Significance of tumor-associated neutrophils, lymphocytes, and neutrophil-to-lymphocyte ratio in non-invasive and invasive bladder urothelial carcinoma
Article information
Abstract
Background
Tumor-infiltrating neutrophils and lymphocytes play essential roles in promoting or combating various neoplasms. This study aimed to investigate the association between tumor-infiltrating neutrophils and lymphocytes and the neutrophil-to-lymphocyte ratio in the progression of urothelial carcinoma.
Methods
A total of 106 patients diagnosed with urothelial carcinoma were was. Pathological examination for tumor grade and stage and for tumor-infiltrating neutrophils, both CD4 and CD8+ T lymphocytes, as well as the neutrophil-to-lymphocyte ratio were evaluated.
Results
The presence of neutrophils and the neutrophil-to-lymphocyte ratio correlated with high-grade urothelial neoplasms. In both low- and high-grade tumors, the lymphocytes increased during progression from a non-invasive neoplasm to an early-invasive neoplasm. CD8+ T lymphocytes increased in low-grade non–muscle-invasive tumors compared to non-invasive tumors. Additionally, there was a significant decrease in CD8+ T lymphocytes during progression to muscle-invasive tumors.
Conclusions
Our results suggest that tumor-infiltrating neutrophils and CD8+ T lymphocytes have a significant effect on tumor grade and progression.
Bladder cancer accounts for 3% of global cancer diagnoses and is estimated to be the 10th most common cancer worldwide [1]. Urothelial carcinoma is the most common type of bladder cancer, constituting up to 95% of bladder malignancies [2]. Most patients with urothelial carcinoma present with either non-invasive papillary neoplasms or superficially invasive tumors limited to the mucosa and submucosa. These neoplasms do not reach the muscle layer (i.e., they constitute non–muscle-invasive urothelial carcinoma [NMIUC; pTa or pT1]). For such neoplasms, transurethral resection of the bladder tumor (TURBT) is followed by adjuvant intravesical instillation therapy [3,4]. However, approximately 30% of patients present with muscle invasion (i.e., muscle-invasive urothelial carcinoma [MIUC]; pT2) [5]. The five-year survival rate of NMIUC ranges from 50% to 70%, whereas that with MIUC, despite radical cystectomy and chemotherapy, is only 30%–40% [4,5]. Moreover, NMIUC progresses to MIUC in approximately 43% of patients [6]. Thus, despite advancements in the management of bladder carcinomas, the outcomes have remained largely unchanged over several decades [7], highlighting the need to identify additional pathological parameters and biomarkers that could affect carcinogenesis and help improve management procedures [8].
The link between tumor microenvironment inflammation and tumor progression has been reported in several malignancies [9–11]. Neutrophils and lymphocytes are the main inflammatory cells observed in the tumor microenvironment [12]. Several studies have highlighted the significance of elevated neutrophil-to-lymphocyte ratio (NLR) in patients with urothelial carcinoma and its association with a higher risk of recurrence and progression in NMIUC, as well as increased mortality and decreased overall survival in patients with MIUC [13,14]. Moreover, tumor-associated lymphocytes (TALs) exhibit diverse functions in various subsets. CD8 T lymphocytes are primarily responsible for attacking tumor cells [15,16]. Meanwhile, CD4 T lymphocytes are considered a double-edged immunologic sword: they can initiate and maintain CD8 lymphocyte anti-cancer immune responses [17] but can also convert anti-tumor activity to pro-tumor activity [18]. This study investigated the presence of tumor-associated neutrophils (TANs) and TALs, both CD4 and CD8, in both NMIUC and MIUC and their relation to well-known clinical and pathological prognostic parameters.
MATERIALS AND METHODS
Patients and specimens
This study included 106 patients pathologically diagnosed with urothelial bladder neoplasm by TURBT at Suez Canal University and Ismailia Oncology Hospitals from December 2021 to May 2022. Only cases with bladder urothelial neoplasms were selected. Clinical and pathological data of age, sex, tumor size (maximum diameter of the tumor) and number, pathological grade, and pTNM stage were recorded. The tumors were classified and graded according to the 2016 World Health Organization/International Society of Urological Pathology classification [19].
Histopathological evaluation
The samples were fixed with 10% formalin and embedded in paraffin. From each block, histological sections of 3-μm thickness were submitted, mounted to a glass slide, stained by hematoxylin and eosin, and reviewed to confirm the diagnosis of urothelial bladder neoplasm and to identify tumor grade, invasion depth, and presence of lympho-vascular invasion. Moreover, TANs and TALs were identified.
Immunohistochemical staining
Sections from the selected paraffin blocks were cut into 4-μm-thick sections for immunohistochemical (IHC) staining. Slides were prepared and incubated with primary anti-CD8 antibody (anti-CD8 alpha; ab4055, Abcam, Cambridge, UK) and anti-CD4 antibody (anti-CD4; ab133616, Abcam) to further sub-classify the tumor-infiltrating lymphocytes (TILs). This was followed by incubation with the appropriate secondary antibody (anti-rabbit IgG; ab205718, Abcam). All slides were lightly counterstained with hematoxylin for 30 seconds prior to dehydration and mounting.
Histopathological and IHC scoring
TANs or TALs were defined as any neutrophils or lymphocytes that were in close proximity to the tumor base in non-invasive neoplasms or between tumor nests in invasive neoplasms. Four fields from the tumor were selected under low magnification (×100), and the neutrophils and lymphocytes were counted at high magnification (× 400) using 2D image analysis software (Olympus CellSense, Tokyo, Japan) on an Olympus BX-46 microscope equipped with an Olympus SC30 digital camera. Care was taken not to count such inflammatory cells in areas of ulceration or erosion. Then, the average number was calculated and scored. TANs and TALs (either CD4 or CD8 T lymphocytes) were identified in the lamina propria just beneath the lower margin of the non-invasive urothelial neoplasm or infiltrated in the cancer nests or stroma.
Statistical analysis and data interpretation
Data were fed to a computer and analyzed using IBM SPSS Statistics for Windows ver. 27.0, released 2020 (IBM Corp., Armonk, NY). Qualitative data were described using number and percentage. The quantitative data were described using median (minimum and maximum) and interquartile range (IQR) for non-parametric data and mean and standard deviation for parametric data after determining normality using the Kolmogrov-Smirnov test. Significance of the obtained results was assigned at the (0.05) level.
Data analysis
Qualitative data were analyzed using chi-square test. If more than 25% of cells had a count less than 5 in the tables, the Monte Carlo test was used. Fisher exact test was employed when more than 25% of cells had a count less than five in 2 × 2 tables. The Mann-Whitney U-test was used to compare two independent groups.
RESULTS
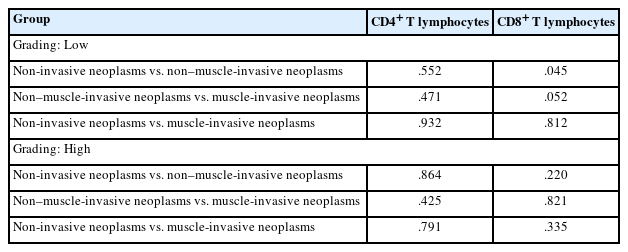
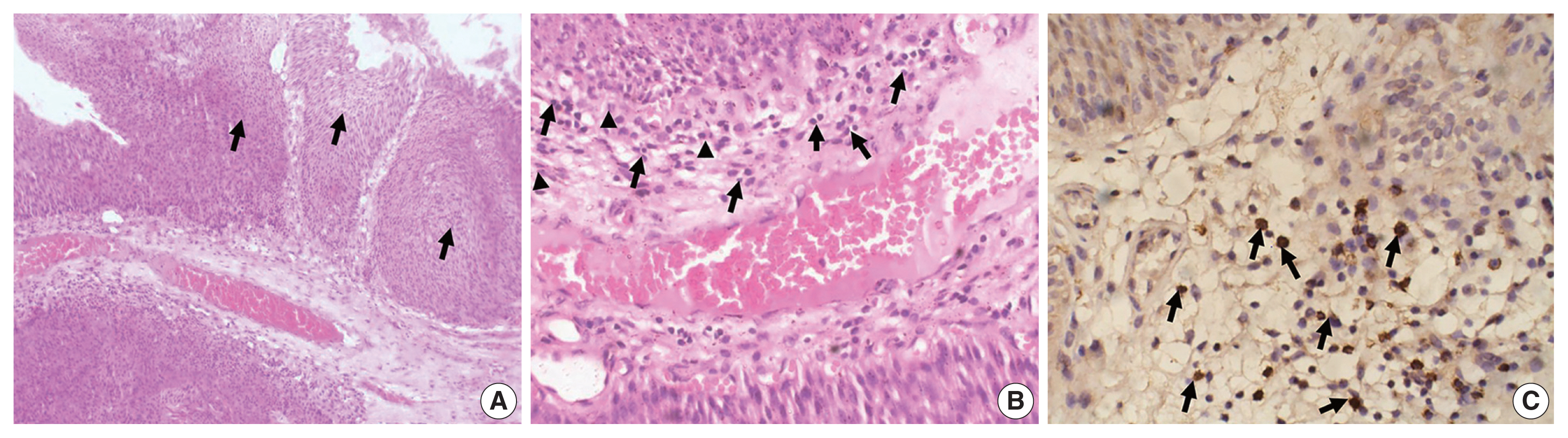
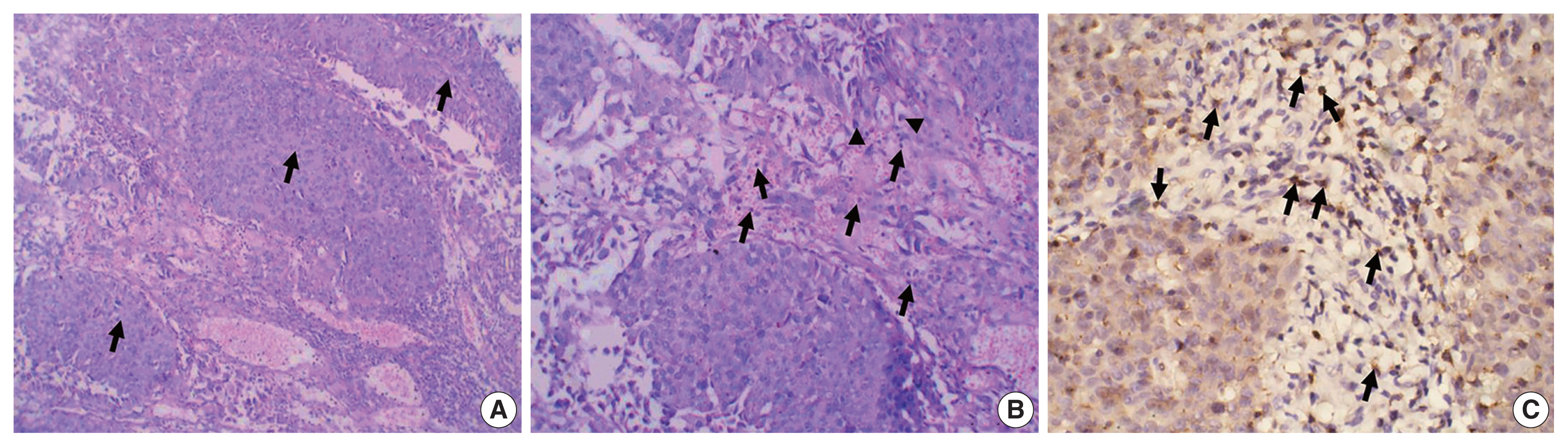
The study included 106 patients with a median age of 58 years and an IQR of 49–66 years. There were 60 males (56.6%) and 46 females (43.4%) who were classified according to the degree of invasion into three groups (Table 1): group 1, non-invasive urothelial carcinoma (NIUC) (36 patients, 34%) (Fig. 1A); group 2, non–muscle-invasive urothelial carcinoma (NMIUC) (38 patients, 35.8%) (Fig. 2A); group 3, MIUC (32 patients, 30.2%) (Fig. 3A).

Numbers of tumor-associated neutrophils and lymphocytes and NLR in the studied groups classified according to tumor grade and state of tumor invasiveness
Tumor-associated neutrophils and CD8+ lymphocytes in non-invasive urothelial carcinoma. (A) Representative hematoxylin and eosin (H&E)-stained section of a low-grade papillary (arrows) non-invasive urothelial carcinoma. (B) Higher magnification of the base of the tumor reveals an abundance of lymphocytes (arrows) with few neutrophils (arrowheads). (C) Immunohistochemically, the lymphocytes express CD8 protein (arrows).
Tumor-associated neutrophils and CD8+ lymphocytes in non–muscle-invasive urothelial carcinoma. (A) Representative hematoxylin and eosin–stained section of a lamina propria (non-muscle) invasive urothelial carcinoma. Tumor tissue grows as nests of malignant urothelial cells (arrows) infiltrating the lamina propria. (B) Higher magnification of the tumor stroma reveals an abundance of lymphocytes (arrows), with few neutrophils (arrowheads). (C) Immunohistochemically, many lymphocytes express CD8 protein (arrows).
Tumor-associated neutrophils and CD8+ lymphocytes in muscle-invasive urothelial carcinoma. (A) Representative hematoxylin and eosin–stained section of muscle-invasive urothelial carcinoma. Tumor tissue grows as nests of malignant urothelial cells (black arrows) infiltrating the muscle layer (red arrows). (B) Higher magnification of the tumor stroma reveals an abundance of neutrophils (black arrows) with few lymphocytes (arrowheads). Immunohistochemically (C), few lymphocytes express CD8 protein (arrowheads).
The patients were further classified according to tumor grade: low-grade urothelial carcinoma (UC) (42 patients, 39.6%), high-grade UC (64 patients, 60.4%).
Significance of neutrophils
The presence of neutrophils correlated with high-grade urothelial neoplasms. Specifically, there was a significant increase in neutrophil number in high-grade UC cases compared to low-grade cases (p = .005) (Table 2).
We found a significant increase in the number of neutrophils in the MIUC (Fig. 3B) compared to the NIUC (Fig. 1B) in high-grade neoplasms (p = .012). Similarly, there was a significant increase in neutrophils in the MIUC (Fig. 3B) compared to the NMIUC (Fig. 2B) in high-grade neoplasms (p = .053) (Table 2).
On the other hand, the number of neutrophils increased in the NMIUC (Fig. 2B) compared to the NIUC (Fig. 1B) in both low- and high-grade neoplasms; however, the increase was not statistically significant (p = .851 and p = .570, respectively) (Table 2).
Significance of lymphocytes
The presence of lymphocytes correlated more significantly with progressive invasion of urothelial neoplasms than with neoplasm grade. Nevertheless, there was a decrease in total lymphocyte count in high-grade neoplasms compared to low-grade neoplasms. However, the decrease was not statistically significant (p = .193).
Moreover, we identified a significant increase in lymphocytes during progression from NIUC (Fig. 1B) to NMIUC (Fig. 2B) in both low- and high-grade neoplasms (p = .007 and p = .013, respectively). Additionally, immunohistochemistry showed that the number of CD8+ lymphocytes in the low-grade neoplasms was significantly increased (p = .045) in the NMIUC group (Fig. 2C) compared to the NIUC group (Table 2, Fig. 1C). Furthermore, we found a significant increase in the lymphocyte count in the MIUC group (Fig. 3B) compared to the NIUC group (Fig. 1B) in both low- and high-grade neoplasms (p = .052 and p = .005, respectively) (Table 2).
On the other hand, when comparing MIUC (Fig. 3B) with NMIUC (Fig. 2B), there was a decrease in lymphocyte number, although it was not statistically significant in either low- or high-grade neoplasms (p = .315 and p = .913, respectively) (Table 2). However, there was a significant decrease in CD8+ lymphocytes in low-grade MIUC (Fig. 3C) compared to low-grade NMIUC (p = .052) (Table 3, Fig. 2C).
Significance of neutrophil-lymphocyte ratio
The NLR correlated with tumor grade; specifically, there was a significant increase in the ratio in high-grade urothelial neoplasms compared to low-grade neoplasms (p = .003) (Table 2). We found a significant increase in the NLR in the MIUC cases compared to the NMIUC cases in the high-grade neoplasms (p = .041), whereas we found no significant change in the ratio in the low-grade neoplasms (Table 2).
Meanwhile, there was a non-significant decrease in the NLR in the NMIUC cases compared to the NIUC cases in both low- and high-grade neoplasms (p = .152 and p = .232, respectively) (Table 2). Similarly, there was a non-significant decrease in the NLR when comparing the NIUC group to the MIUC group in both low- and high-grade neoplasms (p = .128 and p = .792, respectively) (Table 2).
DISCUSSION
Inflammation within a tumor microenvironment plays an important role in tumor progression. Neutrophils are recognized for their antimicrobial activities and are found in many types of tumors. Early studies have suggested that TANs, which are short-lived, have no role in cancer progression. However, it has recently become evident that TANs with associated inflammation play a significant role in malignancy progression [20]. Neutrophils within tumor nests can induce anti-tumoral immune memory. Alternatively, they may have a pro-tumoral phenotype that promotes angiogenesis, invasion, metastasis, and immunosuppression [21]. This study revealed that the presence of neutrophils correlated with high-grade urothelial neoplasms, as there was a significant increase in neutrophil count in high-grade UC cases compared to low-grade cases (p = .005). There was also a significant increase in the number of neutrophils in MIUC cases compared to NIUC cases in high-grade neoplasms (p = .012). Similarly, there was a significant increase in neutrophil count in MIUC cases compared to NMIUC cases in high-grade neoplasms (p = .053).
The increased number of neutrophils in high-grade tumors and deeply invasive tumors may suggest that TANs are related to a poorer prognosis. Liu et al. [21] revealed similar results in their study on localized bladder cancer; i.e., an increased count of TANs was related to deeper tumor invasion and higher grade. This finding coincided with many studies on various tumor types in different organs, such as renal cell carcinoma, head and neck squamous cell carcinoma, and pancreatic adenocarcinoma [22–24]. Such pro-tumor activity of neutrophils could be attributed to released matrix of metalloproteinase 9, which frees vascular endothelial growth factor from the extracellular matrix to enhance angiogenesis, and to secreted arginase 1, which suppresses CD8 T lymphocytes. Moreover, TANs generate reactive oxygen species, which induce tumor progression [25,26]. Similarly, TALs have a dual regulatory role as they can induce an anti-tumor immune response by inhibiting tumor growth and tumor progression by creating a microenvironment that stimulates tumor outgrowth [27].
We found a significant increase in lymphocytes during progression from NIUC to NMIUC in both low- and high-grade neoplasms. This finding coincides with a previous study that reported an association between the adaptive immune response and tumor progression [28,29]. Regarding the lymphocyte population, we found that the number of CD8+ lymphocytes in low-grade neoplasms was significantly increased in the NMIUC group compared to the NIUC group. Similarly, Pichler et al. [30] and Masson-Lecomte et al. [31] reported an increased number of CD8+ lymphocytes in T1 bladder cancer compared to Ta bladder cancer. Moreover, we found a significant decrease in CD8+ lymphocytes in low-grade MIUC compared to low-grade NMIUC, indicating a possible role of CD8+ lymphocytes in hindering the progression to muscle invasion. This finding coincides with a recent review that evaluated the prognostic role of CD8+ TILs in cancer patients treated with immune checkpoint inhibitors. That review showed that CD8+ T cells at the invasive margin of tumors were negatively correlated with depth of invasion, and that high CD8+ TALs led to a 48% reduction in risk of disease progression compared with low CD8+ TILs [32].
On the other hand, Faraj et al. [33] found no significant relation between CD8-expressing T lymphocytes and any clinicopathological parameters; however, they did report a significant correlation with overall survival and disease-specific survival. Unlike our results, Hulsen et al. [34] found significantly higher values for CD8+ T-cell infiltration in grade 3 tumors. They also found that increased CD8 expression was related to decreased survival and increased recurrence and was associated with poor prognosis. They only included specimens were of patients diagnosed with T1 urothelial carcinoma, preventing comparison between tumor stages or relation of their findings to tumor progression [34]. Regarding CD4+ lymphocytes, we could not find a significant relation between their number and any of the groups studied. Similar results were obtained by previous studies, which could not show an association between CD4+ T cell density and any of the studied clinicopathological variables, including tumor stage and histological grade [35–37]. The relation between neutrophil counts in blood and lymphocytes has been correlated to clinicopathological parameters and has been suggested as a prognostic factor for urothelial carcinoma in many studies [38–40].
In the present study, we investigated the NLR at the tissue level. We found that the ratio correlated with tumor grade, as there was a significant increase in the ratio in high-grade urothelial neoplasms compared to low-grade neoplasms. We found a significant increase in this ratio in the MIUC cases compared to the NMIUC cases in high-grade neoplasms, confirming the tumor-promoting effect of neutrophils. It has been suggested that TANs are different from circulating neutrophils. Specifically, cytokines within the tumor microenvironment induce a population of neutrophils that have a pro-tumor phenotype that inhibits CD8+ T cells, and its tumor promotion increases with tumor progression [20]. However, Mandelli et al. [41] found no significant association between the tissue NLR and any of the studied clinicopathological variables, including tumor stage and grade. Such a discrepancy in the results highlights the complexity of the inflammatory response in urothelial carcinogenesis, and that other factors can potentiate or attenuate the role of inflammatory cells as anti- or pro-tumor cells. The importance of TALs is clearly demonstrated by the relation between elevated expression of programmed death-ligand 1 by tumor cells and higher TIL density, as well as by the association with higher histological grade and higher pT category [42].
In summary, this study highlighted the significance of inflammatory cells within the tumor environment of bladder urothelial carcinoma. TANs correlated with tumor grade and stage, whereas TALs, especially CD8+ T cells, and the NLR were more likely to be associated with progression of tumor invasion rather than tumor grade. Further prospective multicenter studies with prolonged follow-up are recommended to confirm our results and to elucidate the prognostic role of inflammatory cells in the progression of urothelial carcinoma.
Notes
Ethics Statement
All procedures performed in the current study were approved by the Institutional Ethics Review Board of Suez Canal University (4687-5/12/2021) in accordance with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study. A copy of the written consent is available for review by the Editor-in-Chief of the journal.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: WAH. Data curation: AKE, NN, MEA, HL. Methodology: WAH, MEA, RIA. Project administration: WAH. Resources: WAH, NN, RIA. Supervision: WAH. Validation: WAH, RIA, NN. Visualization: HL, MEA, AKE. Writing—original draft: WAH, NN, RIA, MEA. Writing—review & editing: HL, AKE. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.