Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: its updated diagnostic criteria, preoperative cytologic diagnoses and impact on the risk of malignancy
Article information
Abstract
Due to the extremely indolent behavior, a subset of noninvasive encapsulated follicular variant papillary thyroid carcinomas has been classified as “noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP)” since 2016 and is no longer considered carcinoma. Since the introduction of this new terminology, changes and refinements have been made in diagnostic criteria. Initially, the incidence of NIFTP was estimated substantial. However, the reported incidence of NIFTP varies greatly among studies and regions, with higher incidence in North American and European countries than in Asian countries. Thus, the changes in the risk of malignancy (ROM) in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) differ inevitably among regions. Because more conservative surgery is recommended for NIFTPs, distinguishing NIFTPs from papillary thyroid carcinomas in preoperative fine-needle aspiration cytology became one of the major concerns. This review will provide comprehensive overview of updates on diagnostic criteria, actual incidence and preoperative cytologic diagnoses of NIFTP, and its impact on the ROM in TBSRTC.
As nuclear features of papillary thyroid carcinoma (PTC) have been increasingly recognized, the incidence of follicular variant PTC (FVPTC), especially encapsulated subtype, rose 2- to 3-fold over the past decade in Europe and North America [1]. Because of the indolent behavior reported in a subset of noninvasive encapsulated FVPTCs (noninvasive EFVPTCs), the terminology “noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP)” was proposed in 2016 by the Endocrine Pathology Society working group [2] to avoid overdiagnosis and unnecessary psychological and financial burden on both clinicians and patients.
The advent of this new terminology has brought up certain issues including the proper diagnostic criteria, actual incidence, preoperative fine-needle aspiration cytology (FNAC) diagnosis, and clinical impact of this neoplasm. This review highlights on the changes in diagnostic criteria of NIFTP, actual incidence in different regions, cytologic features, preoperative FNAC diagnostic categories, and its impact on the risk of malignancy in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC).
UPDATES ON DIAGNOSTIC CRITERIA OF NONINVASIVE FOLLICULAR THYROID NEOPLASM WITH PAPILLARY-LIKE NUCLEAR FEATURES
The initial diagnostic criteria proposed by Nikiforov et al. in 2016 [2], the revised criteria suggested in 2018 [3], and the most recent criteria in the 2022 World Health Organization (WHO) classification [4] are summarized in Table 1. In initial criteria, noninvasive EFVPTCs with <1% papillae, no psammoma bodies and <30% solid/trabecular/insular growth pattern were renamed as NIFTP in the absence of necrosis and high mitotic activity [2] (Fig. 1). Among these histologic criteria, the proportion of papillae has become the center of controversy. Above all, it is important to acknowledge that the authors intended to count the proportion of “true papillae”, not rudimentary or hyperplastic type papillae. Although Nikiforov et al. [2] have reported no adverse events in cases with NIFTPs in the initial study, lymph node metastases and even distant metastases of NIFTPs were reported by other researchers [5]. Moreover, some of these cases harbored BRAF V600E mutation, which is rather a hallmark of conventional PTC [5]. These findings led to the revised criteria which restricted the diagnosis of NIFTP to cases without any well-formed papillae (true papillae) [3]. Also, absence of BRAF V600E or other highrisk mutations involving TP53 or TERT promoter, were additionally described as helpful but not required features of NIFTP [3]. However, larger number of studies demonstrated lack of metastasis and disease recurrence in cases harboring <1% papillae [6-9]. In the study by Xu et al. [8], lymph node metastasis was only observed in cases with >10% papillae. The 2022 WHO classification endorses the original criteria allowing <1% papillae based on these studies [4]. However, lymph node metastases [5,10], and even distant metastases [10] were found in NIFTPs with 0% papillae, despite thorough microscopic examination. Authors of these studies underscored the low-risk malignant nature of NIFTP and the necessity of including NIFTP in cancer registry [5,10]. Although cases less than 1cm or showing oncocytic features that are otherwise consistent with NIFTP were not included in the initial study [2], the new WHO classification will also include these scenarios because previous studies have confirmed similar behavior as NIFTPs without these features [9,11].

Diagnostic criteria for noninvasive follicular thyroid neoplasm with papillary-like nuclear features
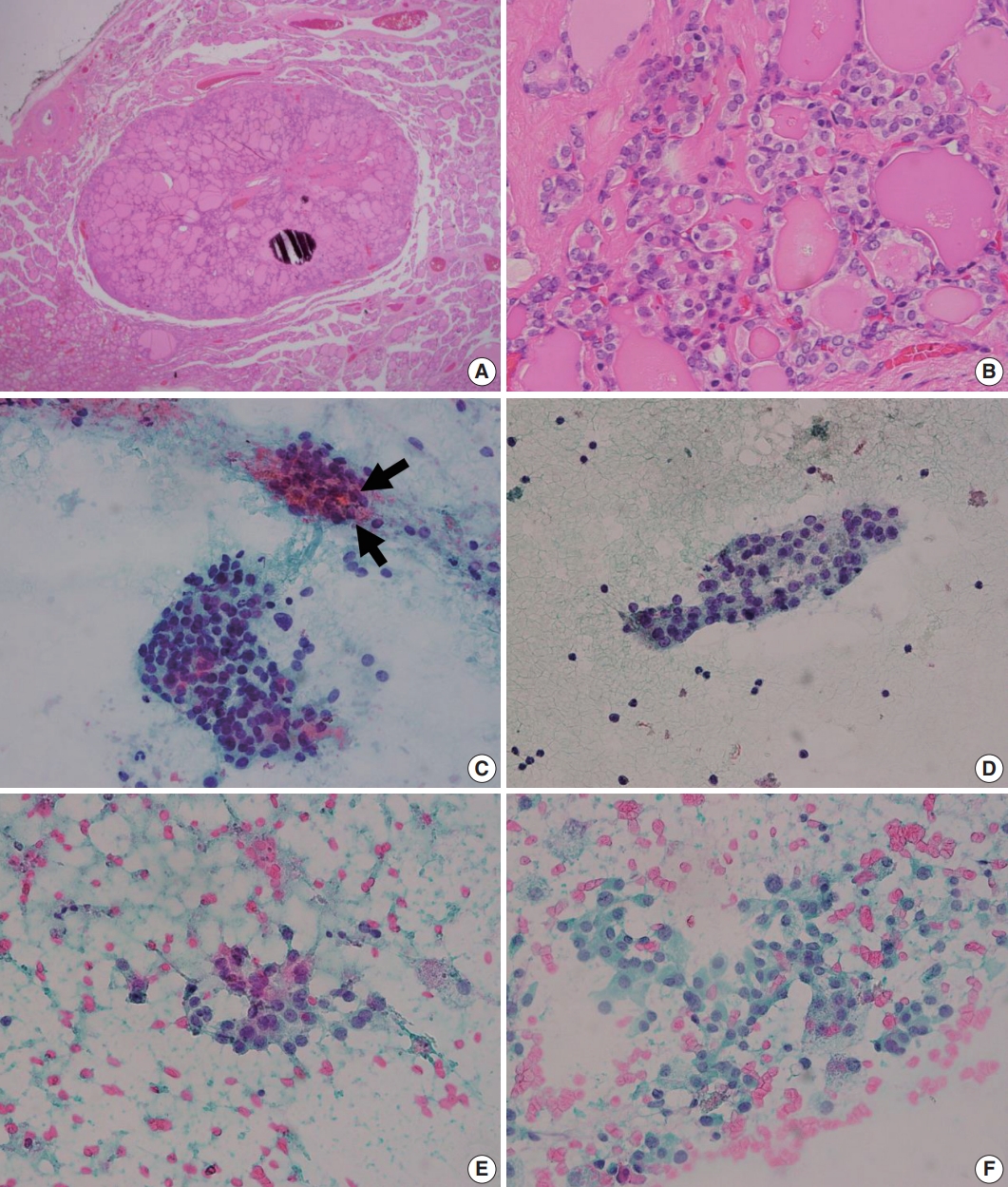
Histologic and cytologic features of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). (A) A well-demarcated mass composed of variable-sized neoplastic follicles is observed in scan view. (B) In high power view, mild nuclear atypia including chromatin clearing, and occasional nuclear grooves which corresponds to “nuclear score 2” is seen (B). (C, D) Fine-needle aspiration cytology of NIFTP generally shows syncytial cell clusters containing microfollicles. Thick colloid can be observed in the microfollicles (arrows). (E, F) Pale chromatin, occasional nuclear grooves, marginal nucleoli are seen. The nuclear atypia is typically mild and patchy. Intranuclear pseudoinclusions, psammomatous calcifications are absent in the presented cases.
ACTUAL INCIDENCE OF NONINVASIVE FOLLICULAR THYROID NEOPLASM WITH PAPILLARY-LIKE NUCLEAR FEATURES
Although it was initially suspected that a substantial proportion of EFVPTCs would be regrouped as NIFTPs, the actual incidence varies greatly according to geographic regions as depicted in recent meta-analyses [12]. The reported incidences of NIFTP range from 1.3% to 23.4% in North America [13-15], from 0.7% to 34.9% in Europe [16-18], and from 0.4% to 29.4% in Asia [19-21]. The pooled incidence of NIFTP was 9.3% and 9.6% in North American and Europe, which was far higher than in Asia with 2.1%, indicating the geographical or ethnic differences [12]. In Korea, the incidence of NIFTP investigated by a multi-institutional study was 0.8%, even lower than Asian average [22]. Of note, the worldwide incidence of NIFTP was 6.0% in the same meta-analysis, suggesting that the impact of NIFTP would not be as considerable as initially estimated [12]. Interestingly, different institutes in the same region also reported widely varying incidences of NIFTP [10,13,15,23], suggesting that interpreting nuclear atypia still lays in subjective area despite the effort to objectifying the nuclear features into three-tiered score.
PREOPERATIVE CYTOLOGIC DIAGNOSES OF NONINVASIVE FOLLICULAR THYROID NEOPLASM WITH PAPILLARY-LIKE NUCLEAR FEATURES
More conservative management is considered for NIFTPs compared with conventional PTCs (cPTCs) which require lymph node dissection and radioactive iodine treatment if indicated. Therefore, it has become a major interest whether NIFTP can be diagnosed preoperatively or not.
Upon the introduction of NIFTP terminology, researchers have investigated the differences in cytologic features of NIFTP and other related lesions such as benign follicular lesion, follicular thyroid adenoma, FVPTC, and cPTCs. The results of studies comparing cytologic features of NIFTP and other lesions are summarized in Table 2. In FNAC, NIFTPs generally show crowded syncytial-like fragments containing microfollicles (Fig. 1) [24,25]. Compared with cPTCs, NIFTPs are more commonly associated with predominant microfollicular pattern in bidimensional clusters and show absent or rare papillary structure [26-30]. Monolayered sheet pattern and tridimensional clusters are more frequent in cPTCs [29,30]. Papillary-like nuclear features, including nuclear enlargement, nuclear elongation, chromatin clearing, intranuclear pseudoinclusion are usually mild and patchy (Fig. 1) [14,26,27,29-32]. Diffuse nuclear change and presence of nuclear score 3 can be observed but are reported to be less frequent than in cPTCs [32]. Regarding the nature of colloid, NIFTPs are associated with thick, dense colloid found both in and out of the microfollicles, while cPTCs tend to show ropy colloid [30]. Psammoma bodies and multinucleated giant cells are also absent or infrequent in NIFTPs compared with cPTCs [14,30]. Indeed, Strickland et al. [33] have demonstrated that NIFTPs and invasive FVPTCs can be efficiently separated from cPTCs in preoperative FNAC when diagnosed according to the criteria as following: (1) cPTC: presence of papillae, pseudoinclusions, or psammomatous calcifications; (2) NIFTP and invasive FVPTC: microfollicle predominance without papillae, pseudoinclusions, or psammomatous calcifications. These criteria surely are not perfect because papillae or pseudoinclusions, by definition, can also be observed in NIFTPs albeit low frequency. Nevertheless, the criteria itself with some additional cytologic features mentioned above appear to be helpful in distinguishing NIFTPs from cPTCs. In addition, some researchers reported that marginal micronucleoli, nuclear grooves, pseudoinclusions, irregular branching sheet and multinucleated giant cells were more common in invasive FVPTCs than in NIFTPs [14,26,28,29,34]. However, these findings are inconsistent among different studies, and others have failed to reveal significant differences between NIFTPs and invasive FVPTCs [32,35]. Pathologists should be aware of the fact that the diagnosis of NIFTP is determined only after the histopathologic examination of surgical specimen, although certain cytologic features are more or less often associated with NIFTP than other lesions.
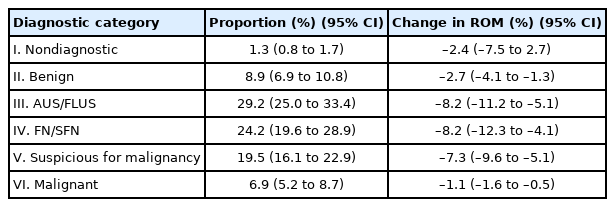
In FNACs, NIFTPs are usually diagnosed as indeterminate categories including atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), follicular neoplasm/suspicious for a follicular neoplasm (FN/SFN), and suspicious for malignancy categories due to microfollicular-predominant architectural pattern and subtle nuclear changes. According to previous studies, distribution of TBSRTC diagnostic categories among NIFTPs range from 0% to 25% in nondiagnostic [12,21,26,36], 0% to 35% in benign [12,26,36,37], 0% to 66.7% in AUS/FLUS [27,29,36,38], 0% to 61.9% in FN/SFN [26,29,36,39], 0% to 83.3% in suspicious for malignancy [12, 27,29,36], 0% to 65.9% in malignant category [29,36,39,40]. A recent meta-analysis showed the pooled distribution of NIFTP cases in FNAC diagnostic categories as follows; 1.3% (95% confidence interval [CI], 0.8 to 1.7) in nondiagnostic, 8.9% (95% CI, 6.9 to 10.8) in benign, 29.2% (95% CI, 25.0 to 33.4) in AUS/FLUS, 24.2% (95% CI, 19.6 to 28.9) in FN/SFN, 19.5% (95% CI, 16.1 to 22.9) in suspicious for malignancy, and 6.9% (95% CI, 5.2 to 8.7) in malignant diagnostic category, respectively (Table 3) [41].
IMPACT OF NIFTP ON THE RISK OF MALIGNANCY IN THE BETHESDA SYSTEM FOR REPORTING THYROID CYTOPATHOLOGY
The impact of NIFTP on the risk of malignancy (ROM) in each TBSRTC diagnostic categories largely depend on distribution of diagnostic categories of NIFTP cases. Therefore, decrease in ROM are more prominent in AUS/FLUS, FN/SFN, and suspicious for malignancy categories compared with other categories. Reported changes in ROM in literature range from 0% to 20.0% in nondiagnostic [12,36,42,43], 0% to 27.6% in benign [19,36, 43,44], 0% to 20.0% in AUS/FLUS [20,36,42,44], 0.2% to 30.8% in FN/SFN [19,36,42,44], 0% to 41.5% in suspicious for malignancy [20,36,42,43], and 0% to 12.8% in malignant diagnostic categories [20,36,44,45]. A recent meta-analysis revealed that the decrease of ROM was 2.4%, 2.7%, 8.2%, 8.2%, 7.3%, and 1.1% in nondiagnostic, benign, AUS/FLUS, FN/SFN, suspicious for malignancy, and malignant diagnostic categories, respectively (Table 3) [41]. While the impact of NIFTP was suspected considerable in European and North American countries due to the high incidence of NIFTP, it does not seem to be the same in Asian counterparts. Compared with European and North American countries, Asian countries generally have reported lower incidence of NIFTPs [22,46,47]. Indeed, results from studies including Asian multi-institutional study performed by Bychkov et al. [48] indicate that magnitude of ROM decrease was slight and not significant [47]. A meta-analysis performed by Vuong et al. [49] compared the ROM decrease in Asian regions to Western counterparts and found that the decrease in ROM in each category is generally lower in Asian countries, with the greatest difference in SM category (5% vs. 18%), followed by AUS/FLUS category (8% vs. 10%).
CONCLUSION
NIFTPs are a group of neoplasm that have been renamed due to the indolent behavior. Although there was a change regarding the amount of papillae in the revised criteria, the initial <1% cutoff is maintained in the 2022 WHO classification based on the recent studies. Diagnosis of NIFTP can only be made according to the strict criteria after thorough pathological examination in surgical specimen. Still, it is important to be aware that some cytological features can be helpful in distinguishing NIFTPs from cPTCs. The impact of NIFTP in cytologic diagnostic categories varies among studies, and regions which hold different incidences.
Notes
Ethics Statement
Not applicable.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Author contributions
Conceptualization: HYN, SYP. Project administration: SYP. Supervision: SYP. Writing—original draft: HYN. Writing—review & editing: HYN, SYP. Approval of final manuscript: all authors.
Conflicts of Interest
SYP, the editor-in-chief of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. Remaining author has declared no conflicts of interest.
Funding Statement
No funding to declare.