Extranodal Follicular Dendritic Cell Sarcoma with Rapid Growth in Parapharynx: A Case Report
Article information
Abstract
Follicular dendritic cell sarcoma (FDCS) is a rare malignancy arising from the antigen-presenting cells in the lymph node and extranodal tissue. We describe a 31-year-old male patient who presented with a swelling of the left parapharynx. The radiologic findings showed a 4.7×4.5×1.9 cm-sized, ill-defined mass in the left parapharyngeal space. A fine-needle aspiration cytology was performed and it showed scattered, irregular, cohesive clusters of tumor cells with a spindle-to-ovoid shape with irregular contours in a background of lymphocytes. Based on these findings, a diagnosis of spindle cell neoplasm was made. The surgically resected tumor was composed of elongated, ovoid or polygonal cells showing positive immunohistochemistry for CD21, CD23, and CD35. Postoperatively, the residual tumor was observed to undergo a rapidly growth. There is an overlap in the cytologic and histologic findings between FDCS of the parapharynx and other tumors. Pathologists should therefore be aware of its characteristics not only to provide an accurate diagnosis but also to recommend the appropriate clinical management.
Follicular dendritic cells are the stromal cells that are present in the germinal centers of the lymphoid tissue. They trap antigens on their surface and then present them to the B cells.1 Follicular dendritic cell sarcoma (FDCS) arising in the lymph nodes was first reported by Monda et al.2 in 1986. FDCSs often arise in lymph nodes, although the extranodal presentations have also been described.3
It is difficult to make a diagnosis of FDCS with a fine-needle aspiration cytology. In addition, it is not generally included in the differential diagnosis of tumors that are composed of spindle and ovoid cells with scattered mature lymphocytes in the head and neck region. Initially, these features suggest a wide variety of other diagnoses such as carcinoma, malignant fibrous histiocytoma, schwannoma and ectopic meningioma.3 In cases of FDCS, the tumor cells show immunoreactivity for the markers specific for follicular dendritic cell differentiation and these include CD21, CD23, and CD35. Combined with characteristic histological features, these immunohistochemical findings allow diagnosis of FDCS.
Primary treatment of FDCS is surgical excision with or without adjuvant therapies, such as radiotherapy or chemotherapy.4 FDCS usually shows an indolent clinical course like other low-grade soft tissue sarcomas.5 It has recently been reported that the recurrence rate of FDCS can be as high as 50%.6,7 Local recurrence of extranodal FDCS is associated with a larger size (≥5 cm), a high-grade histology and a high mitotic count (≥5/10 high power fields [HPFs]).8 However, the precise clinical behaviors and biologic features of this malignancy in the head and neck region remain unknown. We herein report a case of FDCS in the parapharyngeal space which was preoperatively diagnosed using a fine-needle.
CASE REPORT
A 31-year-old man presented with a swelling of the left parapharynx. Physical examination revealed a hard, non-tender mass with round shape in left posterior pharyngeal area with no enlarged lymph nodes in the neck.
On initial computed tomography (CT) scans, there were an ill-defined, heterogeneous mass in the left parapharyngeal space but no remarkable enlarged lymph nodes in the neck (Fig. 1A). On magnetic resonance image scans, there were a 4.7×4.5×1.9 cm-sized lobulated enhancing mass with a hyperintensity on T2WI (Fig. 1B) and an isointensity on T1WI. The impression of radiologists was neurogenic tumor or pleomorphic adenoma. On positron emission tomography-CT scans, the mass of left parapharyngeal area showed a focal hypermetabolic lesion (SUV =6.1) (Fig. 1C).
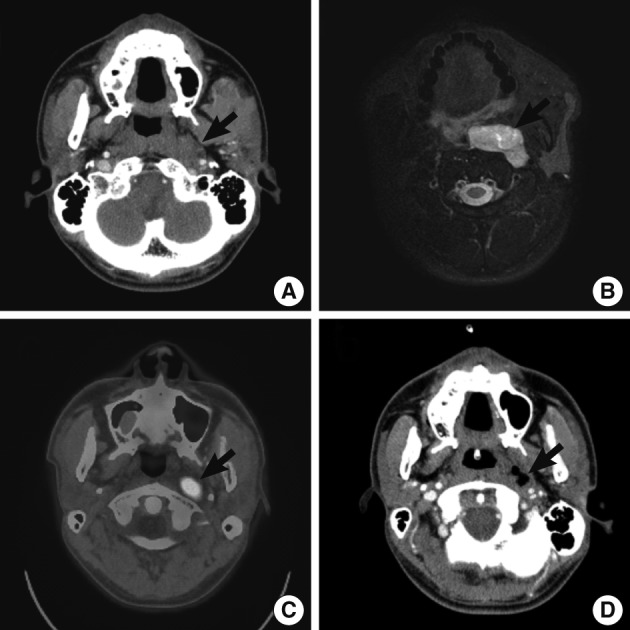
Radiologic findings. Computed tomography (CT) (A, D), magnetic resonance imaging (MRI) (B) and positron emission tomography-CT (PET-CT) (C) demonstrate enhancing mass of left parapharynx (arrow). (A-C) Preoperative CT (A), MRI (B) and PET-CT (C). (D) Follow-up CT scans taken on postoperative day 19.
On the fine-needle aspiration cytology, there was a high cellular smear. The scattered, irregular, cohesive clusters of tumor cells showed irregular contours with mature lymphocytes scattered in the background (Fig. 2A). The spindle-to-ovoid tumor cells had an indistinctive cell border and a moderate amount of pale eosinophilic cytoplasm. In tumor cells, oval-shaped nuclei showed mild atypia with a fine chromatin pattern and they occasionally had small distinctive nucleoli (Fig. 2B). Nuclear pseudoinclusions were seen in some tumor cells (Fig. 2B inset). We made a diagnosis of a spindle cell neoplasm including paraganglioma.
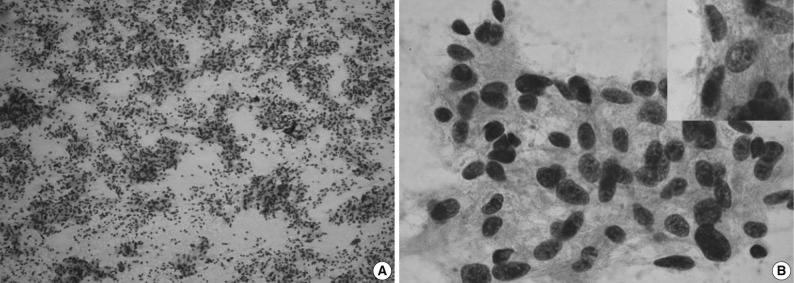
Cytologic findings. (A) The aspiration shows a high cellularity and irregular cohesive clusters. (B) Delicate cytoplasmic processes are extended from these clustered cells with smooth, spindle-shaped nuclei (inset, nuclear pseudoinclusion).
Grossly, the surgically resected specimen showed a fibrotic cut surface with a grayish white color (Fig. 3A). Histopathologically, there was a complete replacement of the normal tissue by the tumor. The tumor was composed of the elongated, ovoid or polygonal cells and it exhibited a fascicular growth pattern in certain areas. The tumor cells had more or less abundant eosinophilic cytoplasm with ill-defined cell borders. They had not only round-to-oval nuclei with a smooth nuclear membrane but also vesicular or granular chromatin and distinct nucleoli. In addition, they also had a nuclear pleomorphism with a sparse mitotic count (0-1/10 HPFs). Small lymphocytes were scattered throughout the tumor (Fig. 3B). Hemorrhages, but not necrosis, were detected in the peripheral area.

Histologic and immunohistochemical findings. (A) Grossly, the mass shows a fibrotic cut surface with a grayish white color. (B) Histopathologically, there is a proliferation of oval to spindle-shaped cells with round, oval or elongated nuclei, vesicular or granular chromatin and an ill-defined margin. (C-E) The immunohistochemical stainings for CD21 (C), CD23 (D), and CD35 (E), markers specific for follicular dendritic cells.
On immunohistochemistry, the tumor cells were positive for CD21, CD23, and CD35, all of which are markers specific for follicular dendritic cell differentiation (Fig. 3C-E). However, the tumor cells were negative for pancytokeratin (AE1/3), cytokeratin 19, human melanoma black-45, chromogranin, synaptophysin, CD56, leukocyte common antigen, smooth muscle actin, desmin, and S-100 protein. The ki-67 labeling index was about 10%.
In the current case, a diagnosis of FDCS of the parapharynx was therefore confirmed, based on the histologic and immunohistochemical findings.
A follow-up CT scan was performed on postoperative day 19, and it revealed a peripheral enhancing lesion with an ill-defined margin on the inferior side of the primary lesion (Fig. 1D). These findings are suggestive of the residual tumor, that was rapidly grew. The tumor extend that its greatest dimension reached 3 cm in the short-term postoperative period. The patient underwent the postoperative adjuvant radiotherapy.
DISCUSSION
FDCS typically arises in lymph nodes of the neck, axilla and mediastinum, and it also occurs less commonly in extranodal tissues from variable sites, including head and neck, liver, spleen, gastrointestinal tract, soft tissue, skin, lung, and breast.
According to a review of English literatures, six cases of FDCS arising in the parapharyngeal space have recently been described. But there is no case reported in Korea.4,5,10-13 The clinical data of previously reported six cases of FDCS of the parapharynx are summarized in Table 1.4,5,10-13 Of these six cases, two were initially diagnosed as FDCS but the remaining four were diagnosed as ectopic meningioma, paraganglioma or others. The tumors of the parapharynx are histopathologically characterized by the elongated, ovoid or polygonal cells with scattered mature lymphocytes. Based on these findings, they can be differentially diagnosed from carcinoma, malignant fibrous histiocytoma or schwannoma. Positive immunohistochemistry for CD21, CD23, and CD35 are helpful to make a differential diagnosis of FDCS from other neoplasm-showing similar morphology. We initially considered a diagnosis of paraganglioma, but established a diagnosis of FDCS on immunohistochemistry.
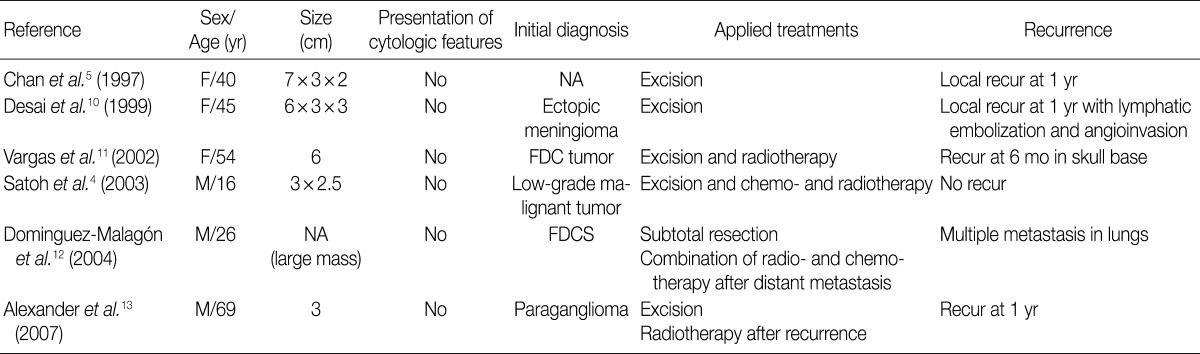
Summary of six cases of extranodal FDCS of the parapharyngeal region that have previously been reported in the literature
Previous studies have shown that a fine-needle aspiration cytology of the FDCS the ovoid or spindle-shaped cells with intermediate-sized nuclei with a smooth border and small, indistinct nucleoli.9 These cytologic features are also seen in other neoplasms including undifferentiated carcinoma, ectopic meningioma and paraganglioma. It would therefore be mandatory to make a differential diagnosis of FDCS on a fine-needle aspiration cytology. In some cases of FDCS of the parapharynx, however, these cytological characteristics are not present.10 In addition, mature lymphocytes in the background are not useful to make a differential diagnosis of FDCS from neoplasms showing similar cytology. Surgical excision should therefore be performed to confirm a diagnosis of FDCS.
Of the six cases of FDCS of the parapharynx, that had been reported previously, five showed both a local recurrence and a distant metastasis. There were two cases of FDCS of the paraphrynx with the greatest diameter of <5 cm, one of which underwent no adjuvant therapy and had a local recurrence.13 Most cases of FDCS of the parapharynx showed a more aggressive behavior with a local recurrence or a distant metastasis as compared with that of other regions.6,8 The current case showed a low mitotic count and a small-sized mass, indicating a better prognosis,8 but it did an aggressive behavior with a local recurrence and a rapid growth.
It is therefore necessary to examine the pathogenesis of FDCS, which would be essential for predicting a prognosis and selecting the optional treatment modalities. There is an overlap in the cytological and histologic findings between FDCS and other tumors. Misdiagnosis can be avoided, however, if pathologists are more aware of its characteristic features.
Notes
No potential conflict of interest relevant to this article was reported.