Expression of c-Met Is Different along the Location and Associated with Lymph Node Metastasis of Head and Neck Carcinoma
Article information
Abstract
Background
Activation of the c-Met pathway is involved in cancer progression and the prognosis. We aimed to identify any association of c-Met protein expression with a number of clinicopathologic variables including infection of human papillomavirus and Epstein-Barr virus (EBV) in head and neck carcinomas (HNCa).
Methods
Eighty-two cases were enrolled in this study. Expression of c-Met and p16 was investigated immunohistochemically. EBV was detected by in situ hybridization and amplification of the c-Met gene by fluorescence in situ hybridization.
Results
The c-Met protein was expressed in 41.5% (34/82), and gene amplification was found in 1.4% (1/71). High expression of c-Met was associated with the primary location of the tumor; the hypopharynx showed the highest expression, followed by the oral cavity, larynx, and nasal cavity. Squamous cell carcinoma expressed c-Met more frequently than undifferentiated carcinoma. Also, p16 immunoreactivity or EBV infection was associated with the tumor location and well-differentiated histologic type, but were not linked to c-Met expression. The patients with positive c-Met expression showed frequent lymph node metastasis.
Conclusions
Activation of the c-Met pathway might be involved in a subset of HNCa. Cases showing positive c-Met expression should be carefully monitored because of the high probability of lymph node metastasis.
Head and neck carcinoma (HNCa) is the 6th most common cancer in the world. In Korea and the United States, HNCa has consistently ranked in the top ten leading cancers among men, with an incidence in Korea of about 3,600 new cases per year.1,2 About two-thirds of the patients are discovered at an advanced stage, and the majority tumor type is squamous cell carcinoma (SCC). Particularly, patients with HNCa have poor functional outcomes due to the indispensability of the anatomic location, and a 5-year survival rate can be achieved in only about 30-40% of advanced cases.3 Various treatment strategies have been attempted for managing advanced HNCa according to the tumor location, histologic type, viral association such as human papillomavirus (HPV) or Epstein-Barr virus (EBV), clinical stage, and many other biomolecular parameters.4
Regarding the pathogenesis or prognostic significance of cancers, the role of the c-Met proto-oncogene has been drawing increased scrutiny. Proto-oncogene c-Met, which is located in the 7q31 locus of chromosome 7, controls several signaling pathways during embryogenesis or wound healing by binding with its ligand, hepatocyte growth factor.4 In cancer, activation of the c-Met pathway is known to promote cancer cell growth, invasion, and metastasis. Activation of c-Met can be caused by genetic alterations such as gene amplification, translocation and activating mutations, transcriptional upregulation, or ligand-dependent manners.5 As a consequence of these alterations, the c-Met protein is aberrantly expressed in a variety of cancers, and is related to disease progression or poor prognosis.6 More recently, the c-Met inhibitor has been incorporated in several clinical trials as a new therapeutic target for better treatment.7
A few sporadic reports on HNCa have shown the relationship of c-Met expression with clinical parameters.4 These reports have shown that overexpression of c-Met correlated with advanced stage and lymph node involvement; therefore, it was suggested that c-Met confers more invasive characteristics to HNCa.4 However, no study has investigated the association of c-Met expression along with its genetic changes with anatomic location, HPV or EBV status, which are strong etiologic and prognostic factors, or other clinical variables in HNCa. We assessed expression of the c-Met protein and gene amplification in HNCa and correlated it with various clinicopathologic parameters including infection with EBV.
MATERIALS AND METHODS
Patients
This study enrolled a total of 82 patients who had been diagnosed with SCC or undifferentiated carcinoma in the head and neck area at the Seoul National University Boramae Hospital (SNUH-B) between 2006 and 2010. The patients were categorized into five groups according to the locations of the tumor: 1) the nasopharynx and nasal cavity, 2) the oropharynx and palatine tonsil, 3) the oral cavity including the tongue, 4) the hypopharynx, and 5) the larynx. The histopathologic features were reviewed by two pathologists, and clinical information such as clinical stage, treatment methods, response after treatment, recurrence, and follow-up were obtained from the electronic medical records. This study was approved by the Institutional Review Board (IRB) of SNUH-B.
Tissue microarray (TMA) and immunohistochemistry (IHC)
After reviewing the tumor sections of HNCa, one of the representative areas of each donor block was punched-out with a 2 mm diameter core from the formalin-fixed paraffin-embedded blocks and arranged in the new recipient TMA blocks using a trephine apparatus (Superbiochips Laboratories, Seoul, Korea). IHC was performed using an automated immunostainer (Ventana BenchMark XT, Ventana Medical Systems Inc., Tucson, AZ, USA) according to the manufacturer's protocol. In brief, 5 µm-thick sections from the TMA blocks were deparaffinized and rehydrated. Heat-induced epitope retrieval was performed using the CC1 standard protocol, a combination reagent of disodium ethylenediaminetetraacetic acid and boric acid in a Tris buffer for 60 minutes. The endogenous peroxidase was blocked by incubation with 0.3% hydrogen peroxide. After treatment with 10% normal goat serum to block the nonspecific antibody binding, the slides were incubated with primary antibodies, a monoclonal rabbit antibody against c-Met (Ventana Medical Systems Inc.) and a monoclonal mouse antibody against p16 (CINtec, Heidelberg, Germany), for 30 minutes at room temperature. Detection was performed using the Ventana DAB detection kit (Ventana Medical Systems Inc.).
Immunoreactivity was evaluated semi-quantitatively by multiplication of the frequency and the intensity of the positive tumor cells to obtain a final semi-quantitative H-score.8 The frequency of positivity was proportionally scored (0 if <1%, 0.1 if 1-9%, 0.5 if 10-49%, and 1 if 50-100%). The staining intensity was graded from 0 to 3.
EBV in situ hybridization (ISH)
ISH was performed using a fluorescein-conjugated EBV RNA probe (Y5200, DakoCytomation, Glostrup, Denmark). In brief, 5 µm-thick sections from the TMA blocks were deparaffinized and rehydrated, and then the sections were digested with a proteolytic enzyme (proteinase K at 37℃ for 25 minutes). Thereafter, the slides were incubated with the probe at 55℃ for 1.5 hours and washed with a stringent solution. The slides were labeled with an anti-alkaline phosphatase-conjugated antibody to fluorescein. A chromogen (5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium) was then added and counterstained with Mayer's hematoxylin.
Fluorescence in situ hybridization (FISH) assay
The FISH assay was performed on the previously described TMA blocks using a MET/CEP7 dual-color probe (Vysis LSI D7S522 Spectrum Orange/CEP7 Spectrum Green Probes, Abbott Molecular Inc., Chicago, IL, USA). After the pretreatment procedure, the DNA probe kit was applied to the slides, and incubated in humidified air at 73℃ for 5 minutes to denature the target DNA and probes, and subsequently at 37℃ for 19 hours to achieve hybridization. After washing, the slides were counterstained with 4', 6-diamidino-2-phenylindole (DAPI) and p-phenylenediamine.
Statistical analysis
Statistical analyses were performed using statistical software SPSS ver. 19.0 (IBM SPSS Inc., Chicago, IL, USA). The correlations between c-Met expression and the clinicopathological variables were evaluated using the Pearson's χ2 test or Fisher's exact test and multiple logistic regression analysis. Overall survival was defined as the interval between the date of diagnosis and the last follow-up date or death. The progression-free survival was calculated from the end of primary treatment to the last follow-up date without relapse or metastasis. The Kaplan-Meier methods were used to compare the survivals. All of the statistical tests were two-sided, and statistical significance was defined as p<0.05.
RESULTS
Most of the patients were men (male:female=76:6), aged from 11 to 84 years old (mean, 59 years), including 19 smokers. The patients were grouped as follows: 1) 19 patients with a tumor in the nasopharynx or nasal cavity, 2) 17 with a tumor in the oropharynx or palatine tonsil, 3) 13 with a tumor in the oral cavity including the tongue, 4) 3 with a tumor in the hypopharynx, and 5) 29 with a tumor in the larynx. The histologic types of HNCa cases were 66 SCC including 23 well-differentiated (WD), 32 moderately differentiated (MD), 10 poorly differentiated (PD) type, 1 undetermined and 16 nasopharyngeal undifferentiated carcinomas. According to the clinical tumor-node-metastasis (TNM) stage, 40 patients belonged to the advanced status (stage III-IV) and 30 to the limited stage (stage I-II). Lymph node metastasis was found in 44 (58.7%) cases, and distant metastasis was found in 3 (3.9%) at the time of diagnosis. The treatment modalities were extremely variable including laryngomicrosurgery, laser excision, curative radical resection, primary radiotherapy, systemic chemotherapy, concomitant chemotherapy with radiation, and follow-up without treatment. Expression of p16 protein was present in 34 of the 82 (41.5%) patients, and EBV infection was present in 11 of the 82 (13.4%). Other details of the patient characteristics and marker status are summarized in Table 1.
The c-Met protein was expressed in the cytoplasm or cytoplasmic membrane of the HNCa cells in 34 of the 82 cases (41.5%) in variable degrees. In contrast to the tumor cells, the normal squamous epithelial cells showed a very weak or no expression of c-Met. Expression of c-Met was evaluated as either c-Met negative when the H score was ≤1 or c-Met positive when the H score was >1 (Fig. 1).
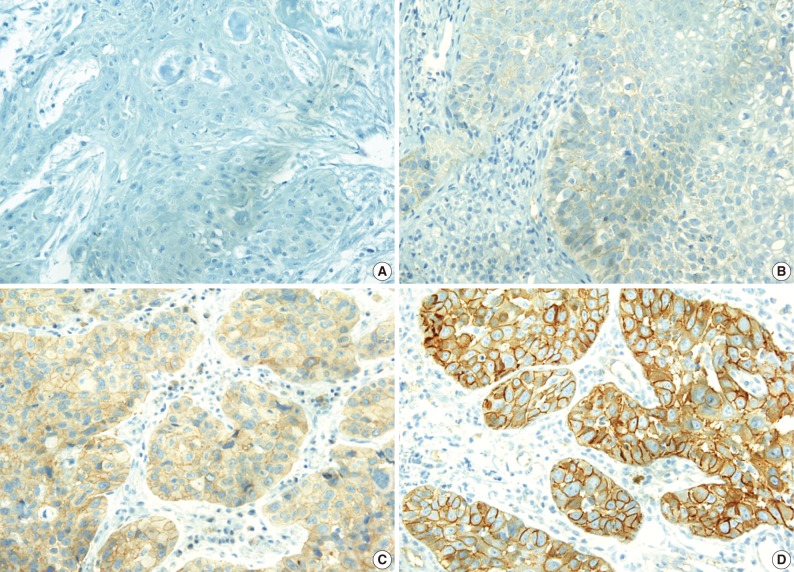
Immunohistochemically, c-Met is positive in the cytoplasm or membrane of head and neck carcinoma with the variable intensity scored as 0 (A), 1 (B), 2 (C), and 3 (D).
Expression of c-Met was significantly different between the anatomic tumor groups (p=0.007) in the Pearson's χ2 test. All 3 of the hypopharyngeal SCC were positive for c-Met (100%). SCC of the oral cavity group showed the second highest rate of c-Met positivity (9/13, 69.2%), followed by the oropharynx group (9/17, 52.9%), larynx group (9/29, 41.0%), and the nasal cavity group (4/19, 21.1%). Expression of c-Met was associated with the histologic type of the tumor, and SCC showed a higher positivity than undifferentiated carcinoma (35/66, 53.0% vs. 3/16, 18.8%, respectively). Moreover, in SCC, the WD tumors and MD tumors expressed a stronger c-Met positivity than the PD tumors (14/23, and 15/32 vs. 1/10, respectively) (p=0.023) (Fig. 2). In multiple logistic regression analysis, the degree of squamous differentiation was associated with c-Met expression (p=0.007).
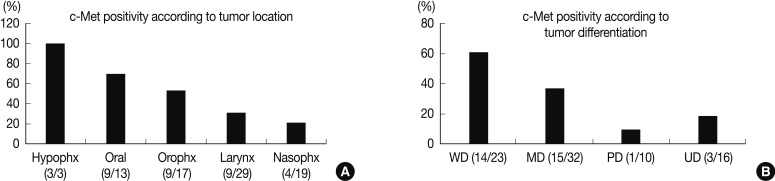
Expression of c-Met is different along the tumor location of the head and neck and in the grade of differentiation. In the hypopharynx, oral cavity, and oropharynx, c-Met is highly expressed, whereas in the larynx and nasopharynx, the expression is quite low in less than a half of the total cases (A). More differentiated tumors also show a more frequent c-Met positivity (B). Hypophx, hypopharynx; Oral, oral cavity; Orophx, oropharynx; Nasphx, nasopharynx; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; UD, undifferentiated carcinoma.
Expression of p16 was strongly associated with the primary location of the tumor (p=0.001), showing the highest expression in the oropharynx group (10/17, 58.8%) and the lowest expression in the nasopharynx group (1/19, 5.3%). Among the clinical variables, p16 positivity correlated with lymph node metastasis (p=0.034). EBV was detected in 11 cases, most of them being nasopharyngeal undifferentiated carcinoma (10/11, 90.9%); however, one SCC of the nasal cavity was also positive for EBV (1/11, 9.1%). EBV positivity was not associated with any clinical parameters.
Expression of p16 or presence of EBV was not linked to c-Met expression. The c-Met was more highly expressed in the cases with lymph node metastasis (24/44, 54.5%) than the node negative tumors (9/31, 29%). In the cases with distant metastasis, 1 case out of the 3 was positive for c-Met, and the other two cases were negative for c-Met. Expression of c-Met was not associated with age, sex, clinical TNM stage, tumor recurrence, or history of smoking.
Amplification of the c-Met gene using FISH analysis was observed in only one of the 71 cases (1.4%), and the other 70 cases showed negative results (Fig. 3). High polysomy was not detected. In the case with c-Met amplification, most of the tumor cells showed clustered red signals, which was interpreted as gene amplification based on the University of Colorado Cancer Center (UCCC) criteria for epidermal growth factor receptor FISH assay (MET to CEP7 ratio≥2 or red signal clusters in ≥10% of the tumor cells) and also the Cappuzzo scoring system for the MET gene (mean≥5 copies/cell in 50 counted cells).9-11 Strong expression of c-Met was also found in this patient showing an H score of 1.5 (a grade 3 of staining intensity in more than 30% of the tumor cells). The patient was a 68-year-old, non-smoking male, who had WD SCC of the larynx with clinical stage I, and no recurrent tumor was found for 33 months after curative resection.
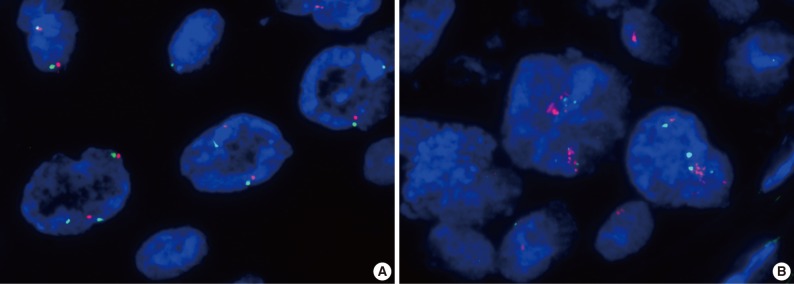
Fluorescence in situ hybridization study with a c-Met gene probe (red) on chromosome 7q31 shows a normal gene copy number in most of the cases (A), except for one case showing clustered red signals indicating gene amplification (B) in head and neck carcinoma.
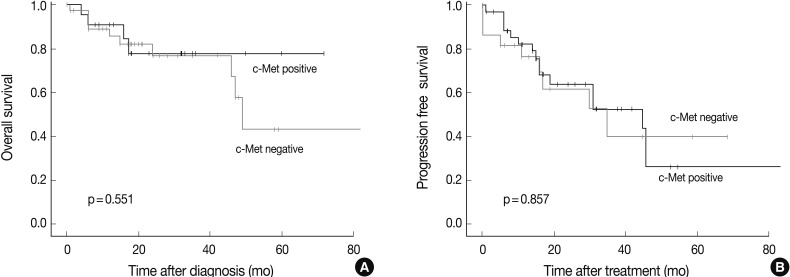
Survival analysis was performed in the 61 patients who could be reached for follow-up data collection at least 6 months after the surgery or biopsy. The median follow-up period was 47 months (range, 6 to 173 months), and the follow-up showed that 41 patients had died and 12 had recurred tumors. There was no prognostic significance measured by the overall survival or progression free survival between the c-Met positive and the c-Met negative groups (Fig. 4).
DISCUSSION
This study aimed to identify the overall expression pattern of c-Met in HNCa according to the anatomic sites and the clinical significance of c-Met expression. Expression of c-Met in HNCa has been mostly investigated in cases limited to oral cavity, tongue, or nasopharynx tumors, which were reported to have a positivity of 42-82%.12-19 We focused on the differences of the c-Met expression pattern along the tumor locations and histologic features of HNCa.
Regarding the anatomic location of HNCa, c-Met was highly expressed in the oral cavity and oropharynx, but was relatively low in the nasopharynx and nasal cavity. This raises a suspicion that the two important etiologic agents of HNCa, HPV and EBV, can be involved in these differences of aberrant expression of c-Met according to the tumor location. It has been well recognized that HPV is frequently detected in oropharyngeal carcinoma, and that EBV is found in the nasopharynx and nasal cavity. These two viruses are thought to participate in the pathogenesis of HNCa and also to have significant clinical impacts, but the relationship with the c-Met pathway has not been thoroughly elucidated. Walker et al.20 suggested that overexpression of c-Met might be involved in tumor progression in HPV-positive uterine cervical carcinoma. Regarding EBV, one study revealed that latent membrane protein-1 can induce c-Met in nasopharyngeal carcinoma,21 but a contradictory result was also reported by another researcher.22 In this study, we aimed to analyze the effect of HPV or EBV status on c-Met expression. Although there are several methods for detecting an HPV infection, IHC for p16 can be very specific and even more sensitive than HPV polymerase chain reaction assay, especially in high-risk HPV infection.23,24 Therefore, we used p16 positivity as a surrogate marker of HPV infection in HNCa. However, we could not find any significant correlation between c-Met expression and p16 expression or EBV. Further studies should be performed in this regard; however, at least we found regional propensity toward c-Met expression in HNCa.
In this study, we demonstrated that c-Met expression was associated with histologic differentiation of HNCa. However, this can be partly affected by the prevalence of certain histologic types according to the anatomic sites. Notably, undifferentiated carcinoma arises most frequently in the nasopharynx and nasal cavity. To clarify the interaction of the anatomic location, histologic type, degree of squamous differentiation, and EBV and HPV on c-Met expression, we performed multiple logistic regression analysis, which revealed a significant association between the degree of differentiation and c-Met expression. However, our results are not in agreement with former studies, which showed a lack of correlation between c-Met expression and histologic differentiation in nasopharyngeal carcinoma,15,16 or in SCC in the head and neck.16,17 These findings suggest that differential expression of c-Met along the anatomic sites of HNCa may have other interacting factors that we could not yet find.
Regarding the positive IHC pattern of c-Met, there have been only a few studies published up to the present. Lo Muzio et al.14 observed a tendency of strong membranous positivity in WD oral SCC instead of a diffuse cytoplasmic reaction in less differentiated tumors. We also observed two distinct patterns of c-Met positivity in HNCa, but no significant difference was found between the staining pattern of c-Met and the clinicopathologic parameters.
A strong correlation of c-Met expression with lymph node metastasis was noted in our study. However, other variables indicative of invasiveness such as distant metastasis, tumor recurrence, advanced stages, overall survival, and progression free survival did not show a significant association with c-Met expression. This could be partly explained by several factors: 1) our cases contained relatively fewer cases of advanced status and 2) there were many options for the treatment of HNCa. Because of the extreme variety of treatment modalities in HNCa, there have been many contradictory reports on c-Met expression and the prognosis of HNCa.12-14,16,19 However, the only constant result that can be gathered from the previous studies is the correlation of c-Met expression and lymph node metastasis, as shown in this study.
There are several mechanisms involved in the activation of the c-Met pathway. One of them is gene amplification, which has been reported in non-small cell lung cancer (NSCLC) and other cancers.10 We found c-Met amplification in only one case out of 71, which was a much lower incidence than a study by Seiwert et al.25 who reported a c-Met gene amplification of 13% (3/23) in HNCa. Taken together with the low frequency of c-Met amplification in NSCLC among Koreans,11 this low incidenceof c-Met amplification can reflect a real frequency of c-Met amplification in HNCa in Korea. Although c-Met was highly expressed in HNCa, gene amplification might not contribute highly to the aberrancy of c-Met pathway regulation. Among the other mechanisms of c-Met regulation, ligand-mediated paracrine stimulation has been reported in HNCa by some researchers.4,6
In conclusion, we found that c-Met was differentially expressed among the various locations of HNCa, and was associated with WD histologic features and frequent lymph node metastases. Based on these findings, positive expression of c-Met can be an indicator of the likelihood of lymph node metastasis; therefore, a more meticulous data gathering and aggressive treatment should be applied in such cases. Although c-Met overexpression was frequently detected in HNCa, gene amplification rarely occurred in our study.
Acknowledgments
This study was supported by a research fund from Seoul National University Hospital (0520110070).
Notes
No potential conflict of interest relevant to this article was reported.