Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 57(2); 2023 > Article
-
Original Article
Significance of tumor-associated neutrophils, lymphocytes, and neutrophil-to-lymphocyte ratio in non-invasive and invasive bladder urothelial carcinoma -
Wael Abdo Hassan1,2
 , Ahmed Kamal ElBanna2,3
, Ahmed Kamal ElBanna2,3 , Noha Noufal1,4
, Noha Noufal1,4 , Mohamed El-Assmy5
, Mohamed El-Assmy5 , Hany Lotfy2,6
, Hany Lotfy2,6 , Rehab Ibrahim Ali1,7
, Rehab Ibrahim Ali1,7
-
Journal of Pathology and Translational Medicine 2023;57(2):88-94.
DOI: https://doi.org/10.4132/jptm.2022.11.06
Published online: January 10, 2023
1Department of Pathology, Faculty of Medicine, Suez Canal University, El Sheikh Zayed, Egypt
2Department of Basic Sciences, College of Medicine, Suliman Al Rajhi University, Al Bukairiyah, Saudi Arabia
3Department of Anatomy, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
4Department of Basic Medical Sciences, College of Medicine, Dar Al Uloom University, Riyadh, Saudi Arabia
5Department of Clinical Sciences, Suliman Al Rajhi University, Bukayriah, Saudi Arabia
6Department of Microbiology and Immunology, Faculty of Medicine, Mansoura University, Mansoura, Egypt
7Department of Pathology, College of Medicine, Jouf University, Al-Jawf, Saudi Arabia
- Corresponding Author: Wael Abdo Hassan, PhD, Department of Basic Sciences, College of Medicine, Sulaiman Al Rajhi University, Al Bukairiyah 51941, PO Box 777, Saudi Arabia, Tel: +966-507091876, Fax: +966-163169090, E-mail: w.hassan@sr.edu.sa
© 2023 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Tumor-infiltrating neutrophils and lymphocytes play essential roles in promoting or combating various neoplasms. This study aimed to investigate the association between tumor-infiltrating neutrophils and lymphocytes and the neutrophil-to-lymphocyte ratio in the progression of urothelial carcinoma.
-
Methods
- A total of 106 patients diagnosed with urothelial carcinoma were was. Pathological examination for tumor grade and stage and for tumor-infiltrating neutrophils, both CD4 and CD8+ T lymphocytes, as well as the neutrophil-to-lymphocyte ratio were evaluated.
-
Results
- The presence of neutrophils and the neutrophil-to-lymphocyte ratio correlated with high-grade urothelial neoplasms. In both low- and high-grade tumors, the lymphocytes increased during progression from a non-invasive neoplasm to an early-invasive neoplasm. CD8+ T lymphocytes increased in low-grade non–muscle-invasive tumors compared to non-invasive tumors. Additionally, there was a significant decrease in CD8+ T lymphocytes during progression to muscle-invasive tumors.
-
Conclusions
- Our results suggest that tumor-infiltrating neutrophils and CD8+ T lymphocytes have a significant effect on tumor grade and progression.
- Patients and specimens
- This study included 106 patients pathologically diagnosed with urothelial bladder neoplasm by TURBT at Suez Canal University and Ismailia Oncology Hospitals from December 2021 to May 2022. Only cases with bladder urothelial neoplasms were selected. Clinical and pathological data of age, sex, tumor size (maximum diameter of the tumor) and number, pathological grade, and pTNM stage were recorded. The tumors were classified and graded according to the 2016 World Health Organization/International Society of Urological Pathology classification [19].
- Histopathological evaluation
- The samples were fixed with 10% formalin and embedded in paraffin. From each block, histological sections of 3-μm thickness were submitted, mounted to a glass slide, stained by hematoxylin and eosin, and reviewed to confirm the diagnosis of urothelial bladder neoplasm and to identify tumor grade, invasion depth, and presence of lympho-vascular invasion. Moreover, TANs and TALs were identified.
- Immunohistochemical staining
- Sections from the selected paraffin blocks were cut into 4-μm-thick sections for immunohistochemical (IHC) staining. Slides were prepared and incubated with primary anti-CD8 antibody (anti-CD8 alpha; ab4055, Abcam, Cambridge, UK) and anti-CD4 antibody (anti-CD4; ab133616, Abcam) to further sub-classify the tumor-infiltrating lymphocytes (TILs). This was followed by incubation with the appropriate secondary antibody (anti-rabbit IgG; ab205718, Abcam). All slides were lightly counterstained with hematoxylin for 30 seconds prior to dehydration and mounting.
- Histopathological and IHC scoring
- TANs or TALs were defined as any neutrophils or lymphocytes that were in close proximity to the tumor base in non-invasive neoplasms or between tumor nests in invasive neoplasms. Four fields from the tumor were selected under low magnification (×100), and the neutrophils and lymphocytes were counted at high magnification (× 400) using 2D image analysis software (Olympus CellSense, Tokyo, Japan) on an Olympus BX-46 microscope equipped with an Olympus SC30 digital camera. Care was taken not to count such inflammatory cells in areas of ulceration or erosion. Then, the average number was calculated and scored. TANs and TALs (either CD4 or CD8 T lymphocytes) were identified in the lamina propria just beneath the lower margin of the non-invasive urothelial neoplasm or infiltrated in the cancer nests or stroma.
- Statistical analysis and data interpretation
- Data were fed to a computer and analyzed using IBM SPSS Statistics for Windows ver. 27.0, released 2020 (IBM Corp., Armonk, NY). Qualitative data were described using number and percentage. The quantitative data were described using median (minimum and maximum) and interquartile range (IQR) for non-parametric data and mean and standard deviation for parametric data after determining normality using the Kolmogrov-Smirnov test. Significance of the obtained results was assigned at the (0.05) level.
- Data analysis
- Qualitative data were analyzed using chi-square test. If more than 25% of cells had a count less than 5 in the tables, the Monte Carlo test was used. Fisher exact test was employed when more than 25% of cells had a count less than five in 2 × 2 tables. The Mann-Whitney U-test was used to compare two independent groups.
MATERIALS AND METHODS
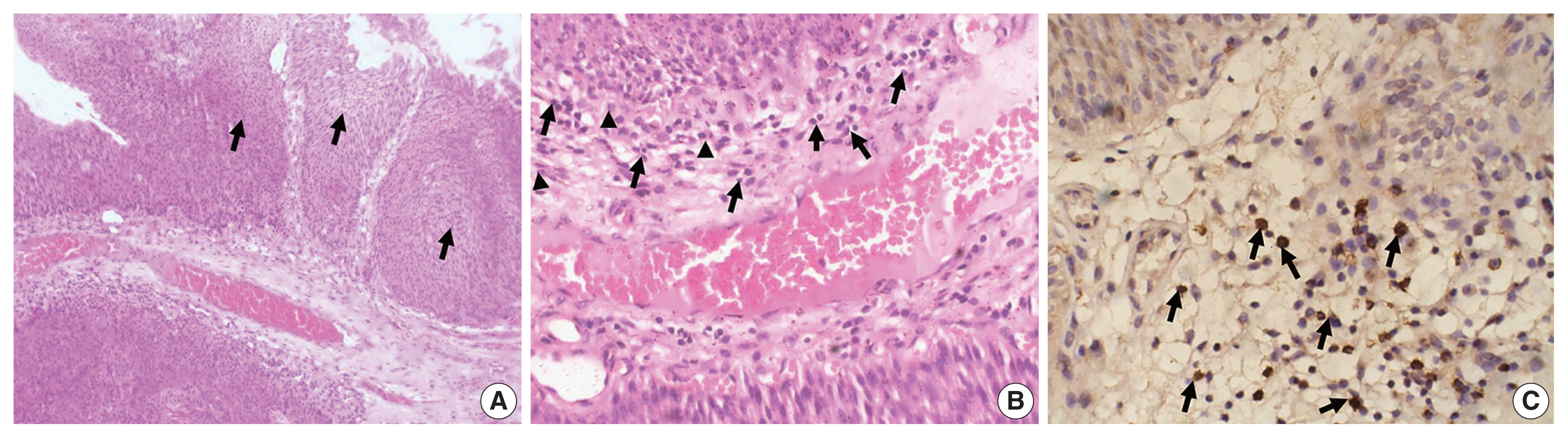
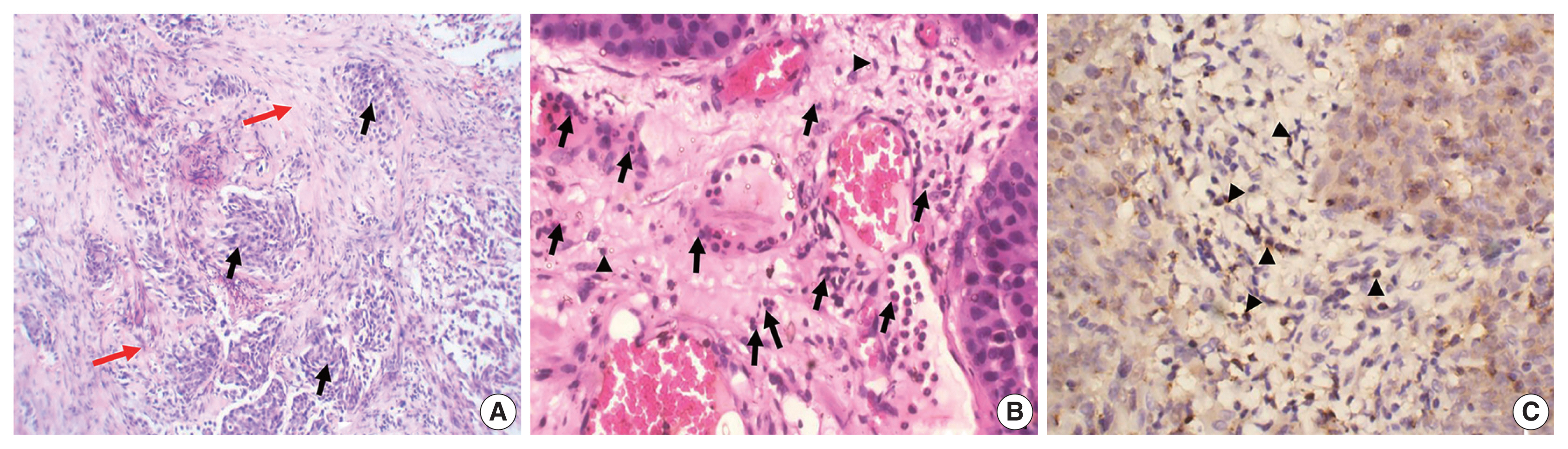
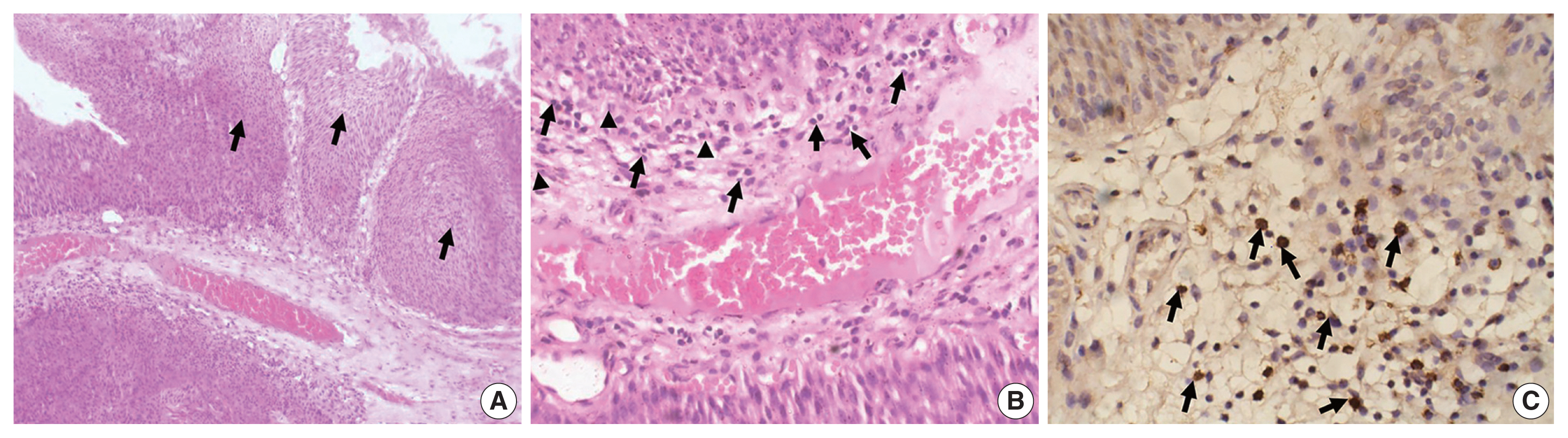
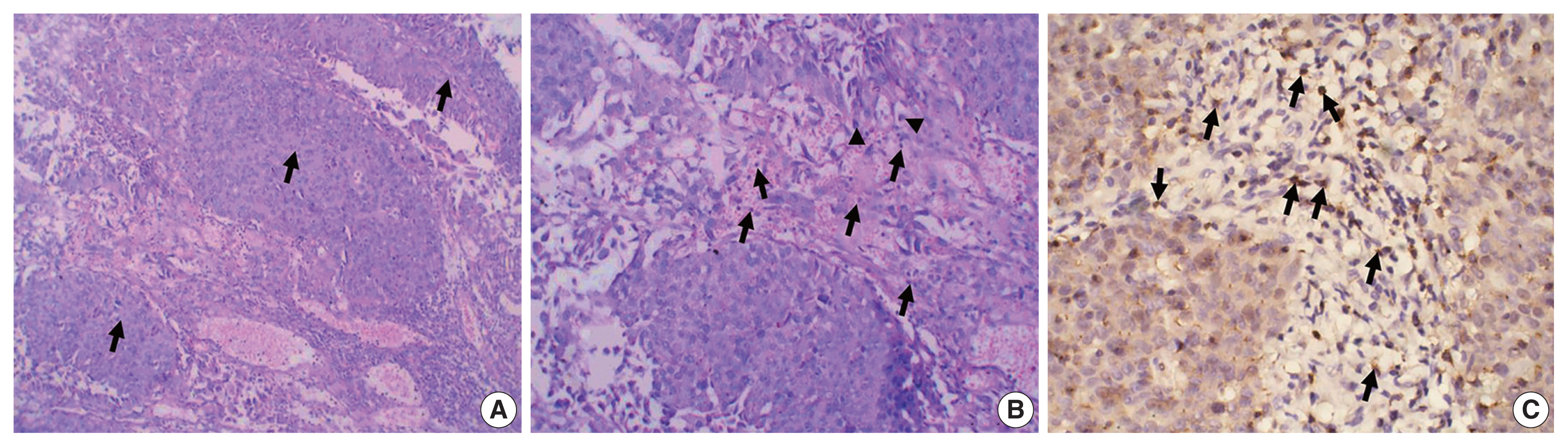
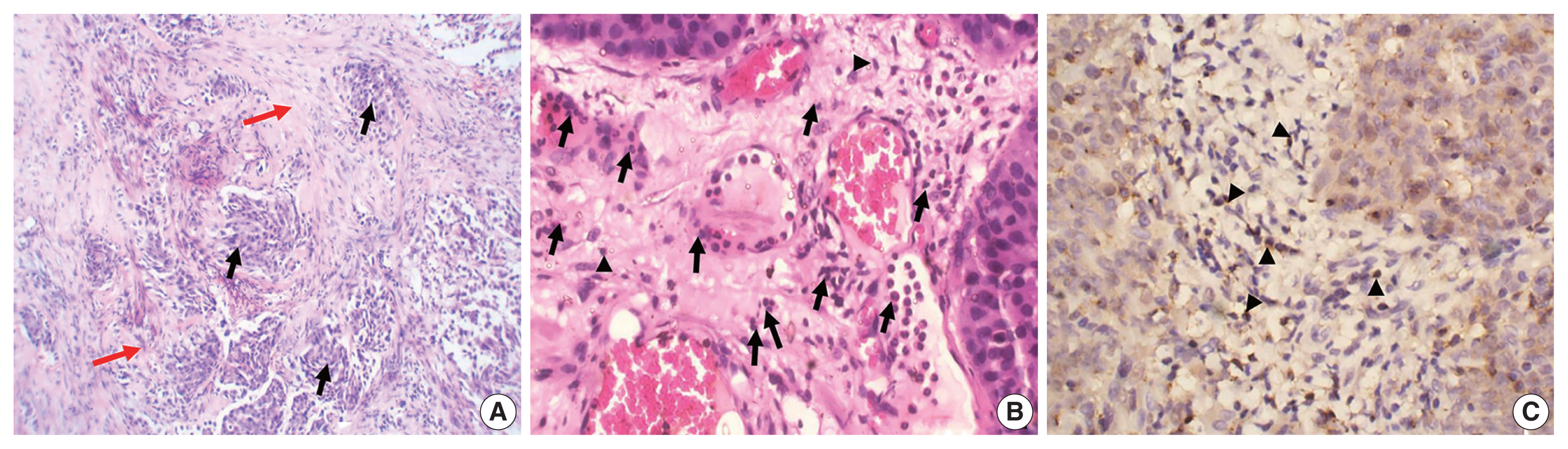
- The study included 106 patients with a median age of 58 years and an IQR of 49–66 years. There were 60 males (56.6%) and 46 females (43.4%) who were classified according to the degree of invasion into three groups (Table 1): group 1, non-invasive urothelial carcinoma (NIUC) (36 patients, 34%) (Fig. 1A); group 2, non–muscle-invasive urothelial carcinoma (NMIUC) (38 patients, 35.8%) (Fig. 2A); group 3, MIUC (32 patients, 30.2%) (Fig. 3A).
- The patients were further classified according to tumor grade: low-grade urothelial carcinoma (UC) (42 patients, 39.6%), high-grade UC (64 patients, 60.4%).
- Significance of neutrophils
- The presence of neutrophils correlated with high-grade urothelial neoplasms. Specifically, there was a significant increase in neutrophil number in high-grade UC cases compared to low-grade cases (p = .005) (Table 2).
- We found a significant increase in the number of neutrophils in the MIUC (Fig. 3B) compared to the NIUC (Fig. 1B) in high-grade neoplasms (p = .012). Similarly, there was a significant increase in neutrophils in the MIUC (Fig. 3B) compared to the NMIUC (Fig. 2B) in high-grade neoplasms (p = .053) (Table 2).
- On the other hand, the number of neutrophils increased in the NMIUC (Fig. 2B) compared to the NIUC (Fig. 1B) in both low- and high-grade neoplasms; however, the increase was not statistically significant (p = .851 and p = .570, respectively) (Table 2).
- Significance of lymphocytes
- The presence of lymphocytes correlated more significantly with progressive invasion of urothelial neoplasms than with neoplasm grade. Nevertheless, there was a decrease in total lymphocyte count in high-grade neoplasms compared to low-grade neoplasms. However, the decrease was not statistically significant (p = .193).
- Moreover, we identified a significant increase in lymphocytes during progression from NIUC (Fig. 1B) to NMIUC (Fig. 2B) in both low- and high-grade neoplasms (p = .007 and p = .013, respectively). Additionally, immunohistochemistry showed that the number of CD8+ lymphocytes in the low-grade neoplasms was significantly increased (p = .045) in the NMIUC group (Fig. 2C) compared to the NIUC group (Table 2, Fig. 1C). Furthermore, we found a significant increase in the lymphocyte count in the MIUC group (Fig. 3B) compared to the NIUC group (Fig. 1B) in both low- and high-grade neoplasms (p = .052 and p = .005, respectively) (Table 2).
- On the other hand, when comparing MIUC (Fig. 3B) with NMIUC (Fig. 2B), there was a decrease in lymphocyte number, although it was not statistically significant in either low- or high-grade neoplasms (p = .315 and p = .913, respectively) (Table 2). However, there was a significant decrease in CD8+ lymphocytes in low-grade MIUC (Fig. 3C) compared to low-grade NMIUC (p = .052) (Table 3, Fig. 2C).
- Significance of neutrophil-lymphocyte ratio
- The NLR correlated with tumor grade; specifically, there was a significant increase in the ratio in high-grade urothelial neoplasms compared to low-grade neoplasms (p = .003) (Table 2). We found a significant increase in the NLR in the MIUC cases compared to the NMIUC cases in the high-grade neoplasms (p = .041), whereas we found no significant change in the ratio in the low-grade neoplasms (Table 2).
- Meanwhile, there was a non-significant decrease in the NLR in the NMIUC cases compared to the NIUC cases in both low- and high-grade neoplasms (p = .152 and p = .232, respectively) (Table 2). Similarly, there was a non-significant decrease in the NLR when comparing the NIUC group to the MIUC group in both low- and high-grade neoplasms (p = .128 and p = .792, respectively) (Table 2).
RESULTS
- Inflammation within a tumor microenvironment plays an important role in tumor progression. Neutrophils are recognized for their antimicrobial activities and are found in many types of tumors. Early studies have suggested that TANs, which are short-lived, have no role in cancer progression. However, it has recently become evident that TANs with associated inflammation play a significant role in malignancy progression [20]. Neutrophils within tumor nests can induce anti-tumoral immune memory. Alternatively, they may have a pro-tumoral phenotype that promotes angiogenesis, invasion, metastasis, and immunosuppression [21]. This study revealed that the presence of neutrophils correlated with high-grade urothelial neoplasms, as there was a significant increase in neutrophil count in high-grade UC cases compared to low-grade cases (p = .005). There was also a significant increase in the number of neutrophils in MIUC cases compared to NIUC cases in high-grade neoplasms (p = .012). Similarly, there was a significant increase in neutrophil count in MIUC cases compared to NMIUC cases in high-grade neoplasms (p = .053).
- The increased number of neutrophils in high-grade tumors and deeply invasive tumors may suggest that TANs are related to a poorer prognosis. Liu et al. [21] revealed similar results in their study on localized bladder cancer; i.e., an increased count of TANs was related to deeper tumor invasion and higher grade. This finding coincided with many studies on various tumor types in different organs, such as renal cell carcinoma, head and neck squamous cell carcinoma, and pancreatic adenocarcinoma [22–24]. Such pro-tumor activity of neutrophils could be attributed to released matrix of metalloproteinase 9, which frees vascular endothelial growth factor from the extracellular matrix to enhance angiogenesis, and to secreted arginase 1, which suppresses CD8 T lymphocytes. Moreover, TANs generate reactive oxygen species, which induce tumor progression [25,26]. Similarly, TALs have a dual regulatory role as they can induce an anti-tumor immune response by inhibiting tumor growth and tumor progression by creating a microenvironment that stimulates tumor outgrowth [27].
- We found a significant increase in lymphocytes during progression from NIUC to NMIUC in both low- and high-grade neoplasms. This finding coincides with a previous study that reported an association between the adaptive immune response and tumor progression [28,29]. Regarding the lymphocyte population, we found that the number of CD8+ lymphocytes in low-grade neoplasms was significantly increased in the NMIUC group compared to the NIUC group. Similarly, Pichler et al. [30] and Masson-Lecomte et al. [31] reported an increased number of CD8+ lymphocytes in T1 bladder cancer compared to Ta bladder cancer. Moreover, we found a significant decrease in CD8+ lymphocytes in low-grade MIUC compared to low-grade NMIUC, indicating a possible role of CD8+ lymphocytes in hindering the progression to muscle invasion. This finding coincides with a recent review that evaluated the prognostic role of CD8+ TILs in cancer patients treated with immune checkpoint inhibitors. That review showed that CD8+ T cells at the invasive margin of tumors were negatively correlated with depth of invasion, and that high CD8+ TALs led to a 48% reduction in risk of disease progression compared with low CD8+ TILs [32].
- On the other hand, Faraj et al. [33] found no significant relation between CD8-expressing T lymphocytes and any clinicopathological parameters; however, they did report a significant correlation with overall survival and disease-specific survival. Unlike our results, Hulsen et al. [34] found significantly higher values for CD8+ T-cell infiltration in grade 3 tumors. They also found that increased CD8 expression was related to decreased survival and increased recurrence and was associated with poor prognosis. They only included specimens were of patients diagnosed with T1 urothelial carcinoma, preventing comparison between tumor stages or relation of their findings to tumor progression [34]. Regarding CD4+ lymphocytes, we could not find a significant relation between their number and any of the groups studied. Similar results were obtained by previous studies, which could not show an association between CD4+ T cell density and any of the studied clinicopathological variables, including tumor stage and histological grade [35–37]. The relation between neutrophil counts in blood and lymphocytes has been correlated to clinicopathological parameters and has been suggested as a prognostic factor for urothelial carcinoma in many studies [38–40].
- In the present study, we investigated the NLR at the tissue level. We found that the ratio correlated with tumor grade, as there was a significant increase in the ratio in high-grade urothelial neoplasms compared to low-grade neoplasms. We found a significant increase in this ratio in the MIUC cases compared to the NMIUC cases in high-grade neoplasms, confirming the tumor-promoting effect of neutrophils. It has been suggested that TANs are different from circulating neutrophils. Specifically, cytokines within the tumor microenvironment induce a population of neutrophils that have a pro-tumor phenotype that inhibits CD8+ T cells, and its tumor promotion increases with tumor progression [20]. However, Mandelli et al. [41] found no significant association between the tissue NLR and any of the studied clinicopathological variables, including tumor stage and grade. Such a discrepancy in the results highlights the complexity of the inflammatory response in urothelial carcinogenesis, and that other factors can potentiate or attenuate the role of inflammatory cells as anti- or pro-tumor cells. The importance of TALs is clearly demonstrated by the relation between elevated expression of programmed death-ligand 1 by tumor cells and higher TIL density, as well as by the association with higher histological grade and higher pT category [42].
- In summary, this study highlighted the significance of inflammatory cells within the tumor environment of bladder urothelial carcinoma. TANs correlated with tumor grade and stage, whereas TALs, especially CD8+ T cells, and the NLR were more likely to be associated with progression of tumor invasion rather than tumor grade. Further prospective multicenter studies with prolonged follow-up are recommended to confirm our results and to elucidate the prognostic role of inflammatory cells in the progression of urothelial carcinoma.
DISCUSSION
Ethics Statement
All procedures performed in the current study were approved by the Institutional Ethics Review Board of Suez Canal University (4687-5/12/2021) in accordance with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study. A copy of the written consent is available for review by the Editor-in-Chief of the journal.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: WAH. Data curation: AKE, NN, MEA, HL. Methodology: WAH, MEA, RIA. Project administration: WAH. Resources: WAH, NN, RIA. Supervision: WAH. Validation: WAH, RIA, NN. Visualization: HL, MEA, AKE. Writing—original draft: WAH, NN, RIA, MEA. Writing—review & editing: HL, AKE. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394-424. ArticlePubMedPDF
- 2. Miyazaki J, Nishiyama H. Epidemiology of urothelial carcinoma. Int J Urol 2017; 24: 730-4. ArticlePubMedPDF
- 3. Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet 2016; 388: 2796-810. ArticlePubMed
- 4. Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol 2016; 196: 1021-9. ArticlePubMed
- 5. Mathieu R, Lucca I, Klatte T, Babjuk M, Shariat SF. Trimodal therapy for invasive bladder cancer: is it really equal to radical cystectomy? Curr Opin Urol 2015; 25: 476-82. PubMed
- 6. Ceylan C, Doluoglu OG, Keles I, et al. Importance of the neutrophil-to-lymphocyte ratio in muscle-invasive and non-muscle invasive bladder tumors. Urologia 2014; 81: 120-4. ArticlePubMedPDF
- 7. Abdou Hassan W, Shalaby E, Abo-Hashesh M, Ibrahim Ali R. Evaluation of the expression of HER2 and c-KIT proteins as prognostic markers in superficial bladder urothelial carcinoma. Res Rep Urol 2021; 13: 197-206. PubMedPMC
- 8. Lobo N, Mount C, Omar K, Nair R, Thurairaja R, Khan MS. Landmarks in the treatment of muscle-invasive bladder cancer. Nat Rev Urol 2017; 14: 565-74. ArticlePubMedPDF
- 9. Gakis G, Todenhofer T, Stenzl A. The prognostic value of hematological and systemic inflammatory disorders in invasive bladder cancer. Curr Opin Urol 2011; 21: 428-33. ArticlePubMed
- 10. Schepisi G, Santoni M, Massari F, et al. Urothelial cancer: inflammatory mediators and implications for immunotherapy. BioDrugs 2016; 30: 263-73. ArticlePubMedPDF
- 11. Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. 2011: the immune hallmarks of cancer. Cancer Immunol Immunother 2011; 60: 319-26. ArticlePubMedPMC
- 12. Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol 2012; 30: 459-89. ArticlePubMed
- 13. Mano R, Baniel J, Shoshany O, et al. Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non-muscle-invasive bladder cancer. Urol Oncol 2015; 33: 67.Article
- 14. Gondo T, Nakashima J, Ohno Y, et al. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology 2012; 79: 1085-91. ArticlePubMed
- 15. Ho WY, Yee C, Greenberg PD. Adoptive therapy with CD8(+) T cells: it may get by with a little help from its friends. J Clin Invest 2002; 110: 1415-7. ArticlePubMedPMC
- 16. Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005; 102: 18538-43. ArticlePubMedPMC
- 17. Sheu BC, Hsu SM, Ho HN, Lin RH, Torng PL, Huang SC. Reversed CD4/CD8 ratios of tumor-infiltrating lymphocytes are correlated with the progression of human cervical carcinoma. Cancer 1999; 86: 1537-43. ArticlePubMed
- 18. Sharma MD, Hou DY, Liu Y, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood 2009; 113: 6102-11. ArticlePubMedPMCPDF
- 19. Comperat EM, Burger M, Gontero P, et al. Grading of urothelial carcinoma and the new “World Health Organisation classification of tumours of the urinary system and male genital organs 2016”. Eur Urol Focus 2019; 5: 457-66. ArticlePubMed
- 20. Uribe-Querol E, Rosales C. Neutrophils in cancer: two sides of the same coin. J Immunol Res 2015; 2015: 983698.ArticlePubMedPMCPDF
- 21. Liu K, Zhao K, Wang L, Sun E. The prognostic values of tumor-infiltrating neutrophils, lymphocytes and neutrophil/lymphocyte rates in bladder urothelial cancer. Pathol Res Pract 2018; 214: 1074-80. ArticlePubMed
- 22. Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol 2009; 27: 4709-17. ArticlePubMed
- 23. Trellakis S, Bruderek K, Dumitru CA, et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer 2011; 129: 2183-93. ArticlePubMed
- 24. Naso JR, Topham JT, Karasinska JM, et al. Tumor infiltrating neutrophils and gland formation predict overall survival and molecular subgroups in pancreatic ductal adenocarcinoma. Cancer Med 2021; 10: 1155-65. ArticlePubMedPMCPDF
- 25. Lin C, Lin W, Yeh S, Li L, Chang C. Infiltrating neutrophils increase bladder cancer cell invasion via modulation of androgen receptor (AR)/MMP13 signals. Oncotarget 2015; 6: 43081-9. ArticlePubMedPMC
- 26. Joseph M, Enting D. Immune responses in bladder cancer-role of immune cell populations, prognostic factors and therapeutic implications. Front Oncol 2019; 9: 1270.ArticlePubMedPMC
- 27. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011; 331: 1565-70. ArticlePubMed
- 28. Kim HS, Ku JH. Prognostic impact of tumor infiltrating lymphocytes in bladder urothelial carcinoma. Transl Androl Urol 2019; 8(Suppl 3):S291-2. ArticlePubMedPMC
- 29. Rouanne M, Betari R, Radulescu C, et al. Stromal lymphocyte infiltration is associated with tumour invasion depth but is not prognostic in high-grade T1 bladder cancer. Eur J Cancer 2019; 108: 111-9. ArticlePubMed
- 30. Pichler R, Fritz J, Zavadil C, Schafer G, Culig Z, Brunner A. Tumor-infiltrating immune cell subpopulations influence the oncologic outcome after intravesical bacillus Calmette-Guerin therapy in bladder cancer. Oncotarget 2016; 7: 39916-30. ArticlePubMedPMC
- 31. Masson-Lecomte A, Maille P, Pineda S, et al. CD8+ cytotoxic immune infiltrate in non-muscle invasive bladder cancer: a standardized methodology to study association with clinico-pathological features and prognosis. Bladder Cancer 2019; 5: 159-69. ArticlePubMedPMC
- 32. Li F, Li C, Cai X, et al. The association between CD8+ tumor-infiltrating lymphocytes and the clinical outcome of cancer immunotherapy: a systematic review and meta-analysis. EClinicalMedicine 2021; 41: 101134.ArticlePubMedPMC
- 33. Faraj SF, Munari E, Guner G, et al. Assessment of tumoral PD-L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology 2015; 85: 703.ArticlePubMedPMC
- 34. Hulsen S, Lippolis E, Ferrazzi F, et al. High stroma T-cell infiltration is associated with better survival in stage pT1 bladder cancer. Int J Mol Sci 2020; 21: 8407.ArticlePubMedPMC
- 35. Zhang Q, Hao C, Cheng G, et al. High CD4(+) T cell density is associated with poor prognosis in patients with non-muscle-invasive bladder cancer. Int J Clin Exp Pathol 2015; 8: 11510-6. PubMedPMC
- 36. Yu A, Mansure JJ, Solanki S, et al. Presence of lymphocytic infiltrate cytotoxic T lymphocyte CD3+, CD8+, and immunoscore as prognostic marker in patients after radical cystectomy. PLoS One 2018; 13: e0205746.ArticlePubMedPMC
- 37. Shi MJ, Meng XY, Wu QJ, Zhou XH. High CD3D/CD4 ratio predicts better survival in muscle-invasive bladder cancer. Cancer Manag Res 2019; 11: 2987-95. PubMedPMC
- 38. Favilla V, Castelli T, Urzi D, et al. Neutrophil to lymphocyte ratio, a biomarker in non-muscle invasive bladder cancer: a single-institutional longitudinal study. Int Braz J Urol 2016; 42: 685-93. ArticlePubMedPMC
- 39. Kawahara T, Furuya K, Nakamura M, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in bladder cancer patients after radical cystectomy. BMC Cancer 2016; 16: 185.ArticlePubMedPMC
- 40. Tazeh NN, Canter DJ, Damodaran S, et al. Neutrophil to lymphocyte ratio (NLR) at the time of transurethral eesection of bladder tumor: a large retrospective study and analysis of racial differences. Bladder Cancer 2017; 3: 89-94. ArticlePubMedPMCPDF
- 41. Mandelli GE, Missale F, Bresciani D, et al. Tumor infiltrating neutrophils are enriched in basal-type urothelial bladder cancer. Cells 2020; 9: 291.ArticlePubMedPMC
- 42. Nukui A, Kamai T, Arai K, et al. Association of cancer progression with elevated expression of programmed cell death protein 1 ligand 1 by upper tract urothelial carcinoma and increased tumor-infiltrating lymphocyte density. Cancer Immunol Immunother 2020; 69: 689-702. ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

- Prognostic role of the neutrophil/lymphocyte ratio in high‐risk BCG‐naïve non‐muscle‐invasive bladder cancer treated with intravesical gemcitabine/docetaxel
Mohamad Abou Chakra, Riitta Lassila, Nancy El Beayni, Sarah L. Mott, Michael A. O'Donnell
BJU International.2025; 135(1): 125. CrossRef - Understanding the Dual Role of Macrophages in Tumor Growth and Therapy: A Mechanistic Review
Muhammad Summer, Saima Riaz, Shaukat Ali, Qudsia Noor, Rimsha Ashraf, Rana Rashad Mahmood Khan
Chemistry & Biodiversity.2025;[Epub] CrossRef - Cross-Talk Between Cancer and Its Cellular Environment—A Role in Cancer Progression
Eliza Turlej, Aleksandra Domaradzka, Justyna Radzka, Dominika Drulis-Fajdasz, Julita Kulbacka, Agnieszka Gizak
Cells.2025; 14(6): 403. CrossRef - Global trends in tumor-associated neutrophil research: a bibliometric and visual analysis
Shaodong Li, Peng Dong, Xueliang Wu, Zhenhua Kang, Guoqiang Yan
Frontiers in Immunology.2025;[Epub] CrossRef - Tumor-associated neutrophils and neutrophil extracellular traps in lung cancer: antitumor/protumor insights and therapeutic implications
Milad Sheervalilou, Mostafa Ghanei, Masoud Arabfard
Medical Oncology.2025;[Epub] CrossRef - Construction of a column-line graphical model of poor outcome of neoadjuvant regimens for muscle-invasive bladder cancer based on NLR, dNLR and SII indicators
Bo Hu, Longsheng Wang, Shanna Qu, Tao Zhang
World Journal of Surgical Oncology.2025;[Epub] CrossRef - Machine Learning of Urine Cytology Highlights Increased Neutrophil Count in Muscle-Invasive Urothelial Carcinoma
Moe Kameda, Sayaka Kobayashi, Yoshimi Nishijima, Ryosuke Akuzawa, Rio Kaneko, Rio Shibanuma, Seiji Arai, Hayato Ikota, Kazuhiro Suzuki, Hideaki Yokoo, Masanao Saio
Journal of Cytology.2025; 42(3): 124. CrossRef - Tumor-Infiltrating Immune Cells in Non-Muscle-Invasive Bladder Cancer: Prognostic Implications, Predictive Value, and Future Perspectives
Roberta Mazzucchelli, Angelo Cormio, Magda Zanelli, Maurizio Zizzo, Andrea Palicelli, Andrea Benedetto Galosi, Francesca Sanguedolce
Applied Sciences.2025; 15(22): 12032. CrossRef - Immune cell networking in solid tumors: focus on macrophages and neutrophils
Irene Di Ceglie, Silvia Carnevale, Anna Rigatelli, Giovanna Grieco, Piera Molisso, Sebastien Jaillon
Frontiers in Immunology.2024;[Epub] CrossRef - Immunohistochemistry assessment of tissue neutrophil-to-lymphocyte ratio predicts outcomes in melanoma patients treated with anti-programmed cell death 1 therapy
Renan J. Teixeira, Vinícius G. de Souza, Bruna P. Sorroche, Victor G. Paes, Fabiana A. Zambuzi-Roberto, Caio A.D. Pereira, Vinicius L. Vazquez, Lidia M.R.B. Arantes
Melanoma Research.2024; 34(3): 234. CrossRef - Association between alteration of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, cancer antigen-125 and surgical outcomes in advanced stage ovarian cancer patient who received neoadjuvant chemotherapy
Ponganun Tuntinarawat, Ratnapat Tangmanomana, Thannaporn Kittisiam
Gynecologic Oncology Reports.2024; 52: 101347. CrossRef - Prognostic value of neutrophil-to-lymphocyte ratio in patients with non–muscle-invasive bladder cancer with intravesical Bacillus Calmette–Guérin immunotherapy: a systematic review and meta-analysis
Jiaguo Huang, Li Lin, Dikai Mao, Runmiao Hua, Feifei Guan
Frontiers in Immunology.2024;[Epub] CrossRef - Update on the Mechanism of Action of Intravesical BCG Therapy to Treat Non-Muscle-Invasive Bladder Cancer
Mohamad Abou Chakra, Yi Luo, Igor Duquesne, Michael A O'Donnell
Frontiers in Bioscience-Landmark.2024;[Epub] CrossRef - Significant association between high neutrophil-lymphocyte ratio and poor prognosis in patients with hepatocellular carcinoma: a systematic review and meta-analysis
Chunhua Xu, Fenfang Wu, Lailing Du, Yeping Dong, Shan Lin
Frontiers in Immunology.2023;[Epub] CrossRef - Chitinase 3-like-1 Expression in the Microenvironment Is Associated with Neutrophil Infiltration in Bladder Cancer
Ling-Yi Xiao, Yu-Li Su, Shih-Yu Huang, Yi-Hua Chen, Po-Ren Hsueh
International Journal of Molecular Sciences.2023; 24(21): 15990. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1
Fig. 2
Fig. 3
| Group | No. of cases | TAN (median) | TAL (median) | NLR |
|---|---|---|---|---|
| Grading: Low | ||||
| Non-invasive neoplasms | 12 | 5.5 | 13.5 | 0.6 |
| Non–muscle-invasive neoplasms | 18 | 8.0 | 41.0 | 0.8 |
| Muscle-invasive neoplasms | 12 | 4.0 | 35.5 | 1.7 |
| Grading: High | ||||
| Non-invasive neoplasms | 24 | 11.5 | 12.5 | 0.5 |
| Non–muscle-invasive neoplasms | 20 | 17.5 | 28.5 | 1.2 |
| Muscle-invasive neoplasms | 20 | 30.0 | 25.5 | 0.9 |
| Group | TAN | TAL | NLR |
|---|---|---|---|
| Grading: Low | |||
| Non-invasive neoplasms vs. non–muscle-invasive neoplasms | .851 | .007 | .152 |
| Non–muscle-invasive neoplasms vs. muscle-invasive neoplasms | .550 | .315 | > .999 |
| Non-invasive neoplasms vs. muscle-invasive neoplasms | .460 | .052 | .128 |
| Grading: High | |||
| Non-invasive neoplasms vs. non–muscle-invasive neoplasms | .570 | .013 | .232 |
| Non–muscle-invasive neoplasms vs. muscle-invasive neoplasms | .053 | .913 | .041 |
| Non-invasive neoplasms vs. muscle-invasive neoplasms | .012 | .005 | .792 |
| Group | CD4+ T lymphocytes | CD8+ T lymphocytes |
|---|---|---|
| Grading: Low | ||
| Non-invasive neoplasms vs. non–muscle-invasive neoplasms | .552 | .045 |
| Non–muscle-invasive neoplasms vs. muscle-invasive neoplasms | .471 | .052 |
| Non-invasive neoplasms vs. muscle-invasive neoplasms | .932 | .812 |
| Grading: High | ||
| Non-invasive neoplasms vs. non–muscle-invasive neoplasms | .864 | .220 |
| Non–muscle-invasive neoplasms vs. muscle-invasive neoplasms | .425 | .821 |
| Non-invasive neoplasms vs. muscle-invasive neoplasms | .791 | .335 |
NLR, neutrophil-to-lymphocyte ratio; TAN, tumor-associated neutrophils; TAL, tumor-associated lymphocytes.
Significant at p < .05. NLR, neutrophil-to-lymphocyte ratio; TAN, tumor-associated neutrophils; TAL, tumor-associated lymphocytes.
Significant at p < .05.

 E-submission
E-submission