Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 59(5); 2025 > Article
-
Letter to the Editor
Variation in mitotic counting and risk classification practices for gastrointestinal stromal tumors: a survey of pathologists in South Korea -
In Hye Song1
 , Soomin Ahn2
, Soomin Ahn2 , Jeong-Hyeon Jo1
, Jeong-Hyeon Jo1 , Young Soo Park1
, Young Soo Park1
-
Journal of Pathology and Translational Medicine 2025;59(5):348-352.
DOI: https://doi.org/10.4132/jptm.2025.08.14
Published online: September 8, 2025
1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
2Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- Corresponding Author: Young Soo Park, MD, PhD Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea Tel: +82-2-3010-5608, Fax: +82-2-472-7898, E-mail: youngspark@amc.seoul.kr
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,819 Views
- 25 Download
Ethics Statement
Not applicable.
Availability of Data and Material
The data supporting the findings of this study are available from the corresponding author upon reasonable requests.
Code Availability
Not applicable.
Author Contributions
Conceptualization: IHS, SA. Investigation: IHS. Methodology: IHS, SA, JHJ. Project administration: YSP. Supervision: YSP. Visualization: IHS. Writing—original draft: IHS. Writing—review & editing: YSP. Approval of the final manuscript: all the authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
Acknowledgments
We sincerely thank all the survey participants, particularly the Gastrointestinal Pathology Study Group of the Korean Society of Pathologists and Editage (www.editage.co.kr) for their assistance with English language editing.
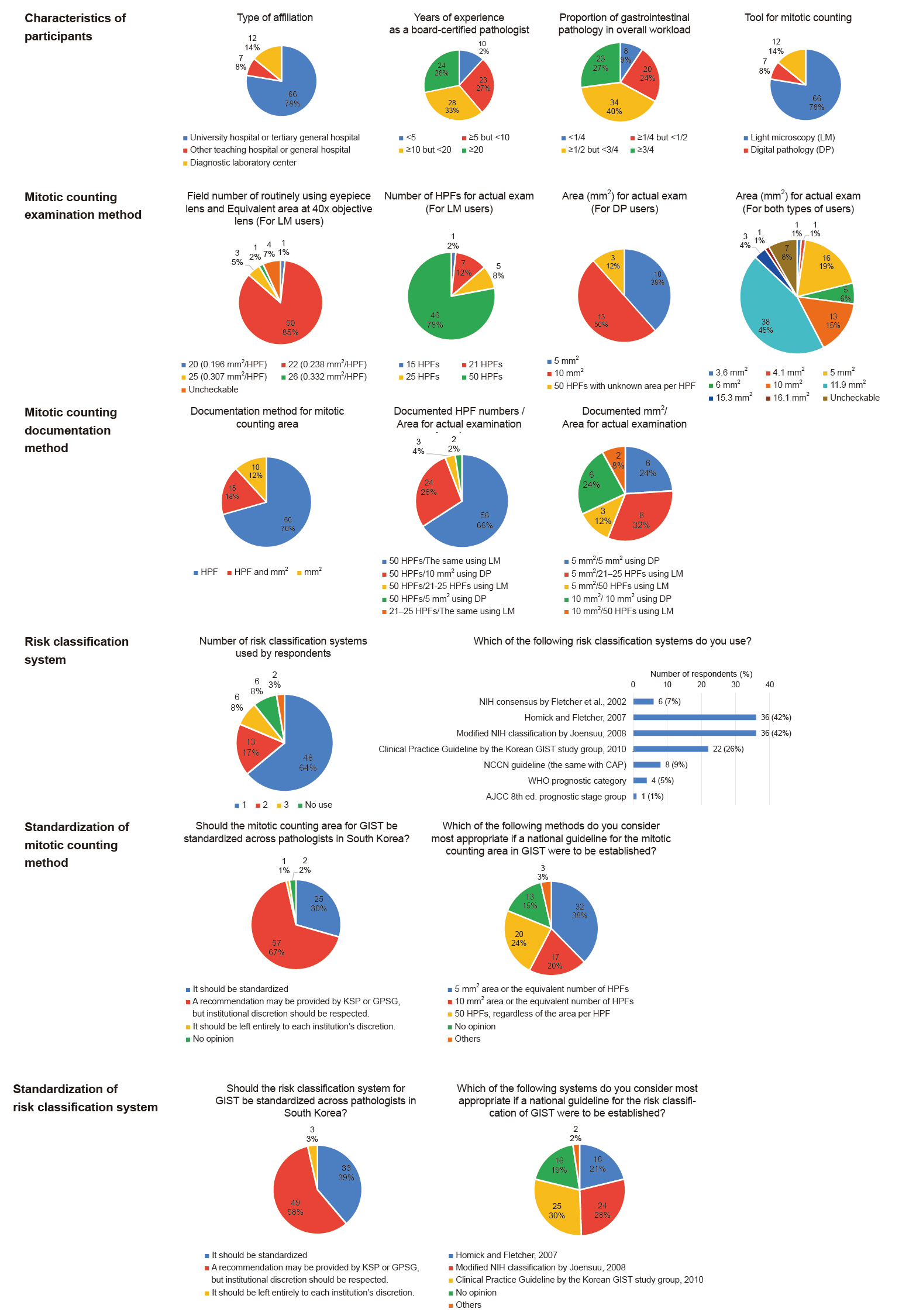
| Year | System | Factors included | Risk categories | Main remarks | ||||
|---|---|---|---|---|---|---|---|---|
| Location | Size | Mitotic rates per 50 HPF or 5 mm2 | Tumor rupture | Nodal or distant metastases | ||||
| 2002 | NIH consensus by Fletcher et al. [1] | × | ○ | <5/6–10/>10 | × | × | Very low/Low/Intermediate/High | Tumor location is not included. |
| Mitotic rates = 5 is not classified as any group. | ||||||||
| 2007 | Hornick and Fletcher [2] | ○ | ○ | <5/≥5 | × | × | Very low/Low/Moderate/High | Mitotic rates = 5 is classified as high. |
| 2008 | Modified NIH classification by Joensuu [3] | ○ | ○ | ≤5/6–10/>10 | ○ | × | Very low/Low/Intermediate/High | Tumor rupture is included. |
| A few groups are classified as higher risk than in 2007, Hornick and Fletcher [2]. | ||||||||
| 2010 | Clinical practice guideline by KGSG [4] | ○ | ○ | ≤5/>5 | × | × | Very low/Low/Intermediate/High | Mostly similar to in 2007, Hornick and Fletcher [2], but a few groups are classified as higher risk. |
| 2017 | AJCC 8th edition prognostic stage group [9] | ○ | ○ | ≤5/>5 | × | ○ | IA/IB/I/II/IIIA/IIIB/IV | Nodal or distant metastases are included. |
| 2019 | WHO prognostic category [6] | ○ | ○ | ≤5/>5 | × | × | 1/2/3a/3b/4/5/6a/6b | Risk categories are defined as numbers without interpretation. |
| 2022 | NCCN guideline/CAP protocol [7,8] | ○ | ○ | ≤5/>5 | × | × | None/Very low/Low/Moderate/High/Insufficient data | Risk categories of “None” and “Insufficient data” exist. |
- 1. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 2002; 33: 459-65. ArticlePubMed
- 2. Hornick JL, Fletcher CD. The role of KIT in the management of patients with gastrointestinal stromal tumors. Hum Pathol 2007; 38: 679-87. ArticlePubMed
- 3. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008; 39: 1411-9. ArticlePubMed
- 4. Kang YK, Kim KM, Sohn T, et al. Clinical practice guideline for accurate diagnosis and effective treatment of gastrointestinal stromal tumor in Korea. J Korean Med Sci 2010; 25: 1543-52. ArticlePubMedPMC
- 5. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006; 23: 70-83. ArticlePubMed
- 6. Tos AP, Hornick JL, Miettinen M. Gastrointestinal stromal tumour. In: WHO Classification of Tumours Tumours Editorial Board, ed. WHO classification of tumours: digestive system tumours. 5th ed. Lyon: International Agency for Research on Cancer, 2019; 439-43.
- 7. von Mehren M, Kane JM, Riedel RF, et al. NCCN Guidelines(R) insights: gastrointestinal stromal tumors, version 2.2022. J Natl Compr Canc Netw 2022; 20: 1204-14. ArticlePubMedPMC
- 8. Protocol for the examination of resection specimens from patients with gastrointestinal stromal tumor (GIST), version 4.3.0.0 [Internet]. Northfield: College of American Pathologists, 2022. Available from: https://documents.cap.org/protocols/Stomach.GIST_4.3.0.0.REL_CAPCP.pdf.
- 9. DeMatteo RP, Maki RG, Agulnik M, et al. Gastrointestinal stromal tumor. In: Amin MB, Edge S, Greene F, et al., ed. AJCC cancer staging manual. 8th ed. New York: Springer, 2017; 523-9.
- 10. Health Insurance Review and Assessment Service. Detailed criteria and methods for the application of health insurance benefits to drugs prescribed and administered to cancer patients [Internet]. Wonju: Health Insurance Review and Assessment Service, 2024 [cited 2025 Jul 18]. Available from: https://repository.hira.or.kr/handle/2019.oak/3272.
- 11. Klimstra DS, Kloppel G, La Rosa S, Rindi G. Classification of neuroendocrine neoplasms of the digestive system. In: WHO Classification of Tumours Editorial Board, ed. WHO classification of tumours: digestive system tumours. 5th ed. Lyon: International Agency for Research on Cancer, 2019; 16-9.
- 12. Jung ES, Kang YK, Cho MY, et al. Update on the proposal for creating a guideline for cancer registration of the gastrointestinal tumors (I-2). Korean J Pathol 2012; 46: 443-53. ArticlePubMedPMC
- 13. Cho MY, Kang YK, Kim KM, et al. Porposal for creating a guideline for cancer registration of the gastrointestinal tumors (I). Korea J Pathol 2008; 42: 140-50.
REFERENCES
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
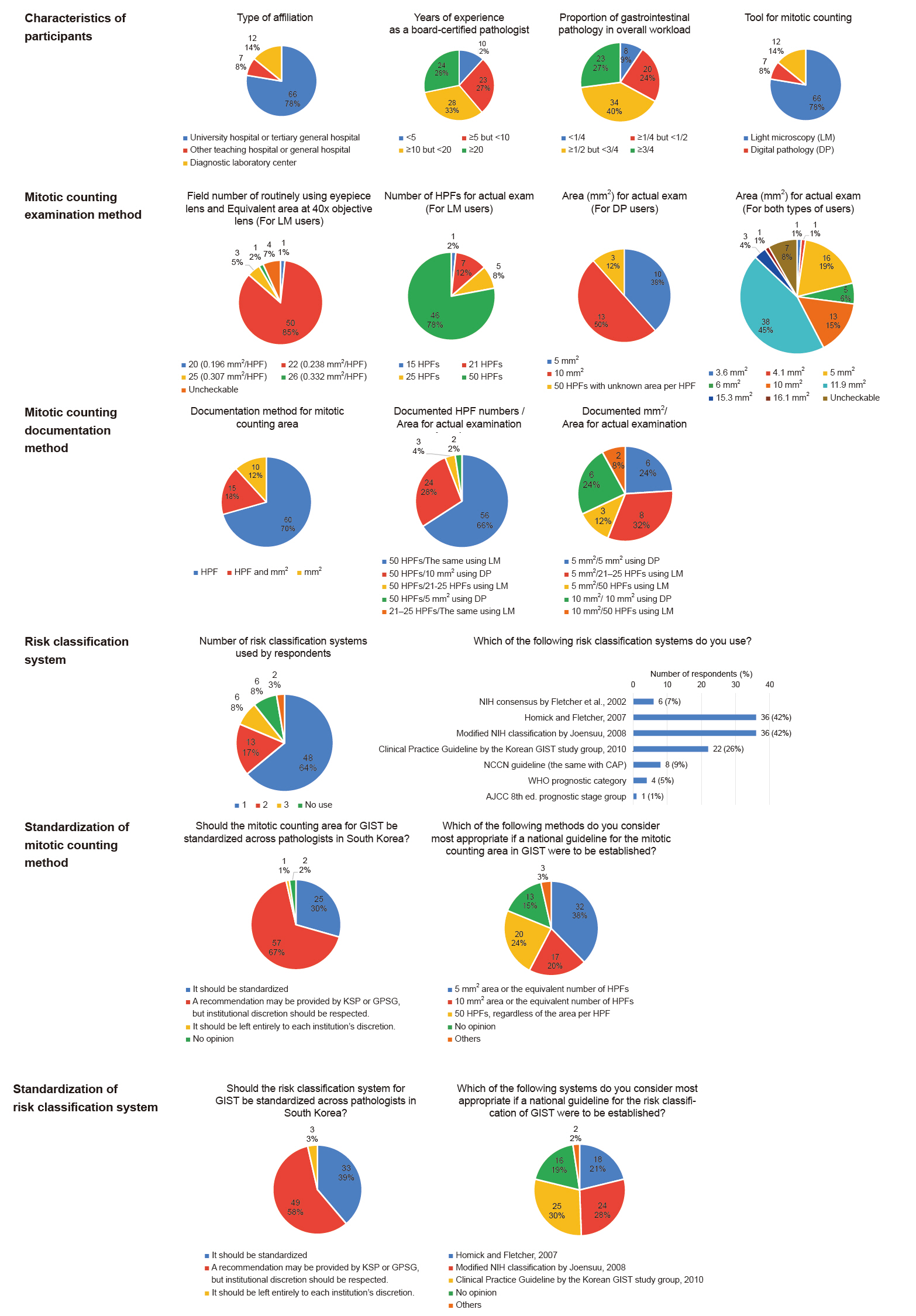
Fig. 1.
| Year | System | Factors included | Risk categories | Main remarks | ||||
|---|---|---|---|---|---|---|---|---|
| Location | Size | Mitotic rates per 50 HPF or 5 mm2 | Tumor rupture | Nodal or distant metastases | ||||
| 2002 | NIH consensus by Fletcher et al. [1] | × | ○ | <5/6–10/>10 | × | × | Very low/Low/Intermediate/High | Tumor location is not included. |
| Mitotic rates = 5 is not classified as any group. | ||||||||
| 2007 | Hornick and Fletcher [2] | ○ | ○ | <5/≥5 | × | × | Very low/Low/Moderate/High | Mitotic rates = 5 is classified as high. |
| 2008 | Modified NIH classification by Joensuu [3] | ○ | ○ | ≤5/6–10/>10 | ○ | × | Very low/Low/Intermediate/High | Tumor rupture is included. |
| A few groups are classified as higher risk than in 2007, Hornick and Fletcher [2]. | ||||||||
| 2010 | Clinical practice guideline by KGSG [4] | ○ | ○ | ≤5/>5 | × | × | Very low/Low/Intermediate/High | Mostly similar to in 2007, Hornick and Fletcher [2], but a few groups are classified as higher risk. |
| 2017 | AJCC 8th edition prognostic stage group [9] | ○ | ○ | ≤5/>5 | × | ○ | IA/IB/I/II/IIIA/IIIB/IV | Nodal or distant metastases are included. |
| 2019 | WHO prognostic category [6] | ○ | ○ | ≤5/>5 | × | × | 1/2/3a/3b/4/5/6a/6b | Risk categories are defined as numbers without interpretation. |
| 2022 | NCCN guideline/CAP protocol [7,8] | ○ | ○ | ≤5/>5 | × | × | None/Very low/Low/Moderate/High/Insufficient data | Risk categories of “None” and “Insufficient data” exist. |
HPF, high power field; NIH, National Institutes of Health; KGSG, Korean GIST Study Group; AJCC, American Joint Committee on Cancer; WHO, World Health Organization; NCCN, National Comprehensive Cancer Network; CAP, College of American Pathologists; GIST, gastrointestinal stromal tumor.

 E-submission
E-submission



