Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 56(6); 2022 > Article
-
Review
Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: its updated diagnostic criteria, preoperative cytologic diagnoses and impact on the risk of malignancy -
Hee Young Na1,2
 , So Yeon Park1,2
, So Yeon Park1,2
-
Journal of Pathology and Translational Medicine 2022;56(6):319-325.
DOI: https://doi.org/10.4132/jptm.2022.09.29
Published online: November 9, 2022
1Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea
2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
- Corresponding Author: So Yeon Park, MD, PhD, Department of Pathology, Seoul National University Bundang Hospital, 82 Gumi-ro 173 Beon-gil, Bundang-gu, Seongnam 13620, Korea Tel: +82-31-787-7712, Fax: +82-31-787-4012, E-mail: sypmd@snu.ac.kr
© 2022 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Abstract
- UPDATES ON DIAGNOSTIC CRITERIA OF NONINVASIVE FOLLICULAR THYROID NEOPLASM WITH PAPILLARY-LIKE NUCLEAR FEATURES
- ACTUAL INCIDENCE OF NONINVASIVE FOLLICULAR THYROID NEOPLASM WITH PAPILLARY-LIKE NUCLEAR FEATURES
- PREOPERATIVE CYTOLOGIC DIAGNOSES OF NONINVASIVE FOLLICULAR THYROID NEOPLASM WITH PAPILLARY-LIKE NUCLEAR FEATURES
- IMPACT OF NIFTP ON THE RISK OF MALIGNANCY IN THE BETHESDA SYSTEM FOR REPORTING THYROID CYTOPATHOLOGY
- CONCLUSION
- NOTES
- REFERENCES
Abstract
- Due to the extremely indolent behavior, a subset of noninvasive encapsulated follicular variant papillary thyroid carcinomas has been classified as “noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP)” since 2016 and is no longer considered carcinoma. Since the introduction of this new terminology, changes and refinements have been made in diagnostic criteria. Initially, the incidence of NIFTP was estimated substantial. However, the reported incidence of NIFTP varies greatly among studies and regions, with higher incidence in North American and European countries than in Asian countries. Thus, the changes in the risk of malignancy (ROM) in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) differ inevitably among regions. Because more conservative surgery is recommended for NIFTPs, distinguishing NIFTPs from papillary thyroid carcinomas in preoperative fine-needle aspiration cytology became one of the major concerns. This review will provide comprehensive overview of updates on diagnostic criteria, actual incidence and preoperative cytologic diagnoses of NIFTP, and its impact on the ROM in TBSRTC.
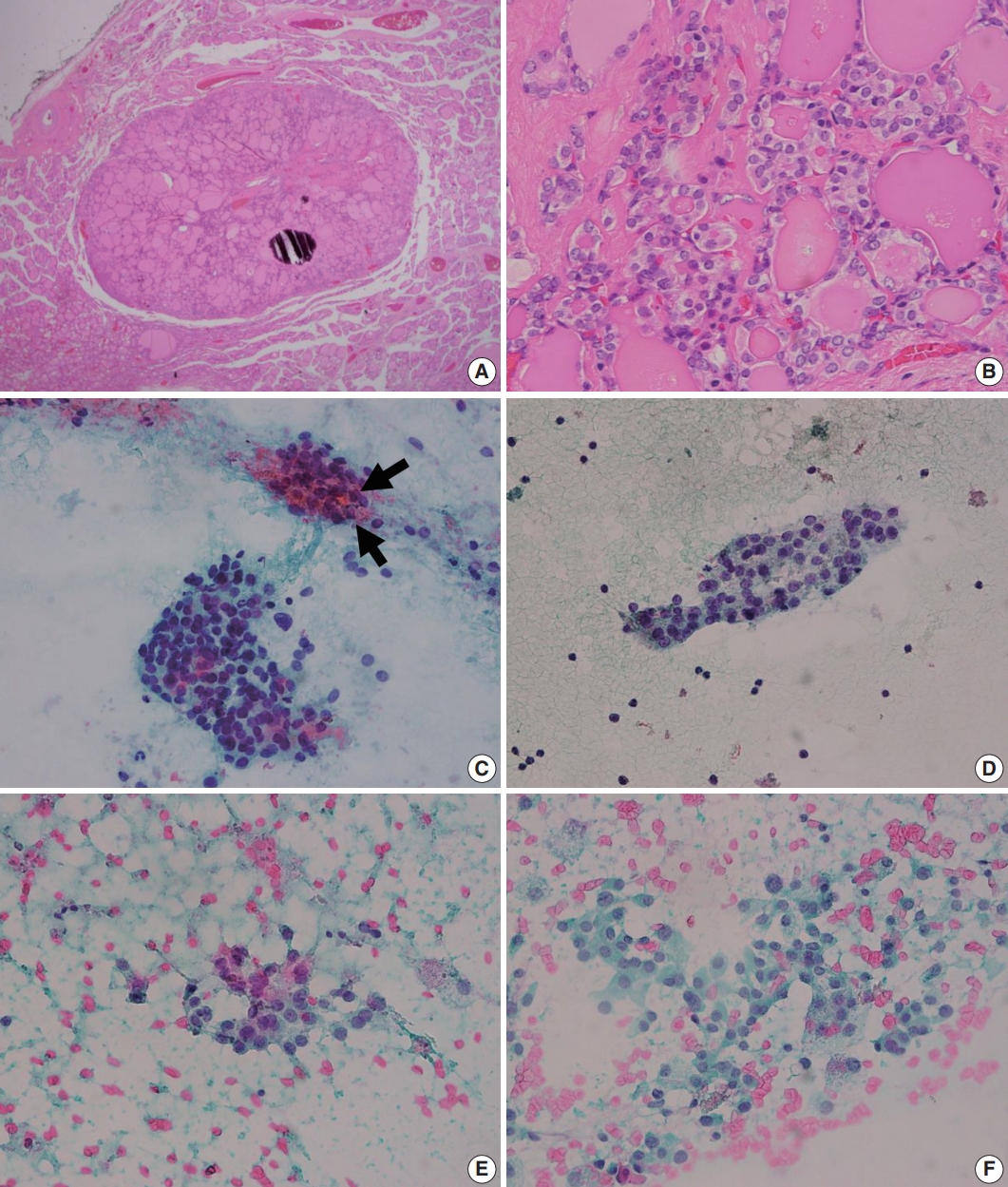
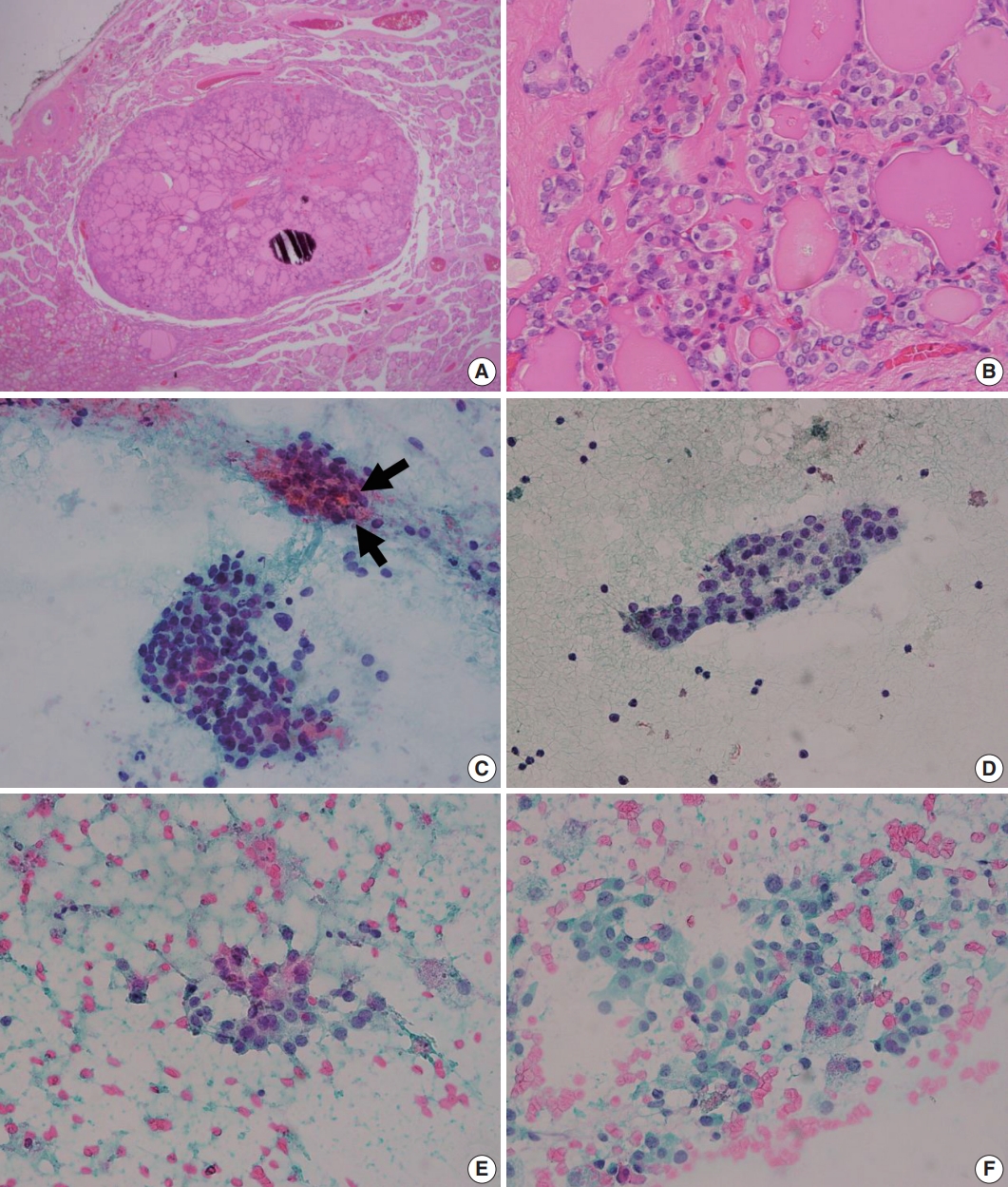
- The initial diagnostic criteria proposed by Nikiforov et al. in 2016 [2], the revised criteria suggested in 2018 [3], and the most recent criteria in the 2022 World Health Organization (WHO) classification [4] are summarized in Table 1. In initial criteria, noninvasive EFVPTCs with <1% papillae, no psammoma bodies and <30% solid/trabecular/insular growth pattern were renamed as NIFTP in the absence of necrosis and high mitotic activity [2] (Fig. 1). Among these histologic criteria, the proportion of papillae has become the center of controversy. Above all, it is important to acknowledge that the authors intended to count the proportion of “true papillae”, not rudimentary or hyperplastic type papillae. Although Nikiforov et al. [2] have reported no adverse events in cases with NIFTPs in the initial study, lymph node metastases and even distant metastases of NIFTPs were reported by other researchers [5]. Moreover, some of these cases harbored BRAF V600E mutation, which is rather a hallmark of conventional PTC [5]. These findings led to the revised criteria which restricted the diagnosis of NIFTP to cases without any well-formed papillae (true papillae) [3]. Also, absence of BRAF V600E or other highrisk mutations involving TP53 or TERT promoter, were additionally described as helpful but not required features of NIFTP [3]. However, larger number of studies demonstrated lack of metastasis and disease recurrence in cases harboring <1% papillae [6-9]. In the study by Xu et al. [8], lymph node metastasis was only observed in cases with >10% papillae. The 2022 WHO classification endorses the original criteria allowing <1% papillae based on these studies [4]. However, lymph node metastases [5,10], and even distant metastases [10] were found in NIFTPs with 0% papillae, despite thorough microscopic examination. Authors of these studies underscored the low-risk malignant nature of NIFTP and the necessity of including NIFTP in cancer registry [5,10]. Although cases less than 1cm or showing oncocytic features that are otherwise consistent with NIFTP were not included in the initial study [2], the new WHO classification will also include these scenarios because previous studies have confirmed similar behavior as NIFTPs without these features [9,11].
UPDATES ON DIAGNOSTIC CRITERIA OF NONINVASIVE FOLLICULAR THYROID NEOPLASM WITH PAPILLARY-LIKE NUCLEAR FEATURES
- Although it was initially suspected that a substantial proportion of EFVPTCs would be regrouped as NIFTPs, the actual incidence varies greatly according to geographic regions as depicted in recent meta-analyses [12]. The reported incidences of NIFTP range from 1.3% to 23.4% in North America [13-15], from 0.7% to 34.9% in Europe [16-18], and from 0.4% to 29.4% in Asia [19-21]. The pooled incidence of NIFTP was 9.3% and 9.6% in North American and Europe, which was far higher than in Asia with 2.1%, indicating the geographical or ethnic differences [12]. In Korea, the incidence of NIFTP investigated by a multi-institutional study was 0.8%, even lower than Asian average [22]. Of note, the worldwide incidence of NIFTP was 6.0% in the same meta-analysis, suggesting that the impact of NIFTP would not be as considerable as initially estimated [12]. Interestingly, different institutes in the same region also reported widely varying incidences of NIFTP [10,13,15,23], suggesting that interpreting nuclear atypia still lays in subjective area despite the effort to objectifying the nuclear features into three-tiered score.
ACTUAL INCIDENCE OF NONINVASIVE FOLLICULAR THYROID NEOPLASM WITH PAPILLARY-LIKE NUCLEAR FEATURES
- More conservative management is considered for NIFTPs compared with conventional PTCs (cPTCs) which require lymph node dissection and radioactive iodine treatment if indicated. Therefore, it has become a major interest whether NIFTP can be diagnosed preoperatively or not.
- Upon the introduction of NIFTP terminology, researchers have investigated the differences in cytologic features of NIFTP and other related lesions such as benign follicular lesion, follicular thyroid adenoma, FVPTC, and cPTCs. The results of studies comparing cytologic features of NIFTP and other lesions are summarized in Table 2. In FNAC, NIFTPs generally show crowded syncytial-like fragments containing microfollicles (Fig. 1) [24,25]. Compared with cPTCs, NIFTPs are more commonly associated with predominant microfollicular pattern in bidimensional clusters and show absent or rare papillary structure [26-30]. Monolayered sheet pattern and tridimensional clusters are more frequent in cPTCs [29,30]. Papillary-like nuclear features, including nuclear enlargement, nuclear elongation, chromatin clearing, intranuclear pseudoinclusion are usually mild and patchy (Fig. 1) [14,26,27,29-32]. Diffuse nuclear change and presence of nuclear score 3 can be observed but are reported to be less frequent than in cPTCs [32]. Regarding the nature of colloid, NIFTPs are associated with thick, dense colloid found both in and out of the microfollicles, while cPTCs tend to show ropy colloid [30]. Psammoma bodies and multinucleated giant cells are also absent or infrequent in NIFTPs compared with cPTCs [14,30]. Indeed, Strickland et al. [33] have demonstrated that NIFTPs and invasive FVPTCs can be efficiently separated from cPTCs in preoperative FNAC when diagnosed according to the criteria as following: (1) cPTC: presence of papillae, pseudoinclusions, or psammomatous calcifications; (2) NIFTP and invasive FVPTC: microfollicle predominance without papillae, pseudoinclusions, or psammomatous calcifications. These criteria surely are not perfect because papillae or pseudoinclusions, by definition, can also be observed in NIFTPs albeit low frequency. Nevertheless, the criteria itself with some additional cytologic features mentioned above appear to be helpful in distinguishing NIFTPs from cPTCs. In addition, some researchers reported that marginal micronucleoli, nuclear grooves, pseudoinclusions, irregular branching sheet and multinucleated giant cells were more common in invasive FVPTCs than in NIFTPs [14,26,28,29,34]. However, these findings are inconsistent among different studies, and others have failed to reveal significant differences between NIFTPs and invasive FVPTCs [32,35]. Pathologists should be aware of the fact that the diagnosis of NIFTP is determined only after the histopathologic examination of surgical specimen, although certain cytologic features are more or less often associated with NIFTP than other lesions.
- In FNACs, NIFTPs are usually diagnosed as indeterminate categories including atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), follicular neoplasm/suspicious for a follicular neoplasm (FN/SFN), and suspicious for malignancy categories due to microfollicular-predominant architectural pattern and subtle nuclear changes. According to previous studies, distribution of TBSRTC diagnostic categories among NIFTPs range from 0% to 25% in nondiagnostic [12,21,26,36], 0% to 35% in benign [12,26,36,37], 0% to 66.7% in AUS/FLUS [27,29,36,38], 0% to 61.9% in FN/SFN [26,29,36,39], 0% to 83.3% in suspicious for malignancy [12, 27,29,36], 0% to 65.9% in malignant category [29,36,39,40]. A recent meta-analysis showed the pooled distribution of NIFTP cases in FNAC diagnostic categories as follows; 1.3% (95% confidence interval [CI], 0.8 to 1.7) in nondiagnostic, 8.9% (95% CI, 6.9 to 10.8) in benign, 29.2% (95% CI, 25.0 to 33.4) in AUS/FLUS, 24.2% (95% CI, 19.6 to 28.9) in FN/SFN, 19.5% (95% CI, 16.1 to 22.9) in suspicious for malignancy, and 6.9% (95% CI, 5.2 to 8.7) in malignant diagnostic category, respectively (Table 3) [41].
PREOPERATIVE CYTOLOGIC DIAGNOSES OF NONINVASIVE FOLLICULAR THYROID NEOPLASM WITH PAPILLARY-LIKE NUCLEAR FEATURES
- The impact of NIFTP on the risk of malignancy (ROM) in each TBSRTC diagnostic categories largely depend on distribution of diagnostic categories of NIFTP cases. Therefore, decrease in ROM are more prominent in AUS/FLUS, FN/SFN, and suspicious for malignancy categories compared with other categories. Reported changes in ROM in literature range from 0% to 20.0% in nondiagnostic [12,36,42,43], 0% to 27.6% in benign [19,36, 43,44], 0% to 20.0% in AUS/FLUS [20,36,42,44], 0.2% to 30.8% in FN/SFN [19,36,42,44], 0% to 41.5% in suspicious for malignancy [20,36,42,43], and 0% to 12.8% in malignant diagnostic categories [20,36,44,45]. A recent meta-analysis revealed that the decrease of ROM was 2.4%, 2.7%, 8.2%, 8.2%, 7.3%, and 1.1% in nondiagnostic, benign, AUS/FLUS, FN/SFN, suspicious for malignancy, and malignant diagnostic categories, respectively (Table 3) [41]. While the impact of NIFTP was suspected considerable in European and North American countries due to the high incidence of NIFTP, it does not seem to be the same in Asian counterparts. Compared with European and North American countries, Asian countries generally have reported lower incidence of NIFTPs [22,46,47]. Indeed, results from studies including Asian multi-institutional study performed by Bychkov et al. [48] indicate that magnitude of ROM decrease was slight and not significant [47]. A meta-analysis performed by Vuong et al. [49] compared the ROM decrease in Asian regions to Western counterparts and found that the decrease in ROM in each category is generally lower in Asian countries, with the greatest difference in SM category (5% vs. 18%), followed by AUS/FLUS category (8% vs. 10%).
IMPACT OF NIFTP ON THE RISK OF MALIGNANCY IN THE BETHESDA SYSTEM FOR REPORTING THYROID CYTOPATHOLOGY
- NIFTPs are a group of neoplasm that have been renamed due to the indolent behavior. Although there was a change regarding the amount of papillae in the revised criteria, the initial <1% cutoff is maintained in the 2022 WHO classification based on the recent studies. Diagnosis of NIFTP can only be made according to the strict criteria after thorough pathological examination in surgical specimen. Still, it is important to be aware that some cytological features can be helpful in distinguishing NIFTPs from cPTCs. The impact of NIFTP in cytologic diagnostic categories varies among studies, and regions which hold different incidences.
CONCLUSION
Ethics Statement
Not applicable.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Author contributions
Conceptualization: HYN, SYP. Project administration: SYP. Supervision: SYP. Writing—original draft: HYN. Writing—review & editing: HYN, SYP. Approval of final manuscript: all authors.
Conflicts of Interest
SYP, the editor-in-chief of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. Remaining author has declared no conflicts of interest.
Funding Statement
No funding to declare.
| Initial criteria [2] | Revised criteria [3] | The 2022 WHO classification [4] | ||||
|---|---|---|---|---|---|---|
| 1. Encapsulation or clear demarcation | 1. Primary | Essential | ||||
| 2. Follicular growth pattern with | Encapsulation or clear demarcation | 1. Encapsulation or clear demarcation | ||||
| < 1% papillae | Follicular growth pattern with no papillae | 2. Follicular growth pattern with | ||||
| No psammoma bodies | No psammoma bodies | < 1% true papillae | ||||
| < 30% solid/trabecular/insular growth pattern | <30% solid/trabecular/insular growth pattern | No psammoma bodies | ||||
| 3. Nuclear score 2–3 | Nuclear score of 2–3 | < 30% solid/trabecular/insular growth | ||||
| 4. No vascular or capsular invasion | No vascular or capsular invasion | 3. Nuclear score of 2–3 | ||||
| 5. No tumor necrosis | No tumor necrosis or high mitotic activity (3 or more mitoses per 10 high power fields) | 4. No vascular or capsular invasion | ||||
| 6. No high mitotic activity | 2. Secondary | 5. No tumor necrosis | ||||
| Lack of BRAF V600E mutation detected by molecular assays or immunohistochemistry | 6. Low mitotic count (< 3 mitoses/2 mm2) | |||||
| Lack of BRAF V600E-like mutations or other high-risk mutations (TERT, TP53) | 7. Lack of cytoarchitectural features of papillary carcinoma variants other than follicular variant | |||||
| Desirable | ||||||
| Immunohistochemistry or molecular testing for BRAF and NRAS mutation | ||||||
| Reference | NIFTP vs. benign/FTA | NIFTP vs. FVPTC | NIFTP vs. cPTC | NIFTP/FVPTC vs. cPTC |
|---|---|---|---|---|
| Legesse et al. [14] | - | Less frequent PIs, marginal micronucleoli, irregular branching sheets, and linear arrangement in NIFTP | Absence of PIs/Less frequent MNGs in NIFTP | - |
| Bizzarro et al. [26] | More frequent scant cytoplasm, NE, nuclear elongation, chromatin clearing, grooves, and membrane irregularities in NIFTP | Less frequent grooves in NIFTP | Less frequent papillae, NE, PIs, grooves, and membrane irregularities in NIFTP | - |
| Brandler et al. [27] | More frequent chromatin clearing, crowding, and NE in NIFTP | - | Less frequent PIs, papillae, crowding, NE, membrane irregularities, chromatin clearing, calcifications, and MNGs in NIFTP | - |
| Chandler et al. [28] | - | More frequent MF predominance/Less frequent nuclear elongation, grooves, and Pis in NIFTP | - | |
| Diaz Del Arco et al. [29] | - | Less frequent nuclear folds in NIFTP | More frequent bidimensional groups and MFs/Less frequent papillary or pseudopapillary architecture, tridimensionality, MNGs, and nuclear folds in NIFTP | - |
| Koshkikawa et al. [30] | - | No differences | - | More frequent MFs, and dense globules of colloids/less frequent PIs, true papillary cell clusters, monolayered cell sheets, ropy colloids, MNGs, psammoma bodies, and cystic background in NIFTP and FVPTC |
| Howitt et al. [31] | - | - | More frequent MFs/Less frequent sheet pattern in NIFTP | - |
| Mahajan et al. [32] | - | No differences in nuclear features/Less frequent 3-dimensional fragments in NIFTP | Less frequent PIs, nuclear score of 3, and diffuse nuclear change in NIFTP | - |
| Selvaggi et al. [34] | - | Less frequent MNGs in NIFTP | - | - |
| Maletta et al. [35] | More frequent NE, membrane irregularities, chromatin clearing, and nuclear molding in NIFTP | No differences | - | - |
| Strickland et al. [33] | - | - | - | MF predominance without papillae, PIs or psammoma bodies in NIFTP and FVPTC |
NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; FTA, follicular thyroid adenoma; FVPTC, follicular variant papillary thyroid carcinoma; cPTC, conventional papillary thyroid carcinoma; PI, pseudoinclusion; MNG, multinucleated giant cell; NE, nucelar enlargement; MF, microfollicle.
- 1. Jung CK, Little MP, Lubin JH, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab 2014; 99: E276-85. ArticlePubMedPMC
- 2. Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2016; 2: 1023-9. ArticlePubMedPMC
- 3. Nikiforov YE, Baloch ZW, Hodak SP, et al. Change in diagnostic criteria for noninvasive follicular thyroid neoplasm with papillarylike nuclear features. JAMA Oncol 2018; 4: 1125-6. ArticlePubMedPMC
- 4. Baloch ZW, Asa SL, Barletta JA, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol 2022; 33: 27-63. ArticlePubMedPDF
- 5. Cho U, Mete O, Kim MH, Bae JS, Jung CK. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol 2017; 30: 810-25. ArticlePubMedPDF
- 6. Rosario PW, Mourao GF, Nunes MB, Nunes MS, Calsolari MR. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Endocr Relat Cancer 2016; 23: 893-7. ArticlePubMed
- 7. Thompson LD. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: a name change to noninvasive follicular thyroid neoplasm with papillary-like nuclear features would help prevent overtreatment. Mod Pathol 2016; 29: 698-707. ArticlePubMedPDF
- 8. Xu B, Serrette R, Tuttle RM, et al. How many papillae in conventional papillary carcinoma? A clinical evidence-based pathology study of 235 unifocal encapsulated papillary thyroid carcinomas, with emphasis on the diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid 2019; 29: 1792-803. ArticlePubMedPMC
- 9. Xu B, Tallini G, Scognamiglio T, Roman BR, Tuttle RM, Ghossein RA. Outcome of large noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid 2017; 27: 512-7. ArticlePubMedPMC
- 10. Parente DN, Kluijfhout WP, Bongers PJ, et al. Clinical safety of renaming encapsulated follicular variant of papillary thyroid carcinoma: is NIFTP truly benign? World J Surg 2018; 42: 321-6. ArticlePubMedPDF
- 11. Xu B, Farhat N, Barletta JA, et al. Should subcentimeter non-invasive encapsulated, follicular variant of papillary thyroid carcinoma be included in the noninvasive follicular thyroid neoplasm with papillarylike nuclear features category? Endocrine 2018; 59: 143-50. ArticlePubMedPMCPDF
- 12. Rana C, Vuong HG, Nguyen TQ, et al. The incidence of noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a meta-analysis assessing worldwide impact of the reclassification. Thyroid 2021; 31: 1502-13. ArticlePubMed
- 13. Cubero Rego D, Lee H, Boguniewicz A, Jennings TA. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) is rare, benign lesion using modified stringent diagnostic criteria: reclassification and outcome study. Ann Diagn Pathol 2020; 44: 151439.ArticlePubMed
- 14. Legesse T, Parker L, Heath J, Staats PN. Distinguishing non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) from classic and invasive follicular-variant papillary thyroid carcinomas based on cytologic features. J Am Soc Cytopathol 2019; 8: 11-7. ArticlePubMed
- 15. Sung S, Margolskee E, Chen D, Tiscornia-Wasserman P. Incidence of noninvasive follicular thyroid neoplasm with papillary-like nuclear features and change in risk of malignancy for “The Bethesda System for Reporting Thyroid Cytology”. J Am Soc Cytopathol 2019; 8: 133-40. ArticlePubMed
- 16. Borda A, Zahan AE, Piciu D, Barbus E, Berger N, Nechifor-Boila A. A 15 year institutional experience of well-differentiated follicular cell-derived thyroid carcinomas; impact of the new 2017 TNM and WHO Classifications of Tumors of Endocrine Organs on the epidemiological trends and pathological characteristics. Endocrine 2020; 67: 630-42. ArticlePubMedPDF
- 17. Boursier L, Clerc Urmes I, Garon J, Klein M, Demarquet L. Ultrasound and cytological characteristics of non-invasive follicular thyroid neoplasm with papillary-like nuclear features compared to papillary carcinomas. Ann Endocrinol (Paris) 2020; 81: 28-33. ArticlePubMed
- 18. Reinke RH, Larsen SR, Mathiesen JS, Godballe C, Londero SC. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features is rare: a population based study of incidence. Head Neck Pathol 2020; 14: 144-9. ArticlePubMedPMCPDF
- 19. Ke J, Jianyong L, Ying L, et al. The use of The Bethesda System for Reporting Thyroid Cytopathology in a Chinese population: an analysis of 13 351 specimens. Diagn Cytopathol 2019; 47: 876-80. ArticlePubMedPDF
- 20. Rana C, Manjunath S, Ramakant P, Singh K, Babu S, Mishra A. Noninvasive follicular neoplasm with papillary like nuclear features: a comprehensive analysis with a diagnostic algorithm. Diagn Cytopathol 2020; 48: 330-41. ArticlePubMedPDF
- 21. Zhu Y, Song Y, Xu G, Fan Z, Ren W. The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC): a report of 2,781 cases in a Chinese population. Chin J Cancer Res 2020; 32: 140-8. ArticlePubMedPMC
- 22. Seo JY, Park JH, Pyo JY, et al. A multi-institutional study of prevalence and clinicopathologic features of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) in Korea. J Pathol Transl Med 2019; 53: 378-85. ArticlePubMedPMCPDF
- 23. Eskander A, Hall SF, Manduch M, Griffiths R, Irish JC. A population-based study on NIFTP incidence and survival: is NIFTP really a “benign” disease? Ann Surg Oncol 2019; 26: 1376-84. ArticlePubMedPDF
- 24. Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology. New York: Springer, 2018; 2nd.
- 25. Yan L, Sethi S, Park JW. Cytologic and clinical features of NIFTP: can we diagnose based on preoperative fine-needle aspiration. Diagn Cytopathol 2019; 47: 1259-66. ArticlePubMedPDF
- 26. Bizzarro T, Martini M, Capodimonti S, et al. Young investigator challenge: the morphologic analysis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features on liquid-based cytology: Some insights into their identification. Cancer Cytopathol 2016; 124: 699-710. ArticlePubMed
- 27. Brandler TC, Zhou F, Liu CZ, et al. Can noninvasive follicular thyroid neoplasm with papillary-like nuclear features be distinguished from classic papillary thyroid carcinoma and follicular adenomas by fine-needle aspiration? Cancer Cytopathol 2017; 125: 378-88. ArticlePubMedPDF
- 28. Chandler JB, Colunga M, Prasad ML, et al. Identification of distinct cytomorphologic features in the diagnosis of NIFTP at the time of preoperative FNA: Implications for patient management. Cancer Cytopathol 2017; 125: 865-75. PubMedPDF
- 29. Diaz Del Arco C, Fernandez Acenero MJ. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: can cytology face the challenge of diagnosis in the light of the new classification? Acta Cytol 2018; 62: 265-72. ArticlePubMedPDF
- 30. Koshikawa T, Fujita N, Ueda N, et al. Important cytological findings for distinction between follicular variant and conventional papillary thyroid carcinoma, including noninvasive follicular thyroid tumors with papillary-like nuclear features. Endocr J 2019; 66: 475-83. ArticlePubMed
- 31. Howitt BE, Chang S, Eszlinger M, et al. Fine-needle aspiration diagnoses of noninvasive follicular variant of papillary thyroid carcinoma. Am J Clin Pathol 2015; 144: 850-7. ArticlePubMed
- 32. Mahajan S, Agarwal S, Kocheri N, Jain D, Mathur SR, Iyer VK. Cytopathology of non-invasive follicular thyroid neoplasm with papillarylike nuclear features: a comparative study with similar patterned papillary thyroid carcinoma variants. Cytopathology 2018; 29: 233-40. ArticlePubMedPDF
- 33. Strickland KC, Vivero M, Jo VY, et al. Preoperative cytologic diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a prospective analysis. Thyroid 2016; 26: 1466-71. ArticlePubMed
- 34. Selvaggi SM. The presence of multinucleated giant cells: noninvasive follicular thyroid neoplasm with papillary-like nuclear features vs the follicular variant of papillary thyroid carcinoma. Diagn Cytopathol 2019; 47: 1007-10. ArticlePubMedPDF
- 35. Maletta F, Massa F, Torregrossa L, et al. Cytological features of “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” and their correlation with tumor histology. Hum Pathol 2016; 54: 134-42. ArticlePubMed
- 36. Kim M, Kim JE, Kim HJ, et al. Cytologic diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features and its impact on the risk of malignancy in the bethesda system for reporting thyroid cytopathology: an institutional experience. J Pathol Transl Med 2018; 52: 171-8. ArticlePubMedPMCPDF
- 37. Ibrahim AA, Wu HH. Fine-needle aspiration cytology of noninvasive follicular variant of papillary thyroid carcinoma is cytomorphologically distinct from the invasive counterpart. Am J Clin Pathol 2016; 146: 373-7. ArticlePubMed
- 38. Ventura M, Melo M, Fernandes G, Carrilho F. Risk of malignancy in thyroid cytology: the impact of the reclassification of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (Niftp). Endocr Pract 2019; 25: 642-7. ArticlePubMed
- 39. Celik M, Bulbul BY, Can N, et al. Comparison of clinicopathological features in patients with noninvasive follicular thyroid neoplasm with papillary-like nuclear features and follicular variant papillary thyroid cancer. Pol Arch Intern Med 2020; 130: 100-5. PubMed
- 40. Hirokawa M, Higuchi M, Suzuki A, Hayashi T, Kuma S, Miyauchi A. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a single-institutional experience in Japan. Endocr J 2017; 64: 1149-55. ArticlePubMed
- 41. Haaga E, Kalfert D, Ludvikova M, Kholova I. Non-invasive follicular thyroid neoplasm with papillary-like nuclear features is not a cytological diagnosis, but it influences cytological diagnosis outcomes: a systematic review and meta-analysis. Acta Cytol 2022; 66: 85-105. ArticlePubMedPMCPDF
- 42. Lau RP, Paulsen JD, Brandler TC, Liu CZ, Simsir A, Zhou F. Impact of the reclassification of “noninvasive encapsulated follicular variant of papillary thyroid carcinoma” to “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” on the Bethesda System for Reporting Thyroid Cytopathology: a large academic institution’s experience. Am J Clin Pathol 2017; 149: 50-4. PubMed
- 43. Strickland KC, Howitt BE, Marqusee E, et al. The impact of noninvasive follicular variant of papillary thyroid carcinoma on rates of malignancy for fine-needle aspiration diagnostic categories. Thyroid 2015; 25: 987-92. ArticlePubMed
- 44. Mito JK, Alexander EK, Angell TE, et al. A modified reporting approach for thyroid FNA in the NIFTP era: a 1-year institutional experience. Cancer Cytopathol 2017; 125: 854-64. ArticlePubMedPDF
- 45. Layfield LJ, Baloch ZW, Esebua M, Kannuswamy R, Schmidt RL. Impact of the reclassification of the non-invasive follicular variant of papillary carcinoma as benign on the malignancy risk of the Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis study. Acta Cytol 2017; 61: 187-93. ArticlePubMedPDF
- 46. Bychkov A, Hirokawa M, Jung CK, et al. Low rate of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Thyroid 2017; 27: 983-4. ArticlePubMed
- 47. Chen CC, Hang JF, Liu CY, Wang YH, Lai CR. Thyroid fine-needle aspiration cytology in Taiwan: a nationwide survey and literature update. J Pathol Transl Med 2020; 54: 361-6. ArticlePubMedPMCPDF
- 48. Bychkov A, Keelawat S, Agarwal S, et al. Impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features on the Bethesda System for Reporting Thyroid Cytopathology: a multi-institutional study in five Asian countries. Pathology 2018; 50: 411-7. ArticlePubMed
- 49. Vuong HG, Tran TTK, Bychkov A, et al. Clinical impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features on the risk of malignancy in the Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis of 14,153 resected thyroid nodules. Endocr Pract 2019; 25: 491-502. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Diagnosis of invasive encapsulated follicular variant papillary thyroid carcinoma by protein-based machine learning
Truong Phan-Xuan Nguyen, Minh-Khang Le, Sittiruk Roytrakul, Shanop Shuangshoti, Nakarin Kitkumthorn, Somboon Keelawat
Journal of Pathology and Translational Medicine.2025; 59(1): 39. CrossRef - Papillae, psammoma bodies, and/or many nuclear pseudoinclusions are helpful criteria but should not be required for a definitive cytologic diagnosis of papillary thyroid carcinoma: An institutional experience of 207 cases with surgical follow up
Tarik M. Elsheikh, Matthew Thomas, Jennifer Brainard, Jessica Di Marco, Erica Manosky, Bridgette Springer, Dawn Underwood, Deborah J. Chute
Cancer Cytopathology.2024; 132(6): 348. CrossRef - ThyroSeq overview on indeterminate thyroid nodules: An institutional experience
Sam Sirotnikov, Christopher C. Griffith, Daniel Lubin, Chao Zhang, Nabil F. Saba, Dehong Li, Amanda Kornfield, Amy Chen, Qiuying Shi
Diagnostic Cytopathology.2024; 52(7): 353. CrossRef - Oncocytic Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features: A Case Report
Kaveripakam Ajay Joseph, Sana Ahuja, Sufian Zaheer
Indian Journal of Surgical Oncology.2024; 15(S4): 606. CrossRef - Cytologic hallmarks and differential diagnosis of papillary thyroid carcinoma subtypes
Agnes Stephanie Harahap, Chan Kwon Jung
Journal of Pathology and Translational Medicine.2024; 58(6): 265. CrossRef - Preoperative evaluation of thyroid nodules – Diagnosis and management strategies
Tapoi Dana Antonia, Lambrescu Ioana Maria, Gheorghisan-Galateanu Ancuta-Augustina
Pathology - Research and Practice.2023; 246: 154516. CrossRef - Reevaluating diagnostic categories and associated malignancy risks in thyroid core needle biopsy
Chan Kwon Jung
Journal of Pathology and Translational Medicine.2023; 57(4): 208. CrossRef - Strategies for Treatment of Thyroid Cancer
Deepika Yadav, Pramod Kumar Sharma, Rishabha Malviya, Prem Shankar Mishra
Current Drug Targets.2023; 24(5): 406. CrossRef - Identification of NIFTP-Specific mRNA Markers for Reliable Molecular Diagnosis of Thyroid Tumors
So-Yeon Lee, Jong-Lyul Park, Kwangsoon Kim, Ja Seong Bae, Jae-Yoon Kim, Seon-Young Kim, Chan Kwon Jung
Endocrine Pathology.2023; 34(3): 311. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

Fig. 1.
| Initial criteria [2] | Revised criteria [3] | The 2022 WHO classification [4] | ||||
|---|---|---|---|---|---|---|
| 1. Encapsulation or clear demarcation | 1. Primary | Essential | ||||
| 2. Follicular growth pattern with | Encapsulation or clear demarcation | 1. Encapsulation or clear demarcation | ||||
| < 1% papillae | Follicular growth pattern with no papillae | 2. Follicular growth pattern with | ||||
| No psammoma bodies | No psammoma bodies | < 1% true papillae | ||||
| < 30% solid/trabecular/insular growth pattern | <30% solid/trabecular/insular growth pattern | No psammoma bodies | ||||
| 3. Nuclear score 2–3 | Nuclear score of 2–3 | < 30% solid/trabecular/insular growth | ||||
| 4. No vascular or capsular invasion | No vascular or capsular invasion | 3. Nuclear score of 2–3 | ||||
| 5. No tumor necrosis | No tumor necrosis or high mitotic activity (3 or more mitoses per 10 high power fields) | 4. No vascular or capsular invasion | ||||
| 6. No high mitotic activity | 2. Secondary | 5. No tumor necrosis | ||||
| Lack of BRAF V600E mutation detected by molecular assays or immunohistochemistry | 6. Low mitotic count (< 3 mitoses/2 mm2) | |||||
| Lack of BRAF V600E-like mutations or other high-risk mutations (TERT, TP53) | 7. Lack of cytoarchitectural features of papillary carcinoma variants other than follicular variant | |||||
| Desirable | ||||||
| Immunohistochemistry or molecular testing for BRAF and NRAS mutation | ||||||
| Reference | NIFTP vs. benign/FTA | NIFTP vs. FVPTC | NIFTP vs. cPTC | NIFTP/FVPTC vs. cPTC |
|---|---|---|---|---|
| Legesse et al. [14] | - | Less frequent PIs, marginal micronucleoli, irregular branching sheets, and linear arrangement in NIFTP | Absence of PIs/Less frequent MNGs in NIFTP | - |
| Bizzarro et al. [26] | More frequent scant cytoplasm, NE, nuclear elongation, chromatin clearing, grooves, and membrane irregularities in NIFTP | Less frequent grooves in NIFTP | Less frequent papillae, NE, PIs, grooves, and membrane irregularities in NIFTP | - |
| Brandler et al. [27] | More frequent chromatin clearing, crowding, and NE in NIFTP | - | Less frequent PIs, papillae, crowding, NE, membrane irregularities, chromatin clearing, calcifications, and MNGs in NIFTP | - |
| Chandler et al. [28] | - | More frequent MF predominance/Less frequent nuclear elongation, grooves, and Pis in NIFTP | - | |
| Diaz Del Arco et al. [29] | - | Less frequent nuclear folds in NIFTP | More frequent bidimensional groups and MFs/Less frequent papillary or pseudopapillary architecture, tridimensionality, MNGs, and nuclear folds in NIFTP | - |
| Koshkikawa et al. [30] | - | No differences | - | More frequent MFs, and dense globules of colloids/less frequent PIs, true papillary cell clusters, monolayered cell sheets, ropy colloids, MNGs, psammoma bodies, and cystic background in NIFTP and FVPTC |
| Howitt et al. [31] | - | - | More frequent MFs/Less frequent sheet pattern in NIFTP | - |
| Mahajan et al. [32] | - | No differences in nuclear features/Less frequent 3-dimensional fragments in NIFTP | Less frequent PIs, nuclear score of 3, and diffuse nuclear change in NIFTP | - |
| Selvaggi et al. [34] | - | Less frequent MNGs in NIFTP | - | - |
| Maletta et al. [35] | More frequent NE, membrane irregularities, chromatin clearing, and nuclear molding in NIFTP | No differences | - | - |
| Strickland et al. [33] | - | - | - | MF predominance without papillae, PIs or psammoma bodies in NIFTP and FVPTC |
| Diagnostic category | Proportion (%) (95% CI) | Change in ROM (%) (95% CI) |
|---|---|---|
| I. Nondiagnostic | 1.3 (0.8 to 1.7) | –2.4 (–7.5 to 2.7) |
| II. Benign | 8.9 (6.9 to 10.8) | –2.7 (–4.1 to –1.3) |
| III. AUS/FLUS | 29.2 (25.0 to 33.4) | –8.2 (–11.2 to –5.1) |
| IV. FN/SFN | 24.2 (19.6 to 28.9) | –8.2 (–12.3 to –4.1) |
| V. Suspicious for malignancy | 19.5 (16.1 to 22.9) | –7.3 (–9.6 to –5.1) |
| VI. Malignant | 6.9 (5.2 to 8.7) | –1.1 (–1.6 to –0.5) |
WHO, World Health Organization.
NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; FTA, follicular thyroid adenoma; FVPTC, follicular variant papillary thyroid carcinoma; cPTC, conventional papillary thyroid carcinoma; PI, pseudoinclusion; MNG, multinucleated giant cell; NE, nucelar enlargement; MF, microfollicle.
CI, confidence interval; ROM, risk of malignancy; AUS/FLUS, atypia of undetermined significance/follicular lesion of undetermined significance; FN/SFN, follicular neoplasm/suspicious for a follicular neoplasm.

 E-submission
E-submission