in the clinical setting [46,56]. Because the thresholds that define high TMB level vary, and reported values also depend on the different techniques used, it is important to harmonize and standardize TMB assay methods and reporting to ensure the successful implementation of clinical TMB testing [57]. Ongoing efforts to ensure reproducible assessment and reporting standards will facilitate the smooth implementation of TMB testing for cancer immunotherapy.
BEYOND PROGRAMMED DEATH-LIGAND 1: IS THERE ANY PREDICTIVE BIOMARKER AS AN ALTERNATIVE TO PROGRAMMED DEATH-LIGAND 1?
- Much remains uncertain about the clinical response to PD-1/PD-L1 blockade therapy in NSCLC; however, it is very clear that one single test cannot be used as a reproducible surrogate to predict the benefit of immunotherapy. Rather, reflecting the clinical complexity of combination multi-modality therapies, the development of a predictive model that takes into account the complex components that affect tumor-host interactions is needed. Although pathologists need to face the practical reality that oncologists will regularly request the PD-L1 IHC results, it should also be considered that there may be room for improvement in terms of the biomarkers for immunotherapy response, and that PD-L1 expression alone is often insufficient for patient stratification for PD-1/PD-L1 blockade therapy.
CONCLUSION
Author Contributions
Conceptualization: HK, JHC.
Data curation: HK, JHC.
Formal analysis: HK.
Funding acquisition: JHC.
Investigation: HK, JHC.
Methodology: HK, JHC.
Supervision: JHC.
Writing—original draft: HK
Writing—review & editing: HK, JHC.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Acknowledgments
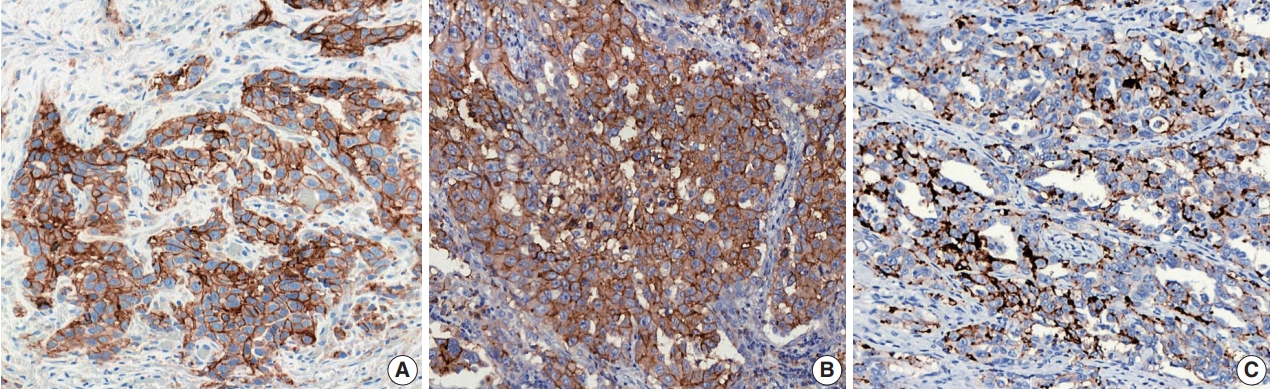
| PD-L1 assay | |||||
|---|---|---|---|---|---|
| Performance | Clone | 22C3 | 28-8 | SP263 | SP142 |
| Developer | Dako | Dako | Ventana | Ventana | |
| Host species | Mouse monoclonal | Rabbit monoclonal | Rabbit monoclonal | Rabbit monoclonal | |
| Epitope location | Extracellular domain | Extracellular domain | Cytoplasmic domain | Cytoplasmic domain | |
| Platform | Link 48 autostainer | Link 48 autostainer | Benchmark ultra | Benchmark ultra | |
| Detection kit | Envision FLEX | Envision FLEX | Optiview | Optiview | |
| Amplification | No | No | No | Yes | |
| Interpretation | Scoring | TC | TC | TC | TC and IC |
| Staining pattern for positivity | Membranous | Membranous | Membranous ± cytoplasmic | Membranous±cytoplasmic | |
| Minimum TC number | 100 | 100 | 100 | 50 with associated stroma | |
| Cut-off (mandatory) | ≥ 50% (≥ 2nd line), ≥ 1% (1st line) | All comer | All comer | All comer | |
| Cut-off (proven survival benefit) | ≥ 1%, ≥ 5%, ≥ 10% | ≥ 25% (for durvalumab), ≥ 10% (for nivolumab)a | TC ≥ 5% or IC ≥ 5% | ||
| Pharma | Immune checkpoint inhibitor | Pembrolizumab | Nivolumab | Durvalumab Nivolumabb | Atezolizumab |
| FDA approval | Companion | Complementary | Complementary | Complementary | |
- 1. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627-39. PubMedPMC
- 2. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123-35. ArticlePubMedPMC
- 3. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823-33. ArticlePubMed
- 4. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540-50. ArticlePubMed
- 5. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837-46. ArticlePubMed
- 6. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255-65. ArticlePubMed
- 7. Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 2017; 18: 599-610. ArticlePubMedPMC
- 8. Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018; 19: 521-36. PubMedPMC
- 9. Non-small cell lung cancer. Version 3.2018 [Internet]. Plymouth Meeting: National Comprehensive Cancer Network, 2018 [cited 2019 Mar 29]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 10. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3: 991-8. ArticlePubMedPDF
- 11. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252-64. ArticlePubMedPMCPDF
- 12. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331: 1565-70. ArticlePubMed
- 13. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455-65. PubMedPMC
- 14. Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 2017; 12: 208-22. ArticlePubMed
- 15. Scheel AH, Dietel M, Heukamp LC, et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod Pathol 2016; 29: 1165-72. ArticlePubMedPDF
- 16. Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol 2017; 3: 1051-8. ArticlePubMedPMC
- 17. Adam J, Le Stang N, Rouquette I, et al. Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer. Ann Oncol 2018; 29: 953-8. ArticlePubMedPDF
- 18. Ratcliffe MJ, Sharpe A, Midha A, et al. Agreement between programmed cell death ligand-1 diagnostic assays across multiple protein expression cutoffs in non-small cell lung cancer. Clin Cancer Res 2017; 23: 3585-91. ArticlePubMedPDF
- 19. Sughayer MA, Alnaimy F, Alsughayer AM, Qamhia N. Comparison of 22C3 PharmDx and SP263 assays to test PD-L1 expression in NSCLC. Appl Immunohistochem Mol Morphol 2018 Jul 17 [Epub]. https://doi.org/10.1097/PAI.0000000000000671. Article
- 20. Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of Blueprint Phase 2 Project. J Thorac Oncol 2018; 13: 1302-11. ArticlePubMedPMC
- 21. Fujimoto D, Sato Y, Uehara K, et al. Predictive performance of four programmed cell death ligand 1 assay systems on nivolumab response in previously treated patients with non-small cell lung cancer. J Thorac Oncol 2018; 13: 377-86. ArticlePubMed
- 22. Marchetti A, Barberis M, Franco R, et al. Multicenter comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) assays to test PD-L1 expression for NSCLC patients to be treated with immune checkpoint inhibitors. J Thorac Oncol 2017; 12: 1654-63. ArticlePubMed
- 23. VENTANA PD-L1 (SP263) Assay (CE IVD) [Internet]. Indianapolis: Roche Diagnostics, 2019 [cited 2019 Mar 29]. Available from: https://diagnostics.roche.com/global/en/products/tests/ventanapd-l1-_sp263-assay2.html.
- 24. Munari E, Rossi G, Zamboni G, et al. PD-L1 assays 22C3 and SP263 are not interchangeable in non-small cell lung cancer when considering clinically relevant cutoffs: an interclone evaluation by differently trained pathologists. Am J Surg Pathol 2018; 42: 1384-9. PubMed
- 25. Hendry S, Byrne DJ, Wright GM, et al. Comparison of four PD-L1 immunohistochemical assays in lung cancer. J Thorac Oncol 2018; 13: 367-76. ArticlePubMed
- 26. Tsao MS, Kerr KM, Dacic SA, Yatabe YA, Hirsch FR. IASLC atlas of PD-L1 immunohistochemistry testing in lung cancer. Aurora: International Association for the Study of Lung Cancer, 2017.
- 27. Herbst RS, Baas P, Perez-Gracia JL, et al. Use of archival versus newly collected tumor samples for assessing PD-L1 expression and overall survival: an updated analysis of KEYNOTE-010 trial. Ann Oncol 2019; 30: 281-9. ArticlePubMedPMCPDF
- 28. Omori S, Kenmotsu H, Abe M, et al. Changes in programmed death ligand 1 expression in non-small cell lung cancer patients who received anticancer treatments. Int J Clin Oncol 2018; 23: 1052-9. ArticlePubMedPDF
- 29. Sheng J, Fang W, Yu J, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in nonsmall cell lung cancer. Sci Rep 2016; 6: 20090.ArticlePubMedPMCPDF
- 30. Shin J, Chung JH, Kim SH, et al. Effect of platinum-based chemotherapy on PD-L1 expression on tumor cells in non-small cell lung cancer. Cancer Res Treat 2018 Nov 5 [Epub]. https://doi.org/10.4143/crt.2018.537. ArticlePDF
- 31. Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 2014; 25: 1935-40. ArticlePubMedPDF
- 32. Han JJ, Kim DW, Koh J, et al. Change in PD-L1 expression after acquiring resistance to gefitinib in EGFR-mutant non-small-cell lung cancer. Clin Lung Cancer 2016; 17: 263-70. e2. ArticlePubMed
- 33. Ilie M, Long-Mira E, Bence C, et al. Comparative study of the PDL1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol 2016; 27: 147-53. ArticlePubMedPDF
- 34. Gniadek TJ, Li QK, Tully E, Chatterjee S, Nimmagadda S, Gabrielson E. Heterogeneous expression of PD-L1 in pulmonary squamous cell carcinoma and adenocarcinoma: implications for assessment by small biopsy. Mod Pathol 2017; 30: 530-8. ArticlePubMedPDF
- 35. Kim H, Kwon HJ, Park SY, Park E, Chung JH. PD-L1 immunohistochemical assays for assessment of therapeutic strategies involving immune checkpoint inhibitors in non-small cell lung cancer: a comparative study. Oncotarget 2017; 8: 98524-32. ArticlePubMedPMC
- 36. Mansfield AS, Murphy SJ, Peikert T, et al. Heterogeneity of programmed cell death ligand 1 expression in multifocal lung cancer. Clin Cancer Res 2016; 22: 2177-82. ArticlePubMedPDF
- 37. Kim S, Koh J, Kwon D, et al. Comparative analysis of PD-L1 expression between primary and metastatic pulmonary adenocarcinomas. Eur J Cancer 2017; 75: 141-9. ArticlePubMed
- 38. Niemeijer AN, Leung D, Huisman MC, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with nonsmall-cell lung cancer. Nat Commun 2018; 9: 4664.ArticlePubMedPMCPDF
- 39. Ilié M, Szafer-Glusman E, Hofman V, et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol 2018; 29: 193-9. ArticlePubMedPDF
- 40. Guibert N, Delaunay M, Lusque A, et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer 2018; 120: 108-12. ArticlePubMed
- 41. Rebelatto MC, Midha A, Mistry A, et al. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol 2016; 11: 95.ArticlePubMedPMCPDF
- 42. Skov BG, Skov T. Paired comparison of PD-L1 expression on cytologic and histologic specimens from malignancies in the lung assessed with PD-L1 IHC 28-8pharmDx and PD-L1 IHC 22C3pharmDx. Appl Immunohistochem Mol Morphol 2017; 25: 453-9. ArticlePubMed
- 43. Heymann JJ, Bulman WA, Swinarski D, et al. PD-L1 expression in non-small cell lung carcinoma: comparison among cytology, small biopsy, and surgical resection specimens. Cancer Cytopathol 2017; 125: 896-907. ArticlePubMedPDF
- 44. Wang H, Agulnik J, Kasymjanova G, et al. Cytology cell blocks are suitable for immunohistochemical testing for PD-L1 in lung cancer. Ann Oncol 2018; 29: 1417-22. ArticlePubMedPDF
- 45. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348: 124-8. PubMedPMC
- 46. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018; 378: 2093-104. ArticlePubMedPMC
- 47. Mazzaschi G, Madeddu D, Falco A, et al. Low PD-1 expression in cytotoxic CD8(+) tumor-infiltrating lymphocytes confers an immuneprivileged tissue microenvironment in NSCLC with a prognostic and predictive value. Clin Cancer Res 2018; 24: 407-19. ArticlePubMedPDF
- 48. Kim H, Kwon HJ, Han YB, et al. Increased CD3+ T cells with a low FOXP3+/CD8+ T cell ratio can predict anti-PD-1 therapeutic response in non-small cell lung cancer patients. Mod Pathol 2019; 32: 367-75. ArticlePubMedPDF
- 49. Postow MA, Manuel M, Wong P, et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J Immunother Cancer 2015; 3: 23.ArticlePubMedPMCPDF
- 50. Weber JS, Sznol M, Sullivan RJ, et al. A serum protein signature associated with outcome after anti-PD-1 therapy in metastatic melanoma. Cancer Immunol Res 2018; 6: 79-86. ArticlePubMedPDF
- 51. Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015; 350: 1084-9. ArticlePubMedPMC
- 52. Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014; 371: 2189-99. ArticlePubMedPMC
- 53. Hellmann MD, Callahan MK, Awad MM, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 2018; 33: 853-61. e4. ArticlePubMedPMC
- 54. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372: 2509-20. PubMedPMC
- 55. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017; 376: 2415-26. ArticlePubMedPMC
- 56. Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol 2018; 36: 633-41. ArticlePubMedPMC
- 57. Deans ZC, Costa JL, Cree I, et al. Integration of next-generation sequencing in clinical diagnostic molecular pathology laboratories for analysis of solid tumours; an expert opinion on behalf of IQN Path ASBL. Virchows Arch 2017; 470: 5-20. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- Impact of patient and tumor characteristics on various aspects of the non-small cell lung cancer patient journey in Romania, Bulgaria, Serbia: A multicenter, non-interventional, retrospective cohort study
Alexandra C. Preda, Rossitza Krasteva, Mihailo Stjepanović, Krassimir Koynov, Assia Konsoulova, Georgeta P. Iorga, Anghel A. Udrea, Anca Zgura, Tudor-Eliade Ciuleanu, Raluca Patru, Jeliazko Arabadjiev, Ivan Tonev, Zoran Andrić, Goran Stojanović, Mihaela P
Cancer Treatment and Research Communications.2026; 46: 101056. CrossRef - Impact of First-Line Maintenance Immunotherapy With or Without Pemetrexed for Non-Squamous Advanced/Metastatic Non-Small Cell Lung Cancer Lacking Targetable Mutations: A Real-World Analysis in England
Fabio Gomes, Niall Gilding, Vivian Tan, Katerina Christoforou, Joanne Rule, Amine Aziez, Adam Januszewski, Anna Minchom
Clinical Lung Cancer.2026;[Epub] CrossRef - A patient similarity-embedded Bayesian approach to prognostic biomarker inference with application to thoracic cancer immunity
Duo Yu, Meilin Huang, Michael J Kane, Brian P Hobbs
Journal of the Royal Statistical Society Series C: Applied Statistics.2025; 74(3): 800. CrossRef - Programmed Cell Death Ligand 1 (PD-L1) and major Histocompatibility Complex Class I (MHC Class I) Expression Patterns and Their Pathologic Associations in triple-Negative Breast Cancer
Ponkrit Kaewkedsri, Piyapharom Intarawichian, Sirawich Jessadapattarakul, Waritta Kunprom, Supinda Koonmee, Malinee Thanee, Ongart Somintara, Anongporn Wongbuddha, Payia Chadbunchachai, Supajit Nawapun, Chaiwat Aphivatanasiri
Breast Cancer: Targets and Therapy.2025; Volume 17: 123. CrossRef - Exploring immune checkpoint inhibitors: Focus on PD-1/PD-L1 axis and beyond
Durre Aden, Samreen Zaheer, Niti Sureka, Monal Trisal, Jai Kumar Chaurasia, Sufian Zaheer
Pathology - Research and Practice.2025; 269: 155864. CrossRef - Prognostic value of HER2 expression in cervical adenocarcinoma: A retrospective cohort study
Qing Xu, Zhuomin Yin, Yueqi Li, Xiu Zhu, Hanmei Lou, Juan Ni
Oncology Letters.2025; 29(5): 1. CrossRef - PD-1/PD-L1 blockade therapy with atezolizumab: a new paradigm in the treatment of non-small cell lung cancer (NSCLC)
Samaneh Moradi, Pedram Sarikhani, Rafid Jihad Albadr, Waam Mohammed Taher, Mariem Alwan, Mahmood Jasem Jawad, Hiba Mushtaq, Niyousha Vakilzadehian
Discover Oncology.2025;[Epub] CrossRef - PD‐L1 Scoring Models for Non‐Small Cell Lung Cancer in China: Current Status, AI‐Assisted Solutions and Future Perspectives
Ziling Huang, Shen Wang, Jiansong Zhou, Haiquan Chen, Yuan Li
Thoracic Cancer.2025;[Epub] CrossRef - Advancing Precision Medicine in PDAC: An Ethical Scoping Review and Call to Action for IHC Implementation
Lyanne A. Delgado-Coka, Lucia Roa-Peña, Andrew Flescher, Luisa F. Escobar-Hoyos, Kenneth R. Shroyer
Cancers.2025; 17(12): 1899. CrossRef - A Prospective Exploratory Study of Functional Immunity Assessed by Pretreatment Interferon Gamma Release Assay in Relation to Different Systemic Therapies in Patients With Advanced-Stage NSCLC
Hsu-Ching Huang, Han-Jhih Chang, Chi-Lu Chiang, Hsin-Yi Huang, Yung-Hung Luo, Yuh-Min Chen, Tsu-Hui Shiao, Chao-Hua Chiu
JTO Clinical and Research Reports.2025; 6(8): 100845. CrossRef - A panorama of lung cancer biomarkers
Ana Sofia Silva Mesquita, Maire Iumi Maeda, Juliana Cabral Duarte Brandão, Nicolle Cavalcante Gaglionone, Igor Campos da Silva, Milena Perez Mak, Ellen Caroline Toledo do Nascimento
Surgical and Experimental Pathology.2025;[Epub] CrossRef - Unraveling Resistance in Lung Cancer Immunotherapy: Clinical Milestones, Mechanistic Insights, and Future Strategies
Maria Vitale, Raffaella Pagliaro, Giuseppe Viscardi, Lucio Pastore, Giuseppe Castaldo, Fabio Perrotta, Susan F. Campbell, Andrea Bianco, Filippo Scialò
International Journal of Molecular Sciences.2025; 26(18): 9244. CrossRef - Precision Oncology in Lung Cancer Surgery
Patrick Bou-Samra, Sunil Singhal
Surgical Oncology Clinics of North America.2024; 33(2): 311. CrossRef - Expression of PD-L1 clones (22C3 and 28-8) in hepatocellular carcinoma: a tertiary cancer care hospital experience
Kashif Asghar, Shaarif Bashir, Muhammad Hassan, Asim Farooq, Muhammad Abu Bakar, Sundus Bilal, Maryam Hameed, Shafqat Mehmood, Asif Loya
Egyptian Liver Journal.2024;[Epub] CrossRef - Engineering Proteus mirabilis improves antitumor efficacy via enhancing cytotoxic T cell responses
Hong Zhang, Yinlin Luo, Xincheng Zhao, Xiande Liu
Molecular Therapy: Oncology.2024; 32(1): 200770. CrossRef - Unlocking the potential of oncology biomarkers: advancements in clinical theranostics
Ankit Kumar Dubey, Ishnoor Kaur, Reecha Madaan, Shikha Raheja, Rajni Bala, Manoj Garg, Suresh Kumar, Viney Lather, Vineet Mittal, Deepti Pandita, Rohit Gundamaraju, Rajeev K. Singla, Rohit Sharma
Drug Metabolism and Personalized Therapy.2024; 39(1): 5. CrossRef - Exploring histological predictive biomarkers for immune checkpoint inhibitor therapy response in non–small cell lung cancer
Uiju Cho, Soyoung Im, Hyung Soon Park
Journal of Pathology and Translational Medicine.2024; 58(2): 49. CrossRef - Predicting efficacy assessment of combined treatment of radiotherapy and nivolumab for NSCLC patients through virtual clinical trials using QSP modeling
Miriam Schirru, Hamza Charef, Khalil-Elmehdi Ismaili, Frédérique Fenneteau, Didier Zugaj, Pierre-Olivier Tremblay, Fahima Nekka
Journal of Pharmacokinetics and Pharmacodynamics.2024; 51(4): 319. CrossRef - Predictions of PD-L1 Expression Based on CT Imaging Features in Lung Squamous Cell Carcinoma
Seong Hee Yeo, Hyun Jung Yoon, Injoong Kim, Yeo Jin Kim, Young Lee, Yoon Ki Cha, So Hyeon Bak
Journal of the Korean Society of Radiology.2024; 85(2): 394. CrossRef - Assessment of Programmed Cell Death Ligand-1 Expression in Non-small Cell Lung Carcinomas by Immunohistochemistry
Sandeep Mani, Archana Lakshmanan, Annapurneswari Subramanyan
Apollo Medicine.2024; 21(1): 57. CrossRef - The efficacy of immune checkpoint inhibitors on low PD‐L1 cervical cancer: A meta‐analysis
Wutao Chen, Nan Zhang, Zhihong He, Qing Li, You Wang, Weihua Lou, Wen Di
Health Science Reports.2024;[Epub] CrossRef - Molecular Characteristics and Pretreatment Neutrophil-to-Lymphocyte Ratio as Predictors of Durable Clinical Benefit from Immune Checkpoint Inhibition in Non-Small Cell Lung Cancer
Arpeet T. Shah, Isabelle Blanchard, Sukhmani K. Padda, Heather A. Wakelee, Joel W. Neal
Clinical Lung Cancer.2024; 25(6): 550. CrossRef - Optimizing immunofluorescence with high-dynamic-range imaging to enhance PD-L1 expression evaluation for 3D pathology assessment from NSCLC tumor tissue
Hsien-Neng Huang, Chun-Wei Kuo, Yu-Ling Hung, Chia-Hung Yang, Yu-Han Hsieh, Yu-Chieh Lin, Margaret Dah-Tsyr Chang, Yen-Yin Lin, Jen-Chung Ko
Scientific Reports.2024;[Epub] CrossRef - The Role of Programmed Cell Death Ligand 1 Expression in Pituitary Tumours: Lessons from the Current Literature
Mariana Lopes-Pinto, Ema Lacerda-Nobre, Ana Luísa Silva, Francisco Tortosa, Pedro Marques
Neuroendocrinology.2024; 114(8): 709. CrossRef - Real‑world evidence of advanced non‑small cell lung carcinoma treated with an immune checkpoint inhibitor plus chemotherapy
Zihan Xu, Huien Zhang, Guikai Ma, Wenjuan Meng, Junliang Du, Xin Wu, Baohong Yang, Ningning Wang, Yanhong Ding, Qingyun Zhang, Na Li, Xuede Zhang, Guohua Yu, Shuzhen Liu, Zhenhua Li
Oncology Letters.2024;[Epub] CrossRef - Clinical impact of concomitant BIO-three use in advanced or recurrent non-small cell lung cancer treated with immune-checkpoint inhibitor
Hitomi Nakatsukasa, Masaya Takahashi, Masahito Shibano, Yusuke Ishigami, Tomoya Kawaguchi, Yasutaka Nakamura, Hiroyasu Kaneda
International Journal of Clinical Oncology.2024; 29(12): 1840. CrossRef - Special issue “The advance of solid tumor research in China”: FGFR4 alterations predict efficacy of immune checkpoint inhibitors in nonsmall cell lung cancer
Guanghui Gao, Longgang Cui, Fei Zhou, Tao Jiang, Wanying Wang, Shiqi Mao, Fengying Wu, Fangli Jiang, Bei Zhang, Ting Bei, Wenchuan Xie, Cheng Zhang, Hougang Zhang, Chan Gao, Xiaochen Zhao, Yuezong Bai, Caicun Zhou, Shengxiang Ren
International Journal of Cancer.2023; 152(1): 79. CrossRef - Molecular Classification of Extrapulmonary Neuroendocrine Carcinomas With Emphasis on POU2F3-positive Tuft Cell Carcinoma
Jiwon Koh, Haeryoung Kim, Kyung Chul Moon, Cheol Lee, Kyoungbun Lee, Han Suk Ryu, Kyeong Cheon Jung, Yoon Kyung Jeon
American Journal of Surgical Pathology.2023; 47(2): 183. CrossRef - Biomarkers for Predicting Response to Personalized Immunotherapy in Gastric Cancer
Moonsik Kim, Ji Yun Jeong, An Na Seo
Diagnostics.2023; 13(17): 2782. CrossRef - PD-L1 Expression in Pituitary Neuroendocrine Tumors/Pituitary Adenomas
Giulia Cossu, Stefano La Rosa, Jean Philippe Brouland, Nelly Pitteloud, Ethan Harel, Federico Santoni, Maxime Brunner, Roy Thomas Daniel, Mahmoud Messerer
Cancers.2023; 15(18): 4471. CrossRef - Prognostic Significance of Programmed Cell Death Ligand 1 Expression in High-Grade Serous Ovarian Carcinoma: A Systematic Review and Meta-Analysis
Jeongwan Kang, Kang Min Han, Hera Jung, Hyunchul Kim
Diagnostics.2023; 13(20): 3258. CrossRef - A targeted expression panel for classification, gene fusion detection and PD-L1 measurements – Can molecular profiling replace immunohistochemistry in non-small cell lung cancer?
Anita Tranberg Simonsen, Amalie Utke, Johanne Lade-Keller, Lasse Westphal Thomsen, Torben Steiniche, Magnus Stougaard
Experimental and Molecular Pathology.2022; 125: 104749. CrossRef - Program death ligand‐1 immunocytochemistry in lung cancer cytological samples: A systematic review
Swati Satturwar, Ilaria Girolami, Enrico Munari, Francesco Ciompi, Albino Eccher, Liron Pantanowitz
Diagnostic Cytopathology.2022; 50(6): 313. CrossRef - Computer-assisted three-dimensional quantitation of programmed death-ligand 1 in non-small cell lung cancer using tissue clearing technology
Yen-Yu Lin, Lei-Chi Wang, Yu-Han Hsieh, Yu-Ling Hung, Yung-An Chen, Yu-Chieh Lin, Yen-Yin Lin, Teh-Ying Chou
Journal of Translational Medicine.2022;[Epub] CrossRef - Correlation Between Pretreatment Neutrophil-to-Lymphocyte Ratio and Programmed Death-Ligand 1 Expression as Prognostic Markers in Non-Small Cell Lung Cancer
Cristina-Florina Pirlog, Horia Teodor Cotan, Andreea Parosanu, Cristina Orlov Slavu, Ana Maria Popa, Cristian Iaciu, Mihaela Olaru, Alexandru Vlad Oprita, Irina Nita, Cornelia Nitipir
Cureus.2022;[Epub] CrossRef - Expert opinion on NSCLC small specimen biomarker testing — Part 2: Analysis, reporting, and quality assessment
Frédérique Penault-Llorca, Keith M. Kerr, Pilar Garrido, Erik Thunnissen, Elisabeth Dequeker, Nicola Normanno, Simon J. Patton, Jenni Fairley, Joshua Kapp, Daniëlle de Ridder, Aleš Ryška, Holger Moch
Virchows Archiv.2022; 481(3): 351. CrossRef - Novel Inflammasome-Based Risk Score for Predicting Survival and Efficacy to Immunotherapy in Early-Stage Non-Small Cell Lung Cancer
Chih-Cheng Tsao, Hsin-Hung Wu, Ying-Fu Wang, Po-Chien Shen, Wen-Ting Wu, Huang-Yun Chen, Yang-Hong Dai
Biomedicines.2022; 10(7): 1539. CrossRef - Comparative Analysis of Mutation Status and Immune Landscape for Squamous Cell Carcinomas at Different Anatomical sites
Wenqi Ti, Tianhui Wei, Jianbo Wang, Yufeng Cheng
Frontiers in Immunology.2022;[Epub] CrossRef - Pan-cancer analysis of the angiotensin II receptor-associated protein as a prognostic and immunological gene predicting immunotherapy responses in pan-cancer
Kai Hong, Yingjue Zhang, Lingli Yao, Jiabo Zhang, Xianneng Sheng, Lihua Song, Yu Guo, Yangyang Guo
Frontiers in Cell and Developmental Biology.2022;[Epub] CrossRef - Molecular imaging of immune checkpoints in oncology: Current and future applications
Shushan Ge, Tongtong Jia, Jihui Li, Bin Zhang, Shengming Deng, Shibiao Sang
Cancer Letters.2022; 548: 215896. CrossRef - PD-L1 expression and association with genetic background in pheochromocytoma and paraganglioma
Katerina Hadrava Vanova, Ondrej Uher, Leah Meuter, Suman Ghosal, Sara Talvacchio, Mayank Patel, Jiri Neuzil, Karel Pacak
Frontiers in Oncology.2022;[Epub] CrossRef - Case report: Variable response to immunotherapy in ovarian cancer: Our experience within the current state of the art
Nicoletta Provinciali, Marco Greppi, Silvia Pesce, Mariangela Rutigliani, Irene Maria Briata, Tania Buttiron Webber, Marianna Fava, Andrea DeCensi, Emanuela Marcenaro
Frontiers in Immunology.2022;[Epub] CrossRef - PD-L1 Is Preferentially Expressed in PIT-1 Positive Pituitary Neuroendocrine Tumours
John Turchini, Loretta Sioson, Adele Clarkson, Amy Sheen, Anthony J. Gill
Endocrine Pathology.2021; 32(3): 408. CrossRef - Comprehensive tumor molecular profile analysis in clinical practice
Mustafa Özdoğan, Eirini Papadopoulou, Nikolaos Tsoulos, Aikaterini Tsantikidi, Vasiliki-Metaxa Mariatou, Georgios Tsaousis, Evgenia Kapeni, Evgenia Bourkoula, Dimitrios Fotiou, Georgios Kapetsis, Ioannis Boukovinas, Nikolaos Touroutoglou, Athanasios Fassa
BMC Medical Genomics.2021;[Epub] CrossRef - CT-Based Hand-crafted Radiomic Signatures Can Predict PD-L1 Expression Levels in Non-small Cell Lung Cancer: a Two-Center Study
Zekun Jiang, Yinjun Dong, Linke Yang, Yunhong Lv, Shuai Dong, Shuanghu Yuan, Dengwang Li, Liheng Liu
Journal of Digital Imaging.2021; 34(5): 1073. CrossRef - Deep Learning of Histopathological Features for the Prediction of Tumour Molecular Genetics
Pierre Murchan, Cathal Ó’Brien, Shane O’Connell, Ciara S. McNevin, Anne-Marie Baird, Orla Sheils, Pilib Ó Broin, Stephen P. Finn
Diagnostics.2021; 11(8): 1406. CrossRef - Molecular Imaging and the PD-L1 Pathway: From Bench to Clinic
David Leung, Samuel Bonacorsi, Ralph Adam Smith, Wolfgang Weber, Wendy Hayes
Frontiers in Oncology.2021;[Epub] CrossRef - A Comparative Study of Immunotherapy as Second-Line Treatment and beyond in Patients with Advanced Non-Small-Cell Lung Carcinoma
Jerónimo Rafael Rodríguez-Cid, Sonia Carrasco-Cara Chards, Iván Romarico González-Espinoza, Vanessa García-Montes, Julio César Garibay-Díaz, Osvaldo Hernández-Flores, Rodrigo Riera-Sala, Anna Gozalishvili-Boncheva, Jorge Arturo Alatorre-Alexander, Luis Ma
Lung Cancer Management.2021;[Epub] CrossRef - Clinical utility of the C‐reactive protein:albumin ratio in non‐small cell lung cancer patients treated with nivolumab
Taisuke Araki, Kazunari Tateishi, Kei Sonehara, Shuko Hirota, Masamichi Komatsu, Manabu Yamamoto, Shintaro Kanda, Hiroshi Kuraishi, Masayuki Hanaoka, Tomonobu Koizumi
Thoracic Cancer.2021; 12(5): 603. CrossRef - Programmed Cell Death Ligand 1‐Transfected Mouse Bone Marrow Mesenchymal Stem Cells as Targeted Therapy for Rheumatoid Arthritis
Qiong-ying Hu, Yun Yuan, Yu-chen Li, Lu-yao Yang, Xiang-yu Zhou, Da-qian Xiong, Zi-yi Zhao, Hiroshi Tanaka
BioMed Research International.2021;[Epub] CrossRef - PD-L1 testing and clinical management of newly diagnosed metastatic non-small cell lung cancer in Spain: MOREL study
Belen Rubio-Viqueira, Margarita Majem Tarruella, Martín Lázaro, Sergio Vázquez Estévez, Juan Felipe Córdoba-Ortega, Inmaculada Maestu Maiques, Jorge García González, Ana Blasco Cordellat, Javier Valdivia-Bautista, Carmen González Arenas, Jose Miguel Sánch
Lung Cancer Management.2021;[Epub] CrossRef - Can Systems Biology Advance Clinical Precision Oncology?
Andrea Rocca, Boris N. Kholodenko
Cancers.2021; 13(24): 6312. CrossRef - Do we need PD‐L1 as a biomarker for thyroid cytologic and histologic specimens?
Marc P. Pusztaszeri, Massimo Bongiovanni, Fadi Brimo
Cancer Cytopathology.2020; 128(3): 160. CrossRef - Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment
Andrea Botticelli, Pamela Vernocchi, Federico Marini, Andrea Quagliariello, Bruna Cerbelli, Sofia Reddel, Federica Del Chierico, Francesca Di Pietro, Raffaele Giusti, Alberta Tomassini, Ottavia Giampaoli, Alfredo Miccheli, Ilaria Grazia Zizzari, Marianna
Journal of Translational Medicine.2020;[Epub] CrossRef - Immune checkpoint inhibitors in advanced non–small cell lung cancer: A metacentric experience from India
Santosh Kumar, Srujana Joga, Bivas Biswas, Deepak Dabkara, Kuruswamy Thurai Prasad, Navneet Singh, Prabhat Singh Malik, Sachin Khurana, Sandip Ganguly, Valliappan Muthu, Ullas Batra
Current Problems in Cancer.2020; 44(3): 100549. CrossRef - Utility of CT radiomics for prediction of PD‐L1 expression in advanced lung adenocarcinomas
Jiyoung Yoon, Young Joo Suh, Kyunghwa Han, Hyoun Cho, Hye‐Jeong Lee, Jin Hur, Byoung Wook Choi
Thoracic Cancer.2020; 11(4): 993. CrossRef - Immune checkpoint inhibitors of the PD-1/PD-L1-axis in non-small cell lung cancer: promise, controversies and ambiguities in the novel treatment paradigm
Lars H. Breimer, Petros Nousios, Louise Olsson, Hans Brunnström
Scandinavian Journal of Clinical and Laboratory Investigation.2020; 80(5): 360. CrossRef - Tumour mutational burden as a biomarker for immunotherapy: Current data and emerging concepts
Jean-David Fumet, Caroline Truntzer, Mark Yarchoan, Francois Ghiringhelli
European Journal of Cancer.2020; 131: 40. CrossRef - Precision Medicine for NSCLC in the Era of Immunotherapy: New Biomarkers to Select the Most Suitable Treatment or the Most Suitable Patient
Giovanni Rossi, Alessandro Russo, Marco Tagliamento, Alessandro Tuzi, Olga Nigro, Giacomo Vallome, Claudio Sini, Massimiliano Grassi, Maria Giovanna Dal Bello, Simona Coco, Luca Longo, Lodovica Zullo, Enrica Teresa Tanda, Chiara Dellepiane, Paolo Pronzato
Cancers.2020; 12(5): 1125. CrossRef - Current status and future perspectives of liquid biopsy in non-small cell lung cancer
Sunhee Chang, Jae Young Hur, Yoon-La Choi, Chang Hun Lee, Wan Seop Kim
Journal of Pathology and Translational Medicine.2020; 54(3): 204. CrossRef - Digital Pathology and PD-L1 Testing in Non Small Cell Lung Cancer: A Workshop Record
Fabio Pagni, Umberto Malapelle, Claudio Doglioni, Gabriella Fontanini, Filippo Fraggetta, Paolo Graziano, Antonio Marchetti, Elena Guerini Rocco, Pasquale Pisapia, Elena V. Vigliar, Fiamma Buttitta, Marta Jaconi, Nicola Fusco, Massimo Barberis, Giancarlo
Cancers.2020; 12(7): 1800. CrossRef - PD-L1 in Systemic Immunity: Unraveling Its Contribution to PD-1/PD-L1 Blockade Immunotherapy
Ana Bocanegra, Ester Blanco, Gonzalo Fernandez-Hinojal, Hugo Arasanz, Luisa Chocarro, Miren Zuazo, Pilar Morente, Ruth Vera, David Escors, Grazyna Kochan
International Journal of Molecular Sciences.2020; 21(16): 5918. CrossRef - PD-1 blockade in recurrent or metastatic cervical cancer: Data from cemiplimab phase I expansion cohorts and characterization of PD-L1 expression in cervical cancer
Danny Rischin, Marta Gil-Martin, Antonio González-Martin, Irene Braña, June Y. Hou, Daniel Cho, Gerald S. Falchook, Silvia Formenti, Salma Jabbour, Kathleen Moore, Aung Naing, Kyriakos P. Papadopoulos, Joaquina Baranda, Wen Fury, Minjie Feng, Elizabeth St
Gynecologic Oncology.2020; 159(2): 322. CrossRef - Atezolizumab: A Review in Extensive-Stage SCLC
James E. Frampton
Drugs.2020; 80(15): 1587. CrossRef - Prognostic and clinicopathological roles of programmed death‐ligand 1 (PD‐L1) expression in thymic epithelial tumors: A meta‐analysis
Hyun Min Koh, Bo Gun Jang, Hyun Ju Lee, Chang Lim Hyun
Thoracic Cancer.2020; 11(11): 3086. CrossRef - Präzisionsmedizin bei NSCLC im Zeitalter der Immuntherapie: Neue Biomarker zur Selektion der am besten geeigneten Therapie oder des am besten geeigneten Patienten
Giovanni Rossi, Alessandro Russo, Marco Tagliamento, Alessandro Tuzi, Olga Nigro, Giacomo Vollome, Claudio Sini, Massimiliano Grassi, Maria Giovanna Dal Bello, Simona Coco, Luca Longo, Lodovica Zullo, Enrica Teresa Tanda, Chiara Dellepiane, Paolo Pronzato
Kompass Pneumologie.2020; 8(6): 300. CrossRef - Association with PD-L1 Expression and Clinicopathological Features in 1000 Lung Cancers: A Large Single-Institution Study of Surgically Resected Lung Cancers with a High Prevalence of EGFR Mutation
Seung Eun Lee, Yu Jin Kim, Minjung Sung, Mi-Sook Lee, Joungho Han, Hong Kwan Kim, Yoon-La Choi
International Journal of Molecular Sciences.2019; 20(19): 4794. CrossRef - Detailed Characterization of Immune Cell Infiltrate and Expression of Immune Checkpoint Molecules PD-L1/CTLA-4 and MMR Proteins in Testicular Germ Cell Tumors Disclose Novel Disease Biomarkers
João Lobo, Ângelo Rodrigues, Rita Guimarães, Mariana Cantante, Paula Lopes, Joaquina Maurício, Jorge Oliveira, Carmen Jerónimo, Rui Henrique
Cancers.2019; 11(10): 1535. CrossRef - Basis of PD1/PD-L1 Therapies
Barbara Seliger
Journal of Clinical Medicine.2019; 8(12): 2168. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

Fig. 1.
| PD-L1 assay | |||||
|---|---|---|---|---|---|
| Performance | Clone | 22C3 | 28-8 | SP263 | SP142 |
| Developer | Dako | Dako | Ventana | Ventana | |
| Host species | Mouse monoclonal | Rabbit monoclonal | Rabbit monoclonal | Rabbit monoclonal | |
| Epitope location | Extracellular domain | Extracellular domain | Cytoplasmic domain | Cytoplasmic domain | |
| Platform | Link 48 autostainer | Link 48 autostainer | Benchmark ultra | Benchmark ultra | |
| Detection kit | Envision FLEX | Envision FLEX | Optiview | Optiview | |
| Amplification | No | No | No | Yes | |
| Interpretation | Scoring | TC | TC | TC | TC and IC |
| Staining pattern for positivity | Membranous | Membranous | Membranous ± cytoplasmic | Membranous±cytoplasmic | |
| Minimum TC number | 100 | 100 | 100 | 50 with associated stroma | |
| Cut-off (mandatory) | ≥ 50% (≥ 2nd line), ≥ 1% (1st line) | All comer | All comer | All comer | |
| Cut-off (proven survival benefit) | ≥ 1%, ≥ 5%, ≥ 10% | ≥ 25% (for durvalumab), ≥ 10% (for nivolumab) |
TC ≥ 5% or IC ≥ 5% | ||
| Pharma | Immune checkpoint inhibitor | Pembrolizumab | Nivolumab | Durvalumab Nivolumab |
Atezolizumab |
| FDA approval | Companion | Complementary | Complementary | Complementary | |
PD-L1, programmed death-ligand 1; TC, tumor cell; IC, immune cell; FDA, Food and Drug Administration. Applied only in Korea; Approved by Conformite Europeenne (CE) and Korea Food and Drug Administration.

 E-submission
E-submission