The frequency of POLE-mutation in endometrial carcinoma and prognostic implications: a systemic review and meta-analysis
Article information
Abstract
Background
Endometrial carcinoma (EC) is classified into four distinct molecular subgroups including ultramutated DNA polymerase epsilon (POLE). POLE-mutated tumors have the best prognosis and are a promising target for immunotherapy. This meta-analysis consolidated the reported variation of POLE-mutant frequency and assessed prognostic value in EC.
Methods
Internet searches explored scientific data bases: EMBASE, PubMed, and the Cochrane Central Register of Controlled Trials databases. Data was extracted from eligible studies including: sample size, number of positive POLE-mutant cases, sequencing information, clinicopathologic data, and survival data. Meta-analysis and a random-effects model produced pooled estimates of POLE frequency and prognostic parameters using 95% confidence intervals (CI), hazard ratios (HR), and odd ratios (OR).
Results
Six thousand three hundred and forty-six EC patient cases were pooled from 25 studies. The pooled proportion of POLE gene mutation in EC was 8.59% (95% CI, 7.01 to 10.32), of which 8.22% (95% CI, 6.27 to 10.42) were type I and 0.93% (95% CI, 0.34 to 1.81) type 2. Clinicopathologic data showed that POLE-mutated tumors are mostly endometrioid. They present at higher levels in earlier stages (I–II) of EC (89.51%; 95% CI, 81.11 to 95.66) at the highest grade III (51.53%; 95% CI, 36.08 to 66.84) with reduced myometrial invasion (OR, 1.48, 95% CI, 0.99 to 2.20). Survival analysis indicated favorable overall survival (HR, 0.90), disease-specific survival (HR, 0.41), and progression-free survival (HR, 0.23) for POLE mutant EC.
Conclusions
Almost one-tenth of EC patients have POLE-mutated tumors. Given their improved prognostic potential, identifying the POLE mutation status is key for the management of EC patients.
Endometrial carcinoma (EC) is the most common cancer of the female reproductive system. In the United States, EC was the sixth-cause of cancer-related death in 2018, accounting for 11,350 deaths. The disease affects an estimated 1 out of every 37 and 49 women respectively during their lifetime in the United States and Australia [1,2]. The outlook for 5-year survival after treatment ranges from 74% to 91% [3,4]. EC has been historically classified into estrogen dependent (type I) and estrogenindependent (type II) cancers [4]. Type II tumors are less common, more clinically aggressive, and may have serous, clear cell or undifferentiated histology. In contrast, type I tumors present in 70%–80% of cases, with endometrioid histology and a more favorable outcome. The updated classification of EC identifies four molecular subtypes according to The Cancer Genomic Atlas (TCGA): “POLE-mutated (ultramutated), microsatellite unstable (hypermutated), copy number low (endometrioid), and copy number high (serous-like)” [1]. The POLE-ultramutated subgroup holds great promise for the outlook of EC patients. The tumors have a more favorable outcome, and are usually noted to be of endometrioid type and associated with lymphoid infiltrates [5].
The POLE gene encodes the catalytic subunit of DNA polymerase epsilon, which synthesizes the leading strand of replicating DNA. The epsilon polymerase recognizes and removes mispaired nucleotides using exonuclease activity. This proofreading capacity of epsilon enables high fidelity DNA replication [6]. Mutations can occur in the exonuclease domain of the polymerase, within hotspot regions [7]. These genetic alterations inactivate or suppress the proofreading abilities of the polymerase, causing increased replicative error rates and the ultra-mutated phenotype. Studies show that ultra-mutated POLE EC harbors up to 10-fold more mutations than the microsatellite instability subgroup [6,7]. In the TCGA study [8], whole genome and exon sequencing of EC tumors uncovered the POLE hotspot mutations of v411L and P286R. Follow-up studies mainly used the Sanger method [9-23], next generation sequencing [13,24] and unspecified sequencing approaches [10,11,25-29] to target the exonuclease domain (exons 9–14) of POLE. The reported proportion of mutated POLE is highly variable [8-31]: ranging from absent in studies of clear cell carcinoma [15,18] to levels as high as 43% in one study of rare undifferentiated/dedifferentiated EC histotypes [28]. In order to consolidate the studies, we conducted a meta-analysis of reported POLE gene mutation in EC to confirm its overall frequency. We also extracted clinicopathologic and survival data, to evaluate how mutated POLE can affect prognosis of EC patients.
MATERIALS AND METHODS
This study was conducted according to the guidelines of Preferred Reporting for Systemic Review and Meta-analysis (PRISMA) statement [32].
Literature search strategy
Searches were conducted according to the guidelines of PRISMA statement 2009 [32]. Two authors (A.S.J. and H.S.A.) searched independently the following data bases from inceptions to October 2019: Embase, PubMed, Cochrane central Register of Controlled trials, and Ovid. The reference lists were also scanned within the articles. There were no language limits and international papers were translated. All pathology and oncology journals indexed in the Scimago directory were reviewed and relevant papers scanned (A.S.J. and M.M.S.). The following search terms were used:
1) ‘Endometrial cancer’ or ‘uterine cancer.’
2) ‘POLE gene’ or ‘ultramutated endometrial carcinoma.’
Inclusion criteria
The following patient inclusion criteria were used:
1) EC or one of its histological variants was present in patients.
2) The expression of POLE gene was reported using genetic
testing (e.g. sequencing, Sanger sequencing, next generation sequencing, polymerase chain reaction).
3) A full paper was published and studies published in abstract format only were excluded.
4) When similar studies were generated from the same patient, only the most recent investigation was included.
Study selection
Studies were identified using different data bases. The title of the paper and abstract were assessed by two independent authors (A.S.J. and H.S.A.). The full texts were also reviewed independently by two authors (M.M.S. and K.A.M.). Any disagreement was resolved under guidance of the senior author (A.A.Y.).
Data collection
The following data was extracted from eligible studies by two authors (A.S.J. and H.S.A.): study information (first author and year of publication), patient characteristics (sample size and gender), site of the study, and test method for POLE gene and proportion detected. Clinicopathologic data extracted included tumor stage and grade, presence of lymphovascular and myometrial invasion, and patient survival. If the relevant data was not available, it was recorded as NR (not reported). All datasets were checked independently (M.M.S.). Any disagreements were resolved by discussion and consultation with the senior author (A.A.Y.).
Meta-analysis and statistical methods
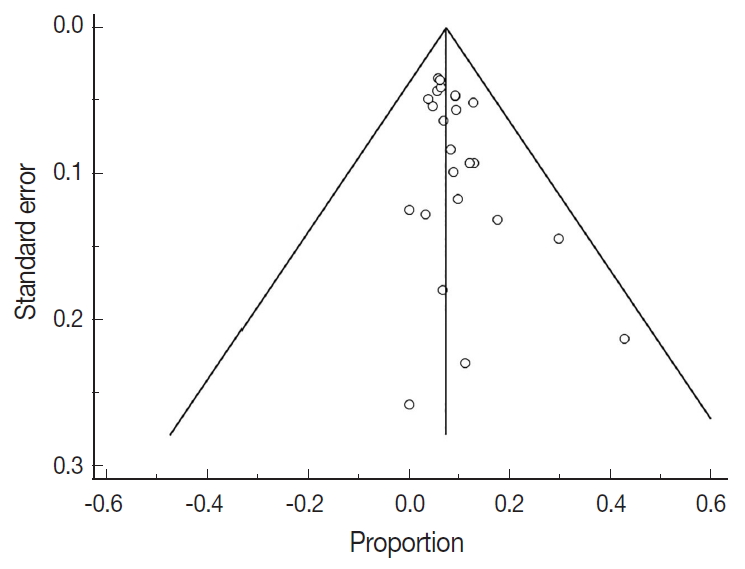
The proportion of endometrial cancers that harbor POLE gene mutations was calculated using medcalc software [33]. The pooled proportion of POLE was calculated using the random effect model [32] for meta-analysis. For clinicopathologic meta-analysis, proportions of tumour stage, grade, lymphovascular invasion, myometrial invasion (MI), and survival analysis (overall survival, disease-free survival, and progression-free survival) were pooled from each study. The variation between datasets was assessed using the heterogeneity test with inconsistency index (I2) and Q statistic. The level of study heterogeneity was considered low at 25% (I2=25%), medium at 50% (I2=50%) or high at 75% (I2= 75%). In regard to the Q statistic, a p-value of less than 0.1 was considered to represent significant heterogeneity. The possibility of publication bias was assessed by visual method using a funnel plot. This determined funnel plot asymmetry resulting from factors such as non-publication of studies with negative results.
Sensitivity and subgroup analysis
Lastly, sensitivity analysis was conducted by omitting each study one-by-one to discover its contribution on the pooled meta-analysis results. Subgroup analysis was performed according to geographical area (Asia, West-Europe, and America) and to different histological types to discover sources of heterogeneity. The subgroup analysis was further extended by using a meta regression model.
RESULTS
Study selection for meta-analysis
The inclusion criteria were met by 1,252 studies, of which 345 studies were excluded for duplication, and 605 studies were excluded as irrelevant by reviewing the title and abstract. The full texts of the remaining 302 studies were considered for review. Approximately 25 studies were left for the final meta-analysis as illustrated in the flowchart diagram (Fig. 1). All of the studies were published from 2013–2019, and there were no unpublished studies of relevance. The study characteristics and POLE gene sequencing methods are listed in (Table 1). The majority used Sanger sequencing to detect tumor mutations in exons 9–14 of the POLE exonuclease domain (Table 1) [8-29].
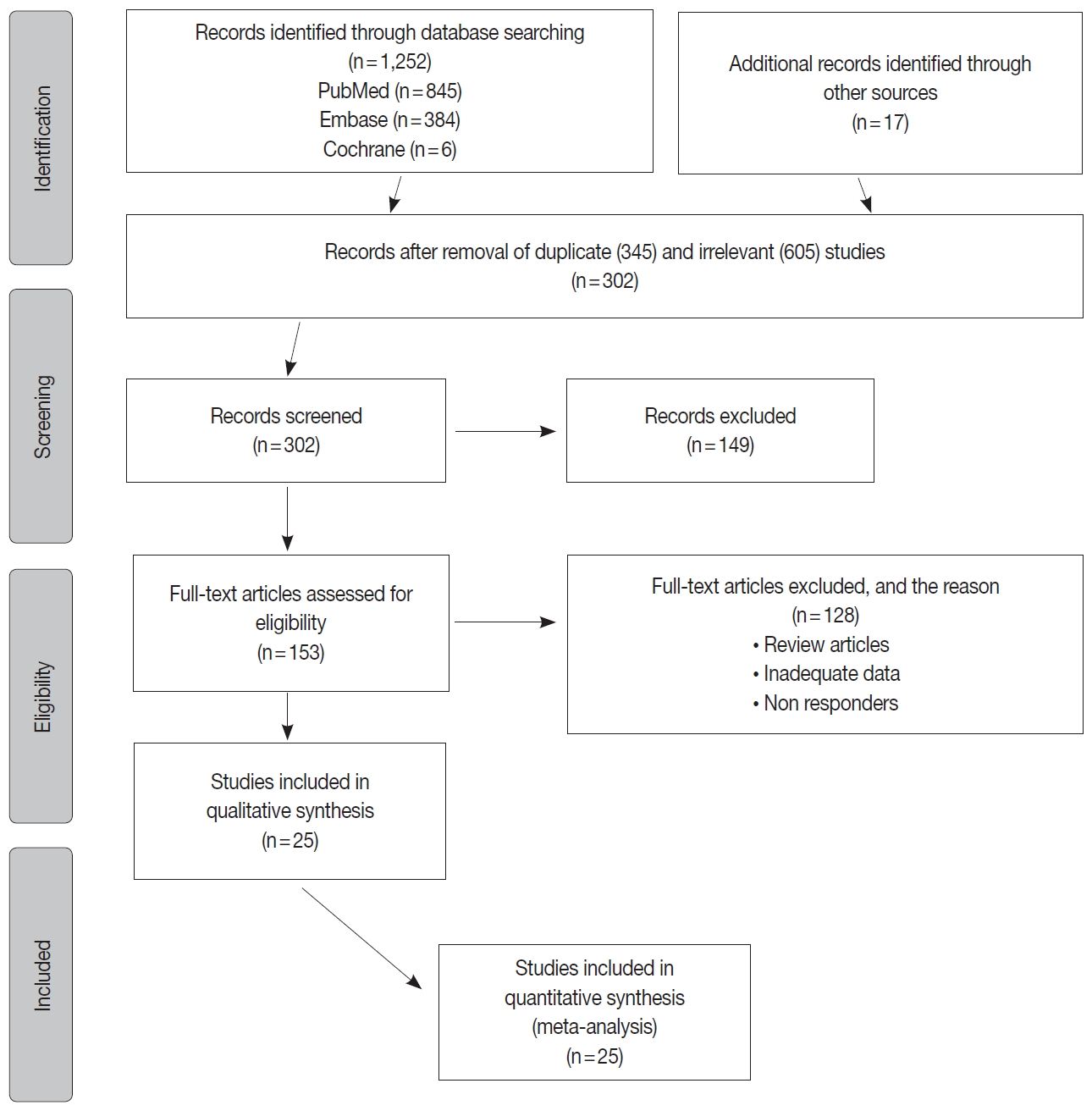
Flow chart of the studies identified, screened and included for final meta-analysis. A total of 1,252 studies met the eligibility criteria for inclusion, of which 26 were selected for final meta-analysis.
Meta-analysis of EC
There were 6,346 EC patient cases pooled from the 25 studies and investigated for POLE gene tumor alterations. All studies (except one) were performed in western countries. The studies were published in English and full texts obtained. There was significant heterogeneity between the studies (Q=109.57, I2 =78.10%; 95% confidence interval [CI] for I2, 68.15 to 84.94; p<.001). The random effect model was used for final meta-analysis because the significant heterogeneity. The pooled proportion of POLE gene mutated in EC was determined at 8.59% (95% CI, 7.01 to 10.32) as shown in Table 2. A forest plot representation of the EC patient cases comprising each study included for meta-analysis is shown in Fig. 2. Publication bias was assessed by funnel plot (Fig. 3) and the visual assessment was symmetrical.

Forest plot representation of the endometrial carcinoma (EC) patient cases for each study included for meta-analysis. POLE, DNA polymerase epsilon.
Analysis of EC histologic variants type I and type II
There were 3,363 patients included for the type 1 meta-analysis using a total of 9 studies (Supplementary Fig. S1). The mutated POLE gene proportion in type I EC was 8.22% (95% CI, 7.01 to 10.32). There was significant heterogeneity between studies (I2 inconsistency=74.88%; 95% CI, 51.43 to 87.00; p<.001) as shown in Table 2. A total of 3,423 patients with EC type II were obtained from 10 studies for the final meta-analysis (Supplementary Fig. S2). The histological types in EC type II are show in (Supplementary Table S1). The pooled proportion of mutated POLE gene in type II was 0.93% (95% CI, 0.34 to 1.81) with a high I2 value (75.32%; 95% CI, 54.08 to 86.74; p<.001).
Subgroup analysis (country of origin)
In order to explore the role of heterogeneity, a subgroup analysis was performed according to the study site (Supplementary Fig. S3, S4). The studies were separated into two groups according to geographical area (USA and Canada vs. European countries). The heterogeneity was higher (I2, inconsistency) for POLE genetic analysis in the European countries (78.41%; 95% CI, 59.27 to 88.55) compared to the USA and Canada (37.41%; 95% CI, 0.00 to 69.22).
Sensitivity analysis
There were 2 identified study outliers [24,28]. Meta-analysis was performed after outlier exclusion. There was still significant heterogeneity (I2=70.39% [95% CI for I2, 54.78 to 80.61; p<.001]) and exclusion did not significantly affect the final pooled proportion (pooled proportion=7.76 [95% CI, 6.45 to 9.18]) (Supplementary Fig. S5). There were also two studies without POLE gene mutated EC [15,18]. Here, the pooled proportion was re-calculated using the random effect model (Supplementary Fig. S6). Significant heterogeneity was still present (I2= 76.31%; 95% CI for I2, 64.98 to 83.97; p<.001). There were 10 studies [9,13,15,17,19-21,23,24,30] identified with a low sample size (<100). These cases were excluded and the pooled proportions re-estimated (Supplementary Fig. S7). There was still significant heterogeneity (I2=74.41%; 95% CI for I2, 53.56 to 85.90; p<.001).
Meta-regression
Meta-regression was performed to identify the source of heterogeneity using the study country of origin and the POLE gene detection method (Supplementary Table S2). There was increased heterogeneity when considering the site of study performance in the European countries (I2=78.41%) compared to United States and Canadian studies (I2=37.41%).
Clinicopathological characteristics
Clinicopathologic data was extracted from eligible studies of POLE mutant EC for meta-analysis (Supplementary Fig. S8-S14). The pooled proportions for stage, grade, lymphovascular invasion (LVI) and MI parameter are reported in Table 2. High heterogeneity was noted for pooled stage, grade, and LVI data. The pooled odd ratios were also calculated for POLE-mutant versus wild type POLE according to each clinicopathologic variable (Table 3).
Tumor stage and grade in POLE mutant EC
The pooled proportion of mutant POLE presented at high levels of 89.51% (95% CI, 81.11 to 95.66) at the earliest EC stages of I–II (Table 2). This reduced to 14.77% by stages III–IV (95% CI, 5.99 to 26.59). The pooled odd ratio of stage I–II POLE mutant EC versus wild type was 3.72 (2.06 to 6.73), while stage III–IV was 0.26 (95% CI, 0.14 to 0.49) (Table 2, Supplementary Fig. S15, S16). The pooled collective proportions of grade I–II POLE mutant tumors was lower at 46.36% (95% CI, 30.66 to 62.43) compared to 51.53 of grade III (95% CI, 36.08 to 66.844) as shown in Table 2. The pooled odd ratio of grade I–II POLE mutant EC versus wild type tumors was 0.40 (95% CI, 0.29 to 0.54) (Supplementary Fig. S17) with low heterogeneity (I2=3.95%, 95% CI, 0.00 to 69.18). Therefore, POLE mutated tumors present with a higher grade but at lower stage than wild type POLE mutant EC.
LVI and MI in POLE-mutant EC
The pooled proportion of LVI was 31.11% (95% CI, 10.44 to 56.86). The pooled odd ratio of LVI positive in POLE mutant EC vs. wild type EC was 0.92 (0.643 to 1.34) (Table 3). The pooled proportion of MI either less or greater than 50% of myometrium in POLE-mutant tumor was equal at 49.90% (95% CI, 43.71 to 56.21) and 49.05% (95% CI, 39.17 to 58.98), respectively. Heterogeneity was low for pooled MI data (<50% myometrium) at 22.10%; 95% CI, 0.00 to 65.16 (Table 2, Supplementary Figs. S13, S14). The pooled odd ratio of MI <50% in POLE mutant EC versus MI < 50% in wild type POLE EC was 1.481 (95% CI, 0.99 to 2.20) with 47.63% heterogeneity (95% CI, 0.00 to 79.24) (Table 3). Overall, these findings imply that POLE mutant EC tumors have reduced ability to progress to myometrial invasion, which is an important prognostic finding.
Endometrioid and non-endometrioid histologic types in POLE mutant EC
POLE mutant tumors were found to mainly present with endometrioid histologic type. The pooled proportions of type I and type II EC are shown in Table 2. The pooled odd ratio of endometrioid (type I) POLE mutant EC vs. endometrioid (type I) in wild type POLE EC was 1.72 (95% CI: 1.11 to 2.66), with very low heterogeneity (I2=0.00%; 95% CI: 0.00 to 68.45) (Table 3, Supplementary Fig. S18).
Survival analysis
The studies used for survival meta-analysis are listed in Table 4. Survival analysis was expressed using overall survival (OS), disease-specific survival (DSS), and progression-free survival (Supplementary Fig. S19–S21). All the survival parameters had a hazard ratio (HR) of less than 1. The estimated HR for OS was 0.90 (95% CI, 0.59 to 1.38) with low heterogeneity 0.00% (95% CI, 0.00 to 73.28). On the other hand, estimated HR for DSS was 0.41 (95% CI, 0.30 to 0.55), also with low heterogeneity (0.00%; 95% CI, 0.00 to 67.34). Likewise, the estimated HR of progression-free survival in POLE-mutant EC was 0.23 (95% CI, 0.08 to 0.64) with a low level of heterogeneity (I2=0.00%, 95% CI, 0.00 to 0.00). These findings indicate better survival and favorable prognosis in POLE mutant EC patients.
DISCUSSION
EC is the most common gynecologic malignancy in the western world [2,3,34], and survival rates are not improving. There is urgent need for strategies to improve outlook for patients with aggressive subtypes and advanced disease. TCGA first identified the very interesting molecular subset of POLE-ultramutated EC [1] that features a favorable prognostic potential, despite high tumor grading. Many follow-up studies investigated POLE mutant EC tumors, however the frequency reported is variable [8-31]. Some studies show ultra-high levels of mutation at 42.9% [28] compared to zero in others [15,18]. This meta-analysis aimed to resolve these datasets to estimate POLE gene mutational frequency in EC and the overall effect on patient prognosis.
Our meta-analysis determined that 8.595% of endometrial tumors harbor POLE gene mutations. The majority have endometrioid histology and present at the earlier stage of disease progression. Paradoxically the POLE mutant tumors present at he highest grade (grade III, 51.5%) and yet have a better outcome with survival analysis. They also have reduced ability to progress to myometrial invasion which is an important prognostic marker. Many studies confirm that POLE mutant tumors have a better prognosis [6-8,13,22,28]. POLE mutations in high grade (grade III) endometrioid EC are shown to be associated with a lower risk of recurrence and death [22]. The presence of POLE mutations even offers a favorable prognosis for rare and aggressive undifferentiated EC [28]. POLE mutation could potentially act as a prognostic biomarker to guide treatment of women with grade III, early-stage disease, with either the common endometrioid or more rare histological types. The lower risk of occurrence means that administration of adjuvant therapy for these patient subsets could be inappropriate. POLE proofreading mutations have also been shown to elicit an anti-tumor response [35]. There is now an emerging link between high mutation burden in tumors, the immune response and improved prognosis in cancer patients. Indeed, ultramutated POLE tumors have been shown to feature higher immune infiltrations and programmed death-1 and programmed death-ligand 1 expression [36]. These immune cells may offset the survival risk caused by higher tumor grades in ultramutated POLE. Overall, mutant POLE is a key proportion of EC patients to target therapeutically for maximizing clinical impact, and a future target for immunotherapy [9].
However, it is important to note that significant heterogeneity was present in this meta-analysis. Indeed, heterogeneity is a key problem for meta-analysis studies. We tried to resolve the heterogeneity sources. Initially, all studies were re-checked with respect to data extraction and entry. The sensitivity analysis was conducted by re-estimating the pooled proportion of POLE gene after exclusion of outlier studies and studies with low sample size. We also adopted a random effect model for final pooled estimation as this model assumed that effects estimated in different studies are not identical. We also tried to perform subgroup analysis with respect to patient age but unfortunately this parameter was not recorded in the majority of studies. Publication bias was investigated; in our study the plot was relatively symmetrical, indicating that publication bias is unlikely [37]. The presence of high heterogeneity was reduced by subgroup and meta-regression according to geographical distribution. European studies were found to be the main contributor to the heterogeneity. Therefore, data obtained from different countries can cause confounding effects and is a potential study limitation. The issue of heterogeneity in genetic studies is also further compounded with recent advances in sequencing technology. The improved yield of genetic testing can increase detection of variants of unknown significance. However in our POLE gene study the lack of standardized clarification of variants of unknown significance may have contributed to heterogeneity. This will be a key area to investigate during future studies.
Conclusion
Our meta-analysis consolidates previous study estimates of POLE-mutated EC frequency and confirms its prognostic benefit for patients. The status and frequencies of the POLE gene mutation in EC has implications for medical management and future administration of immunotherapy. The POLE mutational status serves as an important prognostic marker, and grade III, early-stage disease patients with endometrioid histology could favor a change in medical management.
Supplementary Materials
The Data Supplement is available with this article at https://doi.org/10.4132/jptm.2020.07.23.
Supplementary Table S1
jptm-2020-07-23-suppl1.pdfSupplementary Table S2
jptm-2020-07-23-suppl2.pdfSupplementary Fig. S1
jptm-2020-07-23-suppl3.pdfSupplementary Fig. S2
jptm-2020-07-23-suppl3.pdfSupplementary Fig. S3
jptm-2020-07-23-suppl4.pdfSupplementary Fig. S4
jptm-2020-07-23-suppl4.pdfSupplementary Fig. S5
jptm-2020-07-23-suppl5.pdfSupplementary Fig. S6
jptm-2020-07-23-suppl5.pdfSupplementary Fig. S7
jptm-2020-07-23-suppl6.pdfSupplementary Fig. S8
jptm-2020-07-23-suppl6.pdfSupplementary Fig. S9
jptm-2020-07-23-suppl7.pdfSupplementary Fig. S10
jptm-2020-07-23-suppl7.pdfSupplementary Fig. S11
jptm-2020-07-23-suppl8.pdfSupplementary Fig. S12
jptm-2020-07-23-suppl8.pdfSupplementary Fig. S13
jptm-2020-07-23-suppl9.pdfSupplementary Fig. S14
jptm-2020-07-23-suppl9.pdfSupplementary Fig. S15
jptm-2020-07-23-suppl10.pdfSupplementary Fig. S16
jptm-2020-07-23-suppl10.pdfSupplementary Fig. S17
jptm-2020-07-23-suppl11.pdfSupplementary Fig. S18
jptm-2020-07-23-suppl11.pdfSupplementary Fig. S19
jptm-2020-07-23-suppl12.pdfSupplementary Fig. S20
jptm-2020-07-23-suppl12.pdfSupplementary Fig. S21
jptm-2020-07-23-suppl13.pdfNotes
Ethics Statement
The present study was approved by the Institutional Review Board of the University of Kufa (IRB approval No. UK-2018-0456) in accordance with the 1964 Helsinki declaration and its later amendments. Formal written informed consent was not required with a waiver issued by the Institutional Review Board of the University of Kufa. All the authors will be held responsible for any false statements or failure to follow the ethical guidelines.
Author contributions
Conceptualization: ASJ, MMS. Data curation: ASJ. Formal analysis: ASJ, MMS. Methodology: ASJ, HSA, MMS. Project administration: HSA, MMS. Resources: HSA. Software: ASJ, MMS. Supervision: ASJ, AAY. Validation: MMS, HSA. Visualization: HSA, MMS. Writing—original draft: ASJ, AAY, KAM. Writing—review & editing: AAY, ASJ, KAM. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.