Variation in mitotic counting and risk classification practices for gastrointestinal stromal tumors: a survey of pathologists in South Korea
Article information
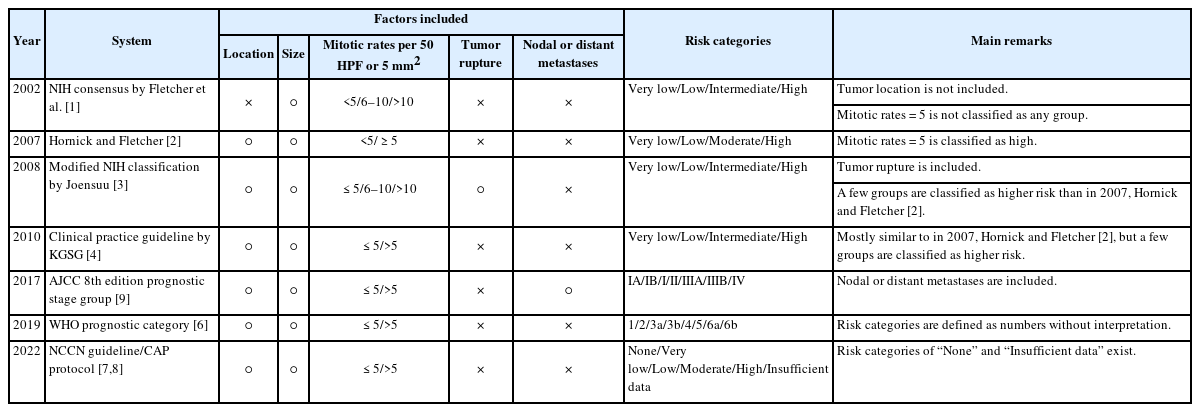
Gastrointestinal stromal tumors (GISTs) are among the most prevalent mesenchymal neoplasms of the gastrointestinal tract. Owing to their variable biological behavior, considerable efforts have been made to predict the prognosis of GIST. Although several risk classification systems have been proposed, most rely on three key parameters: anatomical site, tumor size, and mitotic rate [1-4].
Traditionally, the mitotic rate for GIST risk classification was determined by counting the number of mitoses in 50 high-power fields (HPFs) [5]. However, advances in microscopy have increased the HPF area, and the adoption of digital pathology has further shifted practice toward measuring mitoses in defined areas (mm2) rather than in HPFs. Additionally, measuring mitoses in a defined area using digital pathology offers advantages over light microscopy, as it allows for assessment within a perfectly continuous field without missing or overlapping regions. Consequently, current international guidelines, including the World Health Organization (WHO) classification [6], National Comprehensive Cancer Network (NCCN) [7], College of American Pathologists (CAP) Cancer Protocol [8], and the American Joint Committee on Cancer (AJCC) Cancer Staging Manual [9], recommend assessing the mitotic count within a 5 mm2 area, which corresponds to 50 HPFs in older microscopes, but only 20–25 HPFs in most modern microscopes.
Despite these updates, South Korea’s Health Insurance Review and Assessment Service (HIRA) still mandates mitotic counts based on 50 HPFs in the pathological report of GIST to determine eligibility for adjuvant imatinib [10]. This discrepancy has led to considerable confusion among pathologists regarding the measurement and reporting of mitotic counts. In addition, differences in risk classification systems have created communication gaps among pathologists, clinicians, and insurance authorities.
To assess current practices, we conducted an online survey of South Korean pathologists using Google Forms between June 19 and July 2, 2025. A total of 85 pathologists responded to the survey. The results are demonstrated in Fig. 1. All pathology reports of resected GISTs included the mitotic counts. However, the areas examined for the mitotic count varied significantly. Fifty-nine respondents utilized light microscopy; 85% (50/59) used eyepieces with a field number of 22 (0.238 mm2 per HPF). Among them, 78% (46/59) examined 50 HPFs (approximately 12 mm2), while 12% (7/59) and 8% (5/59) examined 21 and 25 HPFs (approximately 5 mm2), respectively. Among the remaining 26 respondents who used digital pathology, 50% (13/26) examined 10 mm2, and 38% (10/26) examined 5 mm2. Specific reasons for choosing different methods were not addressed in the survey. However, we assume that a possible rationale for examining 10 mm2 to replace 50 HPFs may stem from the WHO classification of neuroendocrine neoplasms of the digestive system, which defines 2 mm2 as equivalent to 10 HPFs [11]. Additionally, 63 respondents (74%) reported performing Ki-67 immunohistochemical staining on resected GISTs, at least occasionally, whereas only 13 (15%) utilized PHH3 immunohistochemical staining.
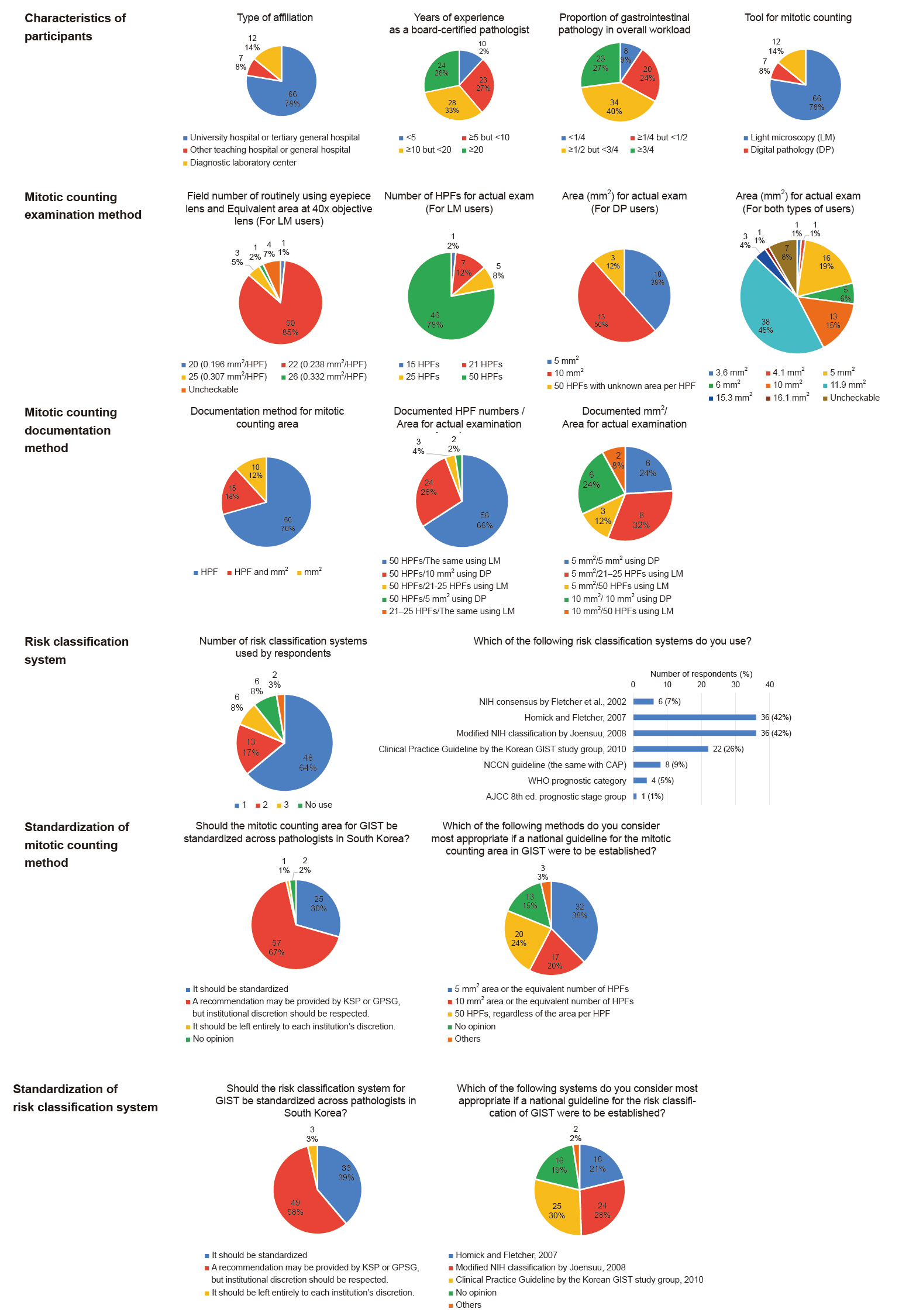
Summary of the survey results. AJCC, American Joint Committee on Cancer; CAP, College of American Pathologists; DP, digital pathology; GIST, gastrointestinal stromal tumor; GPSG, Gastrointestinal Pathology Study Group of the Korean Society of Pathologists; HPF, high-power field; KSP, Korean Society of Pathologists; LM, light microscopy; NCCN, National Comprehensive Cancer Network; NIH, National Institutes of Health; WHO, World Health Organization.
The reporting formats for the areas examined in the mitotic counts varied among the respondents. Seventy percent (60/85) reported the number of HPFs alone, 12% (10/85) reported the area in mm2 alone, and 18% (15/85) reported both. Notably, the interpretation of the reported number of HPFs or mm2 varied considerably, leading to confusion. Among the 73 respondents who documented “50 HPFs” as required by the HIRA, 66% (48/73) examined 50 HPFs using light microscopy, 18% (13/73) assessed 10 mm2 using digital pathology, 8% (6/73) examined 5 mm2 using digital pathology, and 8% (6/73) explored 21–25 HPFs using light microscopy. In contrast, nearly all respondents who reported using measurements in mm² examined areas that aligned with their documentation, except two individuals who documented '5 mm²' but evaluated 50 HPFs (approximately 12 mm²).
Respondents reported using various risk stratification systems for GIST, the remarks of which are summarized in Table 1. The most commonly used was the one proposed by Hornick and Fletcher in 2007 [2], which was recommended by the Korean Society of Pathologists (KSP) in 2012 [12] (42%, 36/85), and the 2008 modified National Institutes of Health (NIH) classification [3], which is currently mandated by HIRA [10] (42%, 36/85). Other systems included the 2010 Clinical Practice Guideline by the Korean GIST study group [4] (26%, 22/85), NCCN guideline [7] which aligns with the CAP protocol [8] (9%, 8/85), 2002 NIH consensus [1] which was recommended by the KSP in 2008 [13] (7%, 6/85), WHO prognostic category [6] (5%, 4/85), and AJCC prognostic stage group [9] (1%, 1/85). Notably, 32% (27/85) of respondents reported concurrently using two or more risk stratification systems.
Regarding the need for a national consensus on mitotic counting and risk classification for GIST, approximately one-third supported standardization, two-thirds preferred recommendations from the KSP or its Gastrointestinal Pathology Study Group with institutional discretion, and fewer than 5% favored complete individual discretion. However, serious disagreements persisted regarding the standardization of methods and systems.
In conclusion, this study highlights the significant variability in mitotic counting practices and risk classification systems used by pathologists in South Korea. National consensus guidelines are urgently needed to standardize diagnosis, reporting, and treatment. However, achieving this requires extensive discussion and coordination among all relevant stakeholders.
Notes
Ethics Statement
Not applicable.
Availability of Data and Material
The data supporting the findings of this study are available from the corresponding author upon reasonable requests.
Code Availability
Not applicable.
Author Contributions
Conceptualization: IHS, SA. Investigation: IHS. Methodology: IHS, SA, JHJ. Project administration: YSP. Supervision: YSP. Visualization: IHS. Writing—original draft: IHS. Writing—review & editing: YSP. Approval of the final manuscript: all the authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
Acknowledgments
We sincerely thank all the survey participants, particularly the Gastrointestinal Pathology Study Group of the Korean Society of Pathologists and Editage (www.editage.co.kr) for their assistance with English language editing.