Founder BRCA1 mutations in Nepalese population
Article information
Abstract
Background
Founder mutation is a heritable genetic alteration observed with high frequency in a geographically and culturally isolated population where one or more ancestors becomes the forebearer of the altered gene. The current study reports two founder mutations in the BRCA1 gene in the Nepalese people.
Methods
Germline BRCA testing in all surface epithelial ovarian cancers and the selected case of breast, prostate, and pancreatic cancers has been the standard practice from 2016 to 2021. One thousand one hundred thirty-three probands were screened for germline BRCA variants by next generation sequencing. The variants were classified as per the American Society of Medical Genetics and Genomics recommendations. Pathogenic (class V) and likely pathogenic (class IV) were considered clinically relevant and utilized for cascade screening.
Results
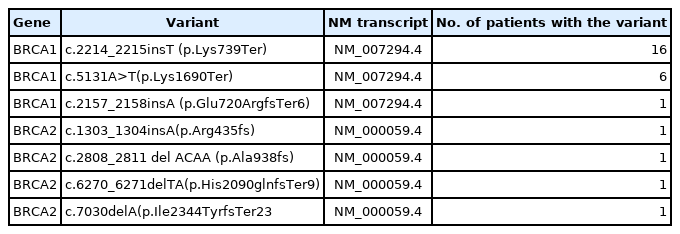
Nepalese population made up a subcohort of 5.12% (58/1,133) of probands tested for germline BRCA1/2 variants. Twenty-seven of these 58 tested harbored pathogenic genetic alterations in BRCA1/2 genes, with 23 being BRCA1 mutant. Sixteen of 23 BRCA1 mutant cases shared one common pathogenic mutation c.2214_2215insT (p.Lys739Ter) (NM_007294.4). Additionally, a second highly recurrent mutation in BRCA1 gene c.5068A>T (p.Lys1690Ter) (NM_007294.4) was noted in six patients from this population.
Conclusions
The overwhelming abundance of the above two variants in a geographically confined population confers these two genetic alterations a status of founder mutations amongst the people of Nepal. A more extensive population-based study to reaffirm these findings will help establish a dual site-specific germline testing similar to the “Multisite-3-assay” in Ashkenazi Jews as the primary screening tool, especially in a resource-constrained environment.
The founder effect amplifies a particular genotypic alteration’ in a geographically or culturally confined population caused by several generations of interbreeding. Deleterious germline alterations of the BRCA1 (OMIM *113705) and BRCA2 (OMIM *600185) gene result in breast and ovarian cancer susceptibility. The lifetime risk of breast cancer is 55%–72% and 45%–69% in mutation carriers of BRCA1 and BRCA2 deleterious germline alterations, respectively [1–4]. Likewise, the risk of ovarian cancer is 39%–44% and 11%–17% in the BRCA1 and BRCA2 germline variants [1–4].
Germline alterations of BRCA2 variants and, to a lesser extent, BRCA1 variants in males predispose them to the breast [5] and prostate carcinoma [6–8]. The inherited pathogenic BRCA1/BRCA2 variants also increase the risk of pancreatic cancers, albeit to a lower extent [9]. The prevalence of BRCA1 or BRCA2 mutations varies considerably between ethnic groups and geographical areas [10,11]. Founder BRCA mutations that are population-specific mutation(s) have been described in Iceland, Netherlands, Sweden, Norway, Germany, France, Spain, countries of central and eastern Europe, and Ashkenazi Jews [10,11]. The prevalence of BRCA mutation carriers in the general population is estimated at 1/800 to 1/1,000; however, the rate of occurrence of BRCA mutation increases several-fold in the population carrying founder mutation, for instance, the rate of a founder mutation in Ashkenazi Jews goes up to 1 in 40 [12,13]. Nepal is a Himalayan country with limited groupings of Tibeto-Burman and Indo-Aryan stock [14]. The geographically confined existence and restricted interaction with the outside world due to rugged terrain and the land-locked nature of the country provided an appropriate milieu for a deleterious mutation to breed, dominate and produce the founder effect. The current study reports the concentration of two particular BRCA1-specific variants in the Nepalese population tested for BRCA1 and BRCA2 deleterious mutations.
MATERIALS AND METHODS
Research setting and subjects
One thousand one hundred thirty-three patients diagnosed with breast/ovarian/prostate/pancreatic cancer and who fulfilled the BRCA testing criteria recommended by National Comprehensive Cancer Network (NCCN) were included in the study [15]. Each eligible subject was explicitly explained and counseled about the pros and cons of undergoing BRCA testing by the institutional genetic counselor, and informed written consent was obtained for testing and using the information for research. The included participants were screened for the mutations in the BRCA genes by next generation sequencing (NGS), and the large genomic rearrangements (Big Indels) were examined through Multiplex Ligation-dependent Probe Amplification (MLPA).
Isolation of DNA from blood, NGS, and data analysis
Genomic DNA was isolated from 2 mL of peripheral blood of the index case using the commercially available DNA isolation kit (Qiagen DNeasy Blood and Tissue kit, Qiagen NV, Hilden, Germany), following the manufacturer’s instructions. Isolated DNA was quantified by Qubit 3.0 Fluorometric quantitation (Thermo Fisher Scientific, Waltham, MA, USA). NGS library was prepared manually with 10 ng of the isolated DNA using Oncomine BRCA assay – A328400 (Thermo Fisher Scientific) as detailed elsewhere [16]. Data generated from the runs was assessed for quality metrics on Torrent Suite Viewer (Ion Torrent Suite 5.10, Thermo Fisher Scientific) for parameters like the number of mapped reads, average base coverage depth, uniformity of coverage, coverage at 1 ×, 20 ×, and 100 ×, strand bias, end to end amplicon reads. Thresholds were employed as enunciated in The National Cancer Institute-Molecular Analysis for Therapy Choice (NCI-MATCH) trial [17]. Mapped reads of > 1,00,000 with 90% uniformity, average base coverage depth ≥ 100, and > 90% coverage at 100× were considered optimal for reporting though the base coverage and mapped reads were usually far more. The variants were classified according to the American Society of Medical Genetics and Genomics recommendations for standards of interpretation and reporting of sequence variations [18].
Cases tested negative for BRCA1/BRCA2 mutations were further investigated for possible large genomic rearrangements (Big Indels) by MLPA assay described previously elsewhere [16].
Statistical analysis
Descriptive statistics were used to summarize the data. The data were analyzed statistically using SPSS ver. 23.0 (IBM Corp., Armonk, NY, USA). The statistical analysis comprised of calculating means and proportions. Appropriate tests of significance were applied, and a p-value of < .05 was considered significant.
RESULTS
One thousand one hundred thirty-three comprehensive BRCA1 and BRCA2 testing for different ethnic populations, comprising the Indian, Nepalese, Bangladeshi, Afghani, Kenyan, Iraqi, and the Myanmarese, were performed at the Molecular Diagnostic laboratory of a tertiary cancer care hospital in India. Of the 1,133 patients tested, 58 were Nepalese descent (5.1%) with a mean age of 47.3 years. Twenty-seven of the 58 Nepalese patients harbored pathogenic BRCA1/BRCA2 genetic alteration, 16 breast, and 11 ovarian carcinoma cases. The mean age was 46.5 years in the Nepalese against 46.8 years in the rest of the cohort (95% confidence interval, −4.288 to 5.088; p = .867). Strikingly 23 of these 27 affected probands carried BRCA1 mutation and, far more significantly, just two recurrent nonsense mutations, as shown in Table 1 below. Only one proband out of 23 with BRCA1 inactivating alteration had a frameshift mutation outside these two genetic alterations. The two recurrent BRCA1 alterations (c.2214_2215insT and c.5068A>T) were not found in any other ethnic population in the study (Fig. 1).
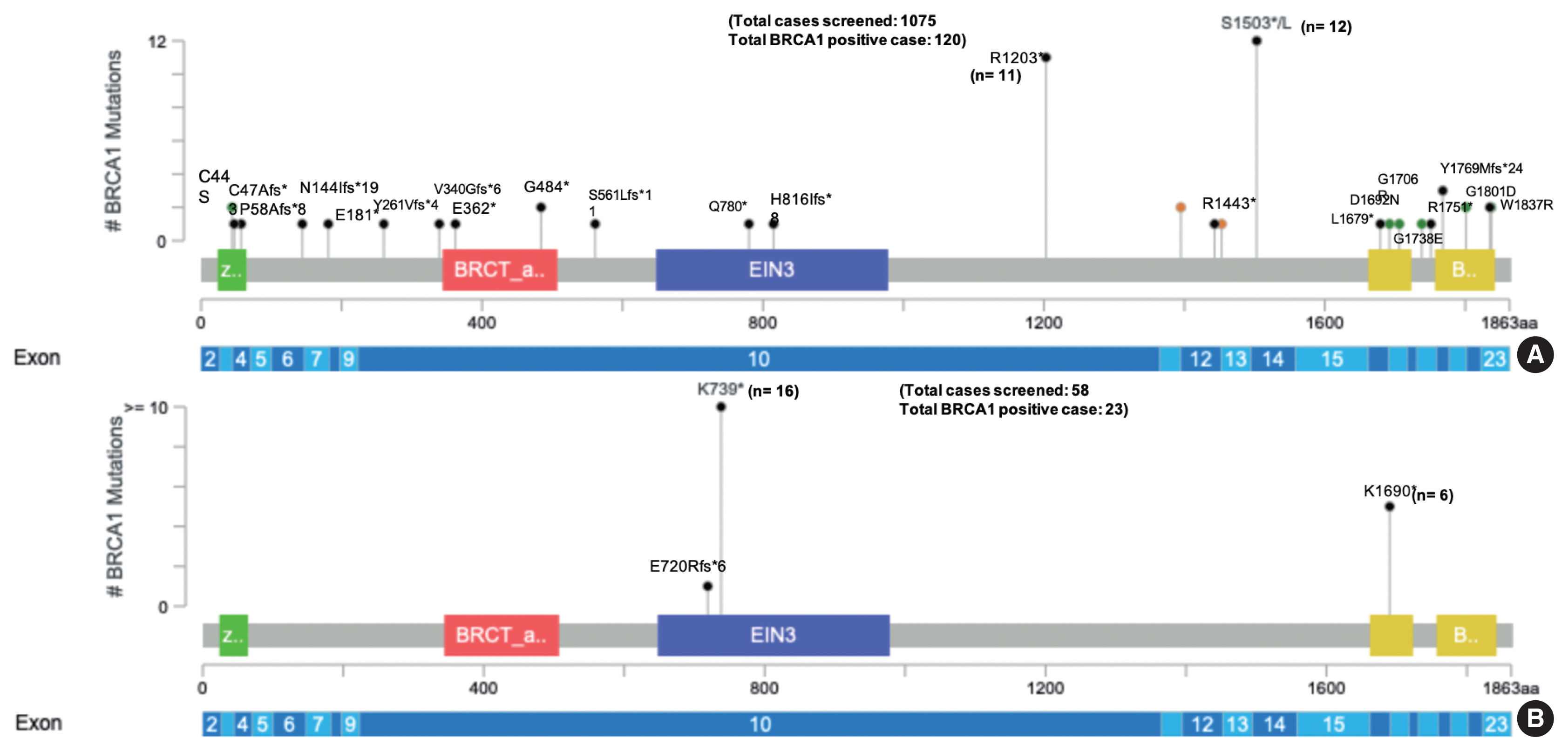
The commonly encountered BRCA1 mutations in the non-Nepalese population (A) and the Nepalese population (B) in the present study [19,20]. Note the dominance of just two genetic alterations in the Nepalese people and contrast to the widespread distribution of pathogenic mutation in the full-length BRCA1 gene in the non-Nepalese population.
A BRCA mutation is seen in 27 Nepalese patients (27/58), accounting for 46.5% of tested patients. In comparison, BRCA mutation is observed in 227 non-Nepalese patients (227/1,075), accounting for 21.1%. The preceding emphasizes that BRCA mutation is significantly higher in the Nepalese population than in the non-Nepalese population (Fisher exact test, statistical value is <0.001). Also, BRCA1 mutation was more common in Nepalese subcohort (23/58) than non-Nepalese subcohort (167/1,075) (Fisher exact test, statistical value is <0.001).
Of the total BRCA mutant cases, c.2214_2215insT is the most frequently observed BRCA1 variant in the Nepalese population (16/27), constituting 59.2% of the total BRCA mutant cases followed by c.5068A>T (6/27) (22.2%). Thirty three point three three percentage of these 27 BRCA mutant cases constituted triple-negative breast carcinoma with 29.6% luminal type breast cancer and 25.9% high-grade serous ovarian carcinoma, as summarized in Table 2. Only 11 (11/27) BRCA mutant cases had a family history of breast/ovarian cancer.
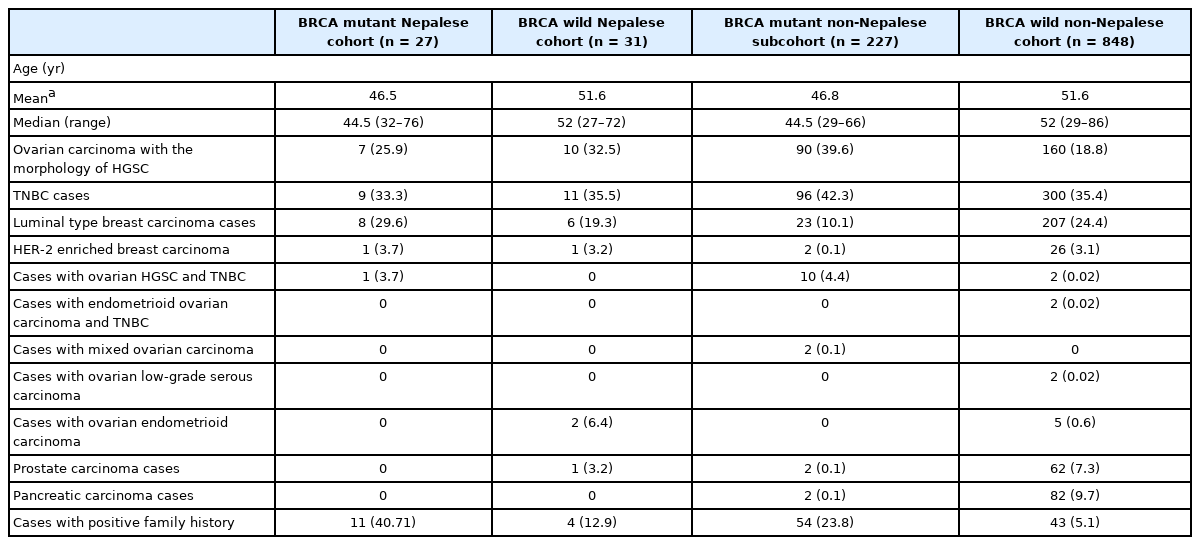
Clinical and morphological profile of BRCA mutant and BRCA wild-type cases in Nepalese and Non-Nepalese subcohorts
One of the probands was tested for somatic alteration in the BRCA1 gene in ovarian carcinoma, and p.Lys1690Ter was detected in the BRCA1 gene at a high variant allele frequency (VAF). Combined with our observation of this variant being highly repetitive in the Nepalese people and the high VAF, a peripheral blood testing was done by an orthogonal method (site-specific Sanger sequencing) and confirmed as germline.
DISCUSSION
BRCA1 and BRCA2 mutations show variable prevalence in different ethnic populations. The prevalence of BRCA1/2 mutation in the general population is estimated to be around 1/800 to 1/1,000 or at the rate of 0.125 to 0.1%. In contrast, the prevalence increases several-fold in the geographically confined ethnic groups with founder mutation as in Ashkenazi Jews, where BRCA mutation is observed at a frequency of 2.5% [12,13]. The present study highlighted a similarly high incidence of BRCA mutation in the Nepalese patient cohort (46.5%), with the BRCA1 gene altered in 39.6% and the BRCA2 in 7%.
The discovery of a founder mutation in Ashkenazi Jews population paved the way to the “Multisite 3 assay” testing for BRCA1 185delAG, BRCA1 5382insC, and BRCA2 6174delT. The present study identifies two founder mutations in the BRCA1 gene (c.2214_2215insT and c.5068A>T) in the Nepalese population. Significantly, none from the non-Nepalese cohort of the 1,075 probands tested positive for these two mutations. This exclusiveness and high frequency of just two variants in an ethnic group support the founder nature of these variants.
These two alterations account for 85.2% of all cases detected with germline BRCA mutation in the Nepalese subcohort, which is far higher than the three mutations that make up 73.2% of all germline BRCA mutations amongst Ashkenazi Jews [21]. Such high occurrence of just two mutations opens up the possibility that, like in Ashkenazi Jews, the first tier of testing can be reduced to dual site-specific testing for BRCA1 c.2214_2215insT and BRCA1 c.5068A>T for the Nepalese people. Failing to identify one of the two nonsense mutations shall prompt further testing by a more extensive NGS-based assay.
One of the index cases of ovarian carcinoma screened for somatic BRCA testing had c. 5068A>T mutation in BRCA1 gene at a high VAF and confirmed as germline mutation on orthogonal testing by site-specific Sanger sequencing on peripheral blood. Further, even when detected in tumor tissue, these two mutations shall get full consideration of germline origin, and germline testing should follow.
A positive family history of breast/ovarian carcinoma was found in 40.71% of BRCA mutant cases in the Nepalese subcohort, clearly far more common than non-Nepalese subcohort (23.8%). The observation of a significantly high BRCA mutant population sans a family history of cancer predisposition thus mandates testing all patients for BRCA irrespective of the family history as emphasized by the NCCN guidelines [15].
c.2214_2215insT is located at chr17:41245334; exon 10 of 24 results in a frameshift that generates a new stop codon in place of Lysine, either causing the formation of a truncated BRCA1 protein or lack of it due to Nonsense-mediated mRNA decay [22,23]. Likewise, c.5068A>T is located at chr17:41219631; exon 17 of 23; position 57 of 78 (on assembly GRCh37) [22] also forms a premature stop codon in place of Lysine with similar effects as for the other founder mutation. These mutations were reported by the first author in the LOVD Database [24]. The variants above are not reported in the population databases (ExAC) [25]. The Clinvar has annotated these variants to be pathogenic [26].
The present hospital-based study on cancer patients acting as probands reveals founder mutations in the Nepalese people. A more extensive population-based study confirming their high-frequency and lack of other randomly distributed deleterious mutations in BRCA genes shall permit the development of a dual site-specific BRCA1 testing as the first-line screen for probands and kindred. Such a strategy shall prevent the need for NGS, drastically reduce the cost of BRCA testing, allow deeper reach, especially in a resource-constrained setting. This strategy shall also provide a fillip to a preventive approach towards cancer.
BRCA1 c.2214_2215insT and BRCA1 c. 5068A>T are the two germline alterations in the Nepalese population with founder effect and incidence of 85.2%. Further confirmation of our findings by a more extensive population-based study shall allow developing a limited dual site-specific germline test as a first-line screening tool, especially in a resource-restricted environment with limited accessibility to NGS. Additionally, Founder mutations can help conclude or rule out an anthropological hypothesis and has immense research potential in that field.
Notes
Ethics Statement
This study was approved by the institutional review board (Rajiv Gandhi Cancer Institute and Research Center), vide the ethical approval letter number RGCIRC/IRB-BHR/41/2020. The study was conducted per the Declaration of Helsinki. Informed written consent was obtained for testing and using the information for research.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: AM. Data curation: GG, HD, SA, SN. Formal analysis: AM, GG, HD, SN. Investigation: AM, HD, GG, SN, SA, SD. Methodology: AM. Project administration: AM. Resources: SD. Supervision: AM. Visualization: HD, SN. Writing—original draft: GG, HD. Writing—review & editing: AM.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.