Finding and Characterizing Mammary Analogue Secretory Carcinoma of the Salivary Gland
Article information
Abstract
Background
A new tumor entity of the salivary glands, mammary analogue secretory carcinoma (MASC) with ETV6-NTRK3 translocation, has recently been proposed. MASC was originally diagnosed as adenocarcinoma, not otherwise specified (ANOS), or acinic cell carcinoma (AciCC) by the current World Health Organization classification. We aimed to identify MASC cases by molecular tests, and to characterize their clinical, histological, and immunohistochemical features.
Methods
Thirty cases of MASC candidates were selected after review of 196 salivary gland tumors, and subjected to break-apart ETV6 fluorescence in situ hybridization (FISH), and immunohistochemical study for S100 protein, gross cystic disease fluid protein 15, DOG1, estrogen receptor, and progesterone receptor.
Results
Valid FISH results were obtained in 23 cases, and 13 positive cases were retrieved. MASCs were histologically varied, and the most frequent features observed in 10 cases were low-grade papillary/cystic/glandular patterns, intraluminal secretory materials, ovoid/wrinkled nuclei, and relatively abundant granular eosinophilic cytoplasms, corresponding to papillary-cystic or follicular types of AciCC. All cases showed diffuse immunopositivity for S100 protein. Three cases developed recurrences, but all patients remained alive.
Conclusions
MASC could be a molecularly well-defined salivary gland neoplasm, encompassing some portions of AciCC and ANOS, but its histological spectrum and clinical implication require further investigation.
A new tumor entity of the salivary gland, mammary analogue secretory carcinoma (MASC) has recently been proposed. The entity was first presented by Skálová et al. in 2010,1 as a distinctive neoplasm with strong similarities to breast secretory carcinoma including ETS variant gene 6 (ETV6)-neurotrophic tyrosine kinase receptor, type 3 (NTRK3) translocation. Since their report, a succession of studies has followed to characterize and distinguish MASC from other salivary gland tumors.1-5 Histologically, the tumors have been reported to show a microcystic, papillary-cystic, glandular, or solid growth patterns, low-grade vesicular nuclei, pale pink granular or vacuolated "bubbly" cytoplasm, and intraluminal or intracellular secretions, featuring subtypes of acinic cell carcinoma (AciCC). According to recent study results, MASCs also mimic other salivary gland tumors, including adenocarcinoma, not otherwise specified (ANOS), mucoepidermoid carcinoma (MEC), and cystadenocarcinoma (CAC).3 Clinically, according to Chiosea et al.,5 MASCs have been known to show a male predilection and more prevalence for non-parotid sites but showed no statistically significant outcome difference, compared to AciCCs. Currently, diagnosing MASCs only by histological features without a molecular study is difficult, and their immunohistochemical characteristics have not been fully investigated.
ETV6-NTRK3 fusion gene is expressed in human secretory breast carcinoma. This gene is the product of a t(12;15)(p13;q25) that fuses the dimerization domain of a transcriptional regulator (ETV6) on chromosome 12 with a membrane receptor tyrosine kinase (NTRK3) on chromosome 15 and has also been reported to associate with infant fibrosarcoma, congenital mesoblastic nephroma and acute myelogenous leukemia. ETV6 is genetically unstable, thus susceptible to chromosomal rearrangements, and is implicated in leukemia, myelodysplastic syndromes and sarcomas, fusing with dozens of genes such as ABL1, FGFR3, PAX5, SYK, and JAK2, in addition to NTRK3. ETV6-NTRK3 gene rearrangement has been proven in MASCs as in other epithelial, mesenchymal, or hematopoietic tumors. Recent studies suggested that the inhibition of ETV6-NTRK3 activation could serve as a therapeutic drug for the treatment of patients with this fusion.6,7
This entity is very new, and to date, no case has been documented in Korea. We aimed to retrospectively identify MASCs using fluorescence in situ hybridization (FISH) for ETV6 rearrangement, and define the histological, clinical, and immunohistochemical characteristics.
MATERIALS AND METHODS
We selected excised salivary gland tumors, excluding biopsy or consultation cases, from the surgical pathology files of the Asan Medical Center from 1990 to 2012. For retrieval of MASC candidates, 196 salivary gland tumors (MEC, n=137; AciCC, n=43; ANOS, n=11; CAC, n=2; other unusual salivary gland neoplasm, n=3) were retrospectively reviewed by 2 pathologists. The selection of study candidates was built on the histological features described by recent literature1,3-5 and sufficient amount of tissue available to construct a tissue microarray (TMA). The criteria for selection among the above tumors included the presence of glandular formation or secretory activity. The final specimens comprised 30 cases initially diagnosed as AciCC (n=16), ANOS (n=6), MEC (n=3), CAC (n=2), salivary duct carcinoma (SDC; n=1), squamous cell carcinoma (n=1), and oncocytic carcinoma (n=1). Additionally, 6 conventional AciCC cases, morphologically showing unequivocal serous acinar differentiation, and 2 SDC cases were selected for reference. For all 38 cases, TMAs were generated using a manual tissue arrayer (Pathology Devices, Westminster, MD, USA). Two 1.0- or 1.5-mm cores were taken from donor blocks and arrayed into recipient blocks. Clinical data were obtained through review of medical records and pathologic staging was based on the Cancer Staging Manual of American Joint Committee.8 Disease-free survival was assessed from the time of histological diagnosis to recurrence or death, based on review of the patients' medical records and information from the National Health Insurance. A t-test was employed to characterize the relationships between quantitative variables and the Fisher's exact test was used to characterize the relationship between categorical variables. Disease-free survival periods with 95% confidence intervals (CI) were estimated using the Kaplan-Meier method, with statistical significance of differences between groups estimated by log-rank test. A p-value of <0.05 was defined as statistically significant. Statistical analysis was performed using SPSS ver. 18 (SPSS Inc., Chicago, IL, USA).
Immunohistochemistry and ETV6 FISH
From TMA blocks, 4-µm sectioned slides were obtained and prepared for immunohistochemistry and FISH. Immunohistochemical stainings were performed using the Ventana autostainer and ultra view DAB detection kit (Ventana, Tucson, AZ, USA) according to manufacturer's instructions. The antibody sources and dilutions are listed in Table 1. FISH was performed using a break-apart probe for the ETV6 gene (Abbott Molecular, Des Plaines, IL, USA) according to the manufacturer's recommendations. FISH slides were examined with an Olympus BX51 fluorescence microscope (Olympus, Tokyo, Japan) using a 100× objective. Through FITC and Texas Red Band pass filters, each image was obtained with an Olympus DP70 camera using DP Controller (ver. 3.3.1.292) acquisition software. Fifty to 100 cells were examined in each case. A single green (or red) signal without a corresponding red (or green) signal in addition to a fused signal was considered negative (non-rearranged) in the present study. Red and green signals that were less than 2 signal diameters apart were considered as a single fused signal. The average percentage of split signal in 6 referential AciCCs, showing unequivocal serous acinar differentiation (conventional AciCC) was 4.565 and standard deviation (SD) 3.042. Thus, we considered cases, if more than 15% (mean+3 SD, rounded up) of examined nuclei showed a split signal, as positive for translocation which is the similar cut-off value used in previous literature.1 The slides were independently interpreted by 2 observers. If 10-20% of the analyzed cells showed a split signal, more cells were enumerated and the first and second cell count readings by the 2 observers were added together and a percentage was calculated. Valid FISH results were obtained in 31 out of 38 cases. The laboratory used breast secretory carcinoma as a positive control.
RESULTS
Verifying MASC with ETV6 translocation and its clinicopathologic characteristics
Among the 31 technically successful cases, 13 cases showed ETV6 translocation, reclassified as MASC, and 10 cases showed intact ETV6, categorized as mimic MASC (Fig. 1). The other 8 cases served as a reference, including 6 conventional AciCCs and 2 SDCs, and showed intact ETV6. On average, 57.65% of examined cells showed a split signal with ETV6 translocation (MASC) in 13 cases, while 9.55% of examined cells showed a split signal in 10 cases with intact ETV6 (mimic MASC). The percentage of cells with a split signal ranged between 20.75% and 78.57% in cases with ETV6 translocation, and between 3.10% and 12.96% in cases with intact ETV6.
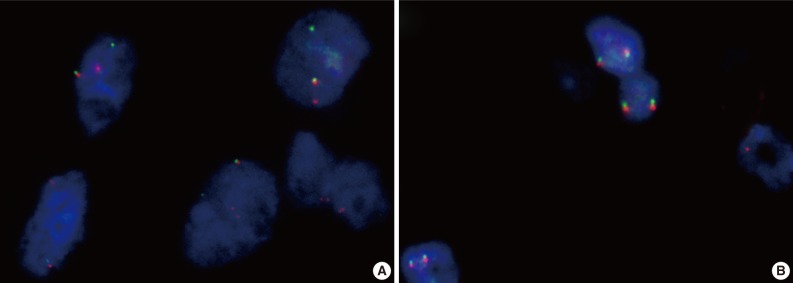
ETV6 fluorescence in situ hybridization (FISH). (A) ETV6 FISH showing 1 fused (yellow) and 1 split (red and green) signal indicative of a translocation. (B) Mimic mammary analogue secretory carcinoma showing a negative ETV6 FISH as evident by 2 intact signals in each cell.
The clinicopathologic features of 23 MASC candidates with ETV6 translocation (MASC, n=13) or with intact ETV6 (mimic MASC, n=10), are summarized in Table 2. For referential comparison, conventional AciCCs (n=15) surgically treated in our institution are also summarized in Table 2.
The 13 MASC cases consisted of 8 males and 5 females. Although no differences in gender distribution were found between MASC and mimic MASC cases, a noticeable male predilection was observed compared with conventional AciCCs, which affected females more frequently than males (F:M=12:3). The average age in the MASCs, mimic MASCs, and conventional AciCCs was in the fifth decade. Both MASCs and mimic MASCs showed relatively even age distribution from the second to the eighth decade. The parotid gland was the most frequently involved site in MASCs, mimic MASCs, and conventional AciCCs and the tumor size in MASCs ranged from 0.7 cm to 2.5 cm (mean, 1.77 cm). The average tumor size was similar to mimic MASC and smaller than conventional AciCC. Only 4 cases of MASC showed extraparenchymal extension, and any lymph node involvement at the time of surgery was not observed. All patients were initially treated by surgery, and 3 MASC, 3 mimic MASC, and 2 conventional AciCC patients received postoperative radiotherapy and all are currently alive. Three MASC patients developed local recurrence (3/13, 23.1%) with median recurrence time of 44 months after diagnosis (range, 10 to 101 months) and 2 mimic MASC patients developed metastases to neck lymph nodes (2/10, 20.0%) at 62 months and 70 months after diagnosis, while an AciCC patient developed local recurrence (1/15, 6.7%) at 39 months after diagnosis. The mean follow-up period was 50 months. In summary, MASC, mimic MASC, and conventional AciCC showed no statistically significant differences in gender, age, site, pathologic T stage, and disease-free survival.
Defining the histological MASC properties and comparison with non-MASC
Histologically, the most frequent features in the 13 MASC cases were well circumscribed, encapsulated, or multinodular masses, showing papillary-cystic, microcystic, solid, follicular or their combination growth pattern. Cytomorphologically, most tumors showed an admixture of variable cell types. Some cells were smaller than typical acinar cells appearing to have an increased nuclear to cytoplasmic ratio, and showed eosinophilic or finely granular cytoplasm and ovoid basophilic nuclei, known as intercalated duct-like cells. Other cells showed a more abundant or vacuolated cytoplasm and low-grade wrinkled nuclei. A small number of cells appeared clear or mucin-containing. Numerous variable-sized spaces were frequently observed and the spaces were usually clear but sometimes contained pinkish- to grayish blue-colored amorphous materials, which were positive for periodic acid-Schiff. As stated in previous literature, the above-mentioned characteristics were predominant features of MASC and corresponded to microcystic, papillary-cystic, or follicular type of AciCC.1-5 These usual morphological MASC features are illustrated in Fig. 2.
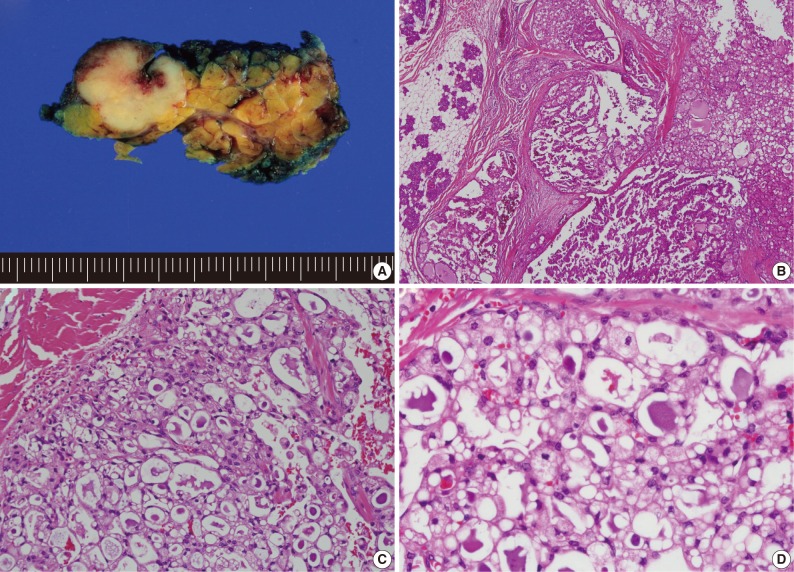
Usual mammary analogue secretory carcinoma (MASC) morphologic features. (A) Gross photograph showing a lobulated and whitish-yellow colored tumor of the parotid gland. (B) MASC showing microcystic and papillary-cystic growth pattern. (C, D) Admixture of variable cell types with eosinophilic or vacuolated cytoplasm and intraluminal secretory material.
However, 3 MASC cases showed unusual histological features. One case was macrocystic, measuring up to 2.5 cm, and partially filled with intraluminal papillary proliferation. A macrocystic tumor with sparse projection could be misidentified as CAC. Nevertheless, epithelial configuration of the cyst lining and papillary projection was similar to the mentioned MASC. Another case showed large but variable-sized cysts interspersed among the fibrous stroma. The tumor showed focal proliferation of the epithelial lining and even cribriform appearance in some relatively small islands. These features were also reminiscent of CAC, however, epithelial lining cells were similar to MASC rather than the cuboidal, clear, tall columnar, or oncocytic features of CAC. Notably, the thin epithelial lining cells often exhibited mucinous cytoplasm (Fig. 3).
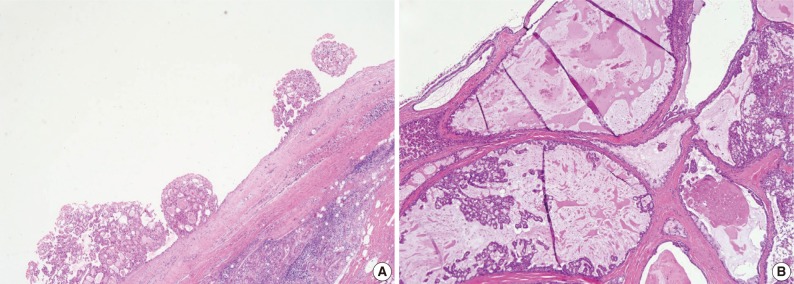
Unusual histologic features of mammary analogue secretory carcinoma. (A) One prominent macrocyst with intraluminal papillary proliferation. (B) Variable-sized cysts among the fibrous stroma and mucinous metaplasia.
The third case showed a distinct morphologic change previously referred to as dedifferentiation or high-grade transformation of AciCC.9 The tumor was composed of 2 distinct areas with an obvious transformation zone. Histological features of one area corresponded to microcystic and papillary-cystic MASC and the other area showed poor zymogen granule containing-basophilic polymorphous cells, forming solid nests with comedonecrosis (Fig. 4).
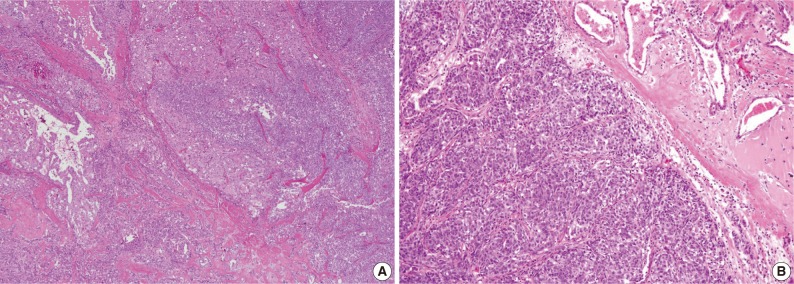
Unusual histologic features of mammary analogue secretory carcinoma. (A) Microcystic growth pattern showing dedifferentiation with transitional area. (B) Solid growth pattern with high-grade cellular features.
A diversity of morphological features with intact ETV6 was also obtained. Among 10 mimic MASCs, 3 cases could be interpreted as CAC. Though the cytomorphologic features were not uniform, the tumor cells were slightly different from MASC, showing cuboidal, tall columnar, clear to oncocytic features. Additionally, the epithelial lining of cysts was more evenly thin in CAC than MASC. Additionally, when papillary projections were present, they were mostly large papillae covered with columnar or tall cuboidal cells. Others showed nonspecific morphologies, corresponding to ANOS. Infiltrating nests of clear cells, glandular growth in the prominent sclerosing background, or solid nests surrounded by very thin fibrovascular septa with reticular and abundant cytoplasm were other examples of mimic MASC. These architectural patterns were obviously different from MASC, but cytomorphological features were similar. Two cases of MECs lacking mucous and epidermoid cells were initially similar to MASC, but careful examination can help in distinguishing MEC from MASC. Simultaneously, oncocytoma lacking characteristic cytoplasm features could also mimic MASC. The above morphological features mimicking MASC are illustrated in Fig. 5.
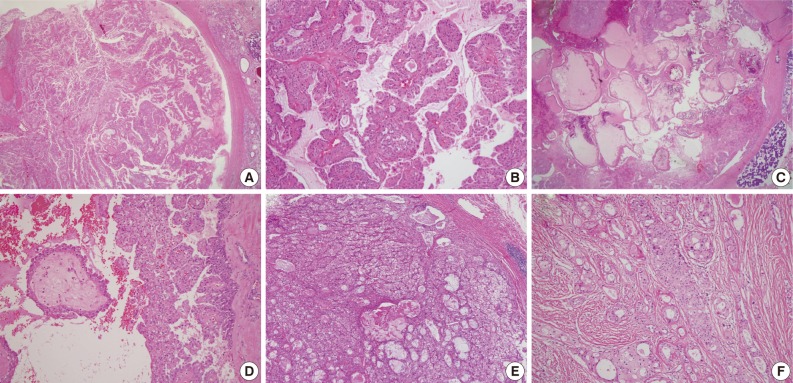
Four examples of histologic features of mimic mammary analogue secretory carcinoma (A and B; C and D; E; F). (A) Macrocyst with extensive papillary proliferation. (B) Papillae showing an eosinophilic but cuboidal to columnar appearance. (C) Macrocyst showing intraluminal papillary proliferation with large papillae. (D) Papillae showing clear to oncocytic cytoplasm. (E) Solid nests with clear to reticular cytoplasm surrounded by very thin fibrovascular septa. (F) Glandular growth pattern in prominent sclerosing stroma.
Immunohistochemical findings
Immunohistochemically, all 13 MASC cases were positive for S100 protein. Most staining patterns were strong and diffuse, except for a case showing dedifferentiation (Fig. 6). Three out of 10 mimic MASCs expressed S100 protein and no expression was observed in 6 conventional AciCCs. Gross cystic disease fluid protein 15 (GCDFP-15) immunostains were focally positive for 2 out of 13 MASCs, 3 out of 10 mimic MASCs, and none of the conventional AciCCs. DOG1 was expressed in 3 conventional AciCCs and 1 MASC, with weak apical or apicoluminal membranous staining. Estrogen receptor (ER) and progesterone receptor (PR) were all negative for MASC, mimic MASC and conventional AciCCs (Table 3).
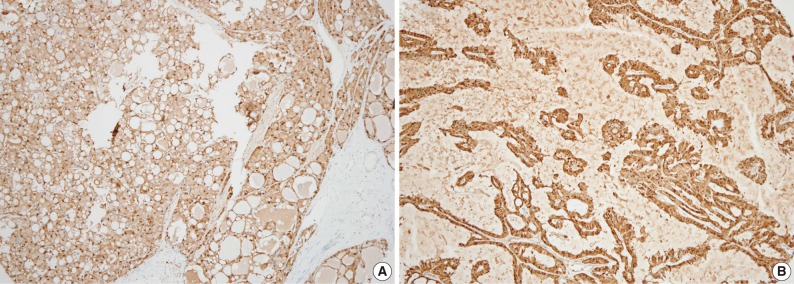
S100 protein immunohistochemistry. (A) Mammary analogue secretory carcinoma (MASC) with strong S100 protein immunostaining. (B) Mimic MASC with strong S100 protein immunostaining.
DISCUSSION
In this study we investigated the new salivary gland tumor, known as MASC, to clarify the clinical, histological, and immunohistochemical characteristics, and to determine any pathognomonic features helpful in diagnosing MASCs.
The first MASC report focused on features reminiscent of breast secretory carcinoma.1 In recent years, there have been several studies extending the clinical, histological, and immunohistochemical properties of MASCs.3,5 However, MASC clinicopathologic features appear to be indistinctive and the molecular demonstration of ETV6 rearrangement is the only diagnostic method. Regardless of the originality of this entity, a salivary gland tumor with ETV6 translocation has yet to be documented in Korea.
The present study compared MASC candidate groups with an intact ETV6 and ETV6 translocation. To evaluate MASCs with an emphasis on easy identification of specific characteristics, consideration of the ideal cut-off value to assess the ETV6 FISH results was necessary. In this study, a 15% cut-off value was determined as acceptable. The negative control cut-off value (mean+3 SD, 13.688) showed a 99.7% CI for intact ETV6. The cut-off value used (15%) was slightly higher than the negative control cut-off value. Moreover, the interpretation of a single colored signal without a corresponding colored signal in addition to a fused signal as negative could decrease the percentage of positive cells for ETV6 translocation. The highest negative value for ETV6 translocation was 12.96% and the lowest positive value was 20.75%. This result shows the importance of determining an accurate cut-off value in a study.
Most MASC features overlapped with subtypes of AciCC. Overall, papillary-cystic, microcystic, or their mixture types were predominantly observed. AciCCs, according to the current World Health Organization classification, are comprised of heterogeneous tumors. Many cell types, including acinar, intercalated duct type, vacuolated, clear, and nonspecific glandular cells, and variable growth patterns such as solid, microcystic, papillary-cystic, and follicular, are described as constituents of AciCC. ANOS is also a heterogeneous tumor group, and their diagnosis is based on the lack of features that characterize other salivary gland adenocarcinomas. According to prior studies and our results, a considerable number of ANOS and zymogen granule-poor AciCCs belong to the MASC group. The concept that MASC is a distinctive entity of salivary gland tumors requires more consideration, however, no zymogen granule-rich AciCC or SDC has shown an ETV6 rearrangement. Our study revealed previously undescribed histology in ETV6-rearranged tumors, such as a solid pattern resembling dedifferentiated AciCC and a large cyst with sparse papillary proliferation. Chiosea et al.4 reported that the results of ETV6 FISH in 4 cases of AciCC with high-grade malformation were all negative for ETV6 translocation. Our results were contrary to the previous result, even in one case.
Apart for a few exceptions, the histological outline of MASC appears evident, and mimic MASC cases without an ETV6 translocation could be differentiated from MASC by careful histological examination. However, MASC diagnosis in routine practice remains difficult by histological examination only. We attempted to identify an immunohistochemical marker for MASC. DOG1, known as a novel marker of salivary acinar and intercalated duct differentiation, was not helpful.10 GCDFP-15, ER, and PR were considered a possibility due to their mammary-like character, but were not expressed in MASC. The S100 protein was highly sensitive, but showed low specificity to differentiate MASC from mimic MASC. Thus, new immunohistochemical markers that could substitute ETV6 FISH are required.
In conclusion, MASC could be a molecularly well-defined salivary gland neoplasm, encompassing some portions of AciCC and ANOS, but its histological spectrum and clinical implication need further investigation.
Notes
No potential conflict of interest relevant to this article was reported.


