Silent Colonic Malakoplakia in a Living-Donor Kidney Transplant Recipient Diagnosed during Annual Medical Examination
Article information
Abstract
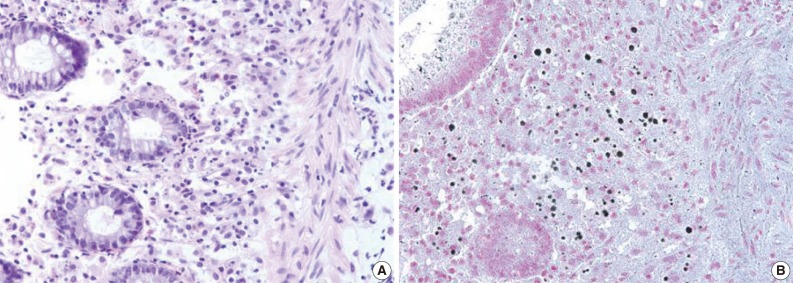
Malakoplakia is a characteristic inflammatory condition, which is usually seen in the urogenital tract, and less frequently in the gastrointestinal tract. We present a case of colonic malakoplakia in an immunocompromised patient. A 55-year-old female visited the outpatient clinic for routine cancer surveillance. Her past medical history was significant for kidney transplantation 11 years ago, and she had been taking immunosuppressants. A colonoscopy revealed several depressed flat lesions and elevated polyps, which were 0.3 to 0.4 cm in size and accompanied by whitish exudates. A biopsy revealed an infiltration of histiocytes with ample granular eosinophilic cytoplasm, with some lymphocytes and plasma cells. Many histiocytes had the characteristic morphology, described as Michaelis-Gutmann bodies: one or several round basophilic structures of approximately 1 to 10 µm in size with some being laminated, some appearing homogeneous, and others having a dense central core with a targetoid appearance. These Michaelis-Gutmann bodies were positively stained on von Kossa stain, and were diagnostic for malakoplakia.
Malakoplakia is a rare chronic inflammatory disease associated with gram-negative bacterial infections, most frequently with Escherichia coli.1 It occurs most commonly in the urinary tract, although all organs, including the gastrointestinal tract, lungs, and skin, can be affected.2 Gastrointestinal malakoplakia is observed in association with a variety of conditions such as ulcerative colitis, diverticular disease, adenomatous polyps, carcinoma, and immune deficiency.3 There were cases of malakoplakia involving the gastrointestinal tract in kidney transplant recipients presenting with acute intestinal perforation or diarrhea.2,4-6 Our case reports an asymptomatic colonic malakoplakia in a 55-year-old woman who underwent kidney transplantation and was on immune suppressants.
CASE REPORT
A 55-year-old female visited the outpatient clinic of the Samsung Medical Center for routine cancer surveillance in May 2011. The patient received a living-donor kidney transplant eleven years ago for end-stage renal disease secondary to IgA nephropathy at Kyunghee University Medical Center. She was on stable doses of immunosuppressive drugs, including prednisone, tacrolimus, and mycophenolate, at the time of the current visit. She had no rejection or other complications of transplantation to date, and was asymptomatic. Her routine cancer surveillance included complete blood counts, a liver function test, abdominal sonogram, gynecologic exam, upper gastrointestinal endoscopy, and colonoscopy. Results were all unremarkable, except or the colonoscopy findings. The colonoscopy revealed several depressed flat lesions and elevated polyps, which were 0.3 to 0.4 cm in size and accompanied by whitish exudates (Fig. 1). The differential diagnosis for the endoscopic lesions included graft-versus-host disease, cytomegalovirus colitis, and amoebiasis. Multiple biopsies were obtained from several sites. Microscopic findings from all biopsy materials revealed the infiltration of many histiocytes with ample granular eosinophilic cytoplasm with some lymphocytes and plasma cells. Many histiocytes had the characteristic morphology described as Michaelis-Gutmann bodies (Fig. 2): one or several round basophilic structures of approximately 1 to 10 µm in size, with some being laminated, some appearing homogeneous, and others having a dense central core with a targetoid appearance. These Michaelis-Gutmann bodies were positively stained on von Kossa stain, and were diagnostic for malakoplakia (Fig. 3). The patient did not receive any treatment. After 1 year, she underwent an annual health examination at Samsung Medical Center in May 2012. She still did not have any symptoms, and had been taking immune suppressive medication. This time she did not receive a colonoscopy. Aspects of the check-up showed results within normal ranges.
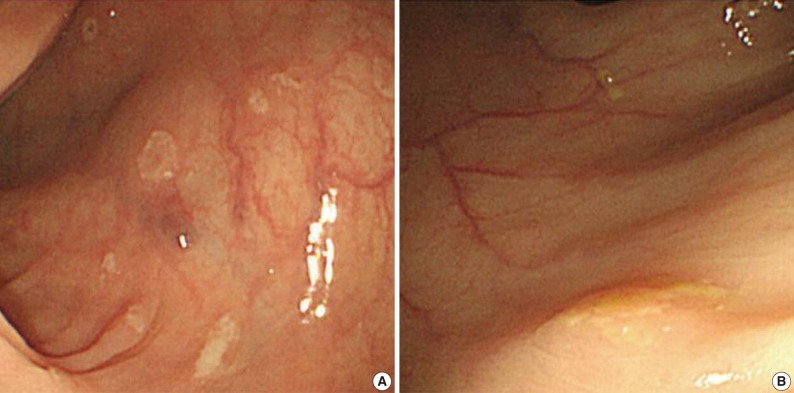
Endoscopic features of the ascending (A) and transverse (B) colon. A colonoscopy reveals several depressed flat lesions and elevated polyps, which are measuring up to 4 mm in size with whitish exudates.
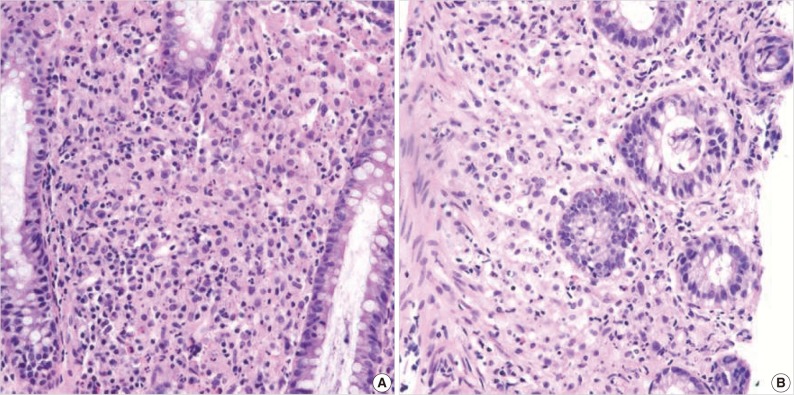
(A) There is an infiltration of many histiocytes with ample granular eosinophilic cytoplasm with some lymphocytes and plasma cells (ascending colon). (B) Round basophilic structures are identified, measuring approximately between 1 µm and 10 µm in size within histiocytes, so-called Michaelis-Gutmann bodies (transverse colon).
DISCUSSION
Malakoplakia is a chronic granulomatous inflammatory disorder frequently involving the genitourinary tract. The gastrointestinal tract is the second most commonly involved system of malakoplakia.3 The etiology of malakoplakia is idiopathic, but is thought to be associated with dysfunctional bacterial clearance by neutrophils and macrophages,7 and thus has been closely associated with states of immunodeficiency.8 Diagnosis is established histologically by the presence of large "Hansemann macrophages," containing laminated calcific spherules, known as Michaelis-Gutmann inclusions.9 The Michaelis-Gutmann body is derived from phagolysosomes containing incompletely destroyed bacteria, and is pathognomonic for malakoplakia.10 Escherichia coli is the most commonly associated bacterial pathogen, and is found in over 90% of affected patients.2
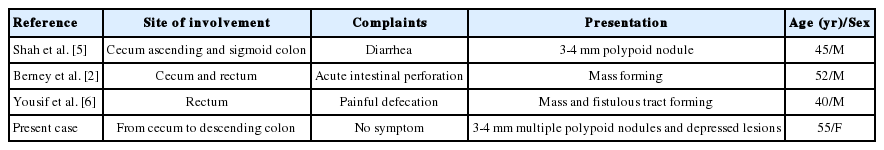
Malakoplakia has been described in post-transplant patients. We performed a Medline search and analyzed all cases reporting colonic malakoplakia in transplant recipients. Only four cases were previously reported in kidney transplant recipients from 1994 to 2010. Other transplant-associated colonic malakoplakia included three cases with liver transplants and one with a cardiac transplant.8,11-13 All patients in the reported cases presented some symptoms, including diarrhea, abdominal pain, fever, and intestinal perforation. Precedent colonic malakoplakia cases associated with kidney transplant are compared in Table 1, which shows patient ages ranging from 40 to 52 years,2,4-6 and all were male. Unlike the previous cases, our case is unique for its demographics, particularly the age and female sex, and for its asymptomatic presentation. This is the fourth case report of colonic malakoplakia identified subsequent to kidney transplantation, and the only case of its asymptomatic presentation.
There are two strategies for the treatment of malakoplakia: antibiotics for the bacterial infection and a cholinergic agonist to improve macrophage function.8 Antibiotics that penetrate and concentrate in macrophages, such as quinolone or trimethoprim-sulfamethoxazole, are currently used with a high cure rate for malakoplakia.14 Antibiotic therapy directed against E. coli in combination with surgery provides the best chance of cure.14 Van Furth et al.15 also reported on two patients with malakoplakia, who were treated with long-term ciprofloxacin and experienced a regression of their lesions. The second approach is an attempt to correct the lysosomal defect by using a cholinergic agonist.14 A cholinergic agonist may correct the decreased cGMP levels that are believed to interfere with bactericidal activity.16 Discontinuation of immunosuppressants is also usually needed to effectively treat malakoplakia.9 However, unlike other previously reported cases, this case did not reveal any symptoms and antecedent infection with gram-negative bacteria despite extensive colonic involvement. This case is unprecedented to date, and therefore there are no available treatment guidelines. Our patient did not require any of the above therapies, given her indolent and asymptomatic presentation.
Notes
No potential conflict of interest relevant to this article was reported.