Sebaceous Carcinoma Arising in Mature Cystic Teratoma of Ovary
Article information
Abstract
Roughly 1% of mature cystic teratomas undergo malignant transformation. In particular, cutaneous-type adnexal neoplasms may occur in mature cystic teratomas. Sebaceous carcinomas, which arise from mature cystic teratomas, have rarely been observed, with only seven cases previously reported. Here, we present a case of a 69-year-old female who had pelvic pain for two weeks and who subsequently underwent bilateral salpingo-oophorectomy and hysterectomy. Her left ovary showed a unilocular cyst, measuring 22.0 cm in diameter, filled with sebaceous material and a few hairs. A luminally-protruding solid mass measuring 4.0 cm in diameter was also noted. Microscopic findings revealed lobular or diffusely arranged basophilic, atypical sebaceous cells connected to a typical mature cystic teratoma. Tumor cells demonstrated positive immunoreactivity for high molecular weight cytokeratin, cytokeratin 7, cytokeratin 19, epithelial membrane antigen, and carcinoembryonic antigen. Here, we present a case of sebaceous carcinoma arising from a mature cystic teratoma along with a review of previously published reports.
Mature cystic teratoma (MCT) is the most common ovarian tumor.1 Malignant transformation of a component of a MCT is a very rare event, occurring in less than 2% of cases,2 with squamous cell carcinoma as the most common malignancy.1,3 Cutaneous-type adnexal neoplasms including basal cell carcinoma, melanoma, and apocrine adenocarcinoma have also been reported as associated malignancies with MCT.4,5 Sebaceous carcinoma arising within a MCT has been rarely reported. To our knowledge, there have been only seven prior reports of sebaceous carcinoma arising within a MCT of the ovary.6-12 Here, we present an additional case of a sebaceous carcinoma, along with a sebaceous adenoma, arising within a MCT, as well as a review of the previous reports.
CASE REPORT
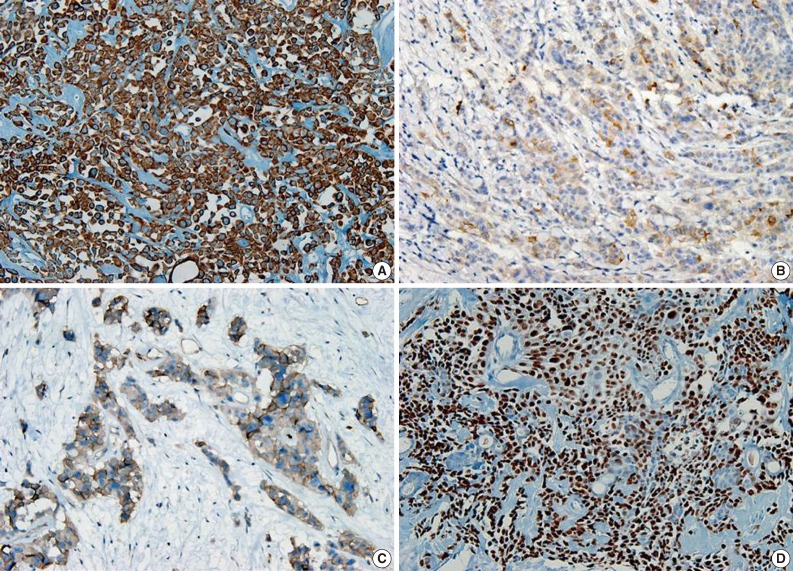
A 69-year-old gravida 4, para 4 Korean woman visited our hospital with a two week history of pelvic pain. Ultrasonography and computed tomography of the abdomen demonstrated a large pelvic mass measuring 22.0 cm in maximal diameter and with a small amount of ascites. Her preoperative serum cancer antigen 125 level was elevated at 430.5 U/mL. Together, these findings were suggestive of malignancy. A total abdominal hysterectomy with bilateral salpingo-oophorectomy and partial omentectomy were performed. A massive left ovarian mass and omental cake were noted in the pelvic cavity, and numerous nodules (1-2 cm in size) were scattered in the peritoneum and along the intestinal serosal surface. A neoplastic implant measuring 1.5 cm in diameter was also observed at the posterior portion of the uterine body. The right ovary was unremarkable. The resected left ovary measured 22.0 cm in diameter and weighed 2,180 g. The outer, capsular surface of the ruptured left ovary appeared ragged with scattered tumor implants measuring up to 1.2 cm in diameter. On the cut section, the left ovary was replaced by a unilocular cyst filled with keratin-like material and brownish-serous fluid. The luminal surface of the cyst was smooth, but a luminally-protruding and outer expanding mass measuring 6.0 cm in diameter was noted. The cut surface of the mass was relatively grayish-white in color and firm in the luminal portion and was tan-colored and friable with hemorrhagic necrosis in the outer, expanding portion (Fig. 1). Microscopically, the smooth cystic wall was lined by stratified squamous epithelium with underlying sebaceous glands and other skin adnexal structures, findings consistent with a typical mature cystic teratoma. Benign squamous epithelium was abruptly replaced by a nodular arrangement of germinative cells with a pushing border, which protruded into the cyst lumen (Fig. 2). The nodular portion showed an alternatively dark and white area, which corresponded to generative cells (dark) and sebaceous cells (light) with cytoplasmic lipid vacuoles. There was no cytologic atypia or sparse mitosis. Taken together, these findings led to a diagnosis of sebaceous adenoma. Beneath the nodular portion, infiltrating trabeculae or nests of atypical cells were noted. The infiltrating portion was mostly separated but was focally contiguous with the sebaceous adenoma (Fig. 3A). Infiltrating cells exhibited conspicuous vacuoles in the cytoplasm and remarkable nuclear pleomorphism, prominent nucleoli, and frequent abnormal mitoses (Fig. 3B). There was no peripheral nuclear palisading or cleft-like spaces between the lobules. Tumor cells were immunohistochemically positive for cytokeratin (CK) 7 (Fig. 4A), CK19, high molecular weight CK, epithelial membrane antigen (EMA) (Fig. 4B), carcinoembryonic antigen (CEA) (Fig. 4C), and p63 (Fig. 4D), but stains were negative for CK20, p53, vimentin, human placental alkaline phosphatase, α-inhibin, S100 protein, c-erbB-2, estrogen receptor, and progesterone receptor. Periodic acid-Schiff (PAS) was negative in the large, cytoplasmic inclusion. The uterine myometrium showed direct infiltration of the sebaceous carcinoma, and the omental mass also revealed the same histology. The patient developed sepsis two weeks after diagnosis and was discharged home with palliative care.
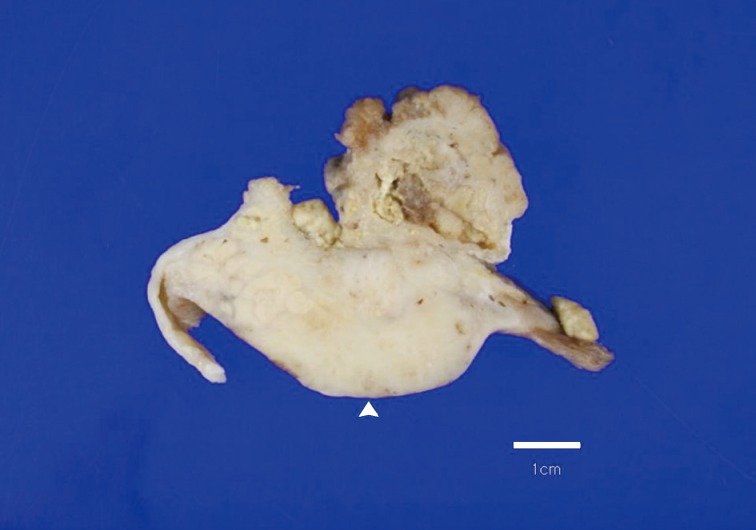
Overall findings of the left ovary demonstrate a unilocular cyst filled with keratin-like material. A luminally-protruding (arrow head) and outer, expanding mass measuring 6.0 cm is noted. The cut surface of the mass is relatively grayish-white and firm in the luminal portion and was tan-colored and friable with hemorrhagic necrosis in the outer, expanding region.
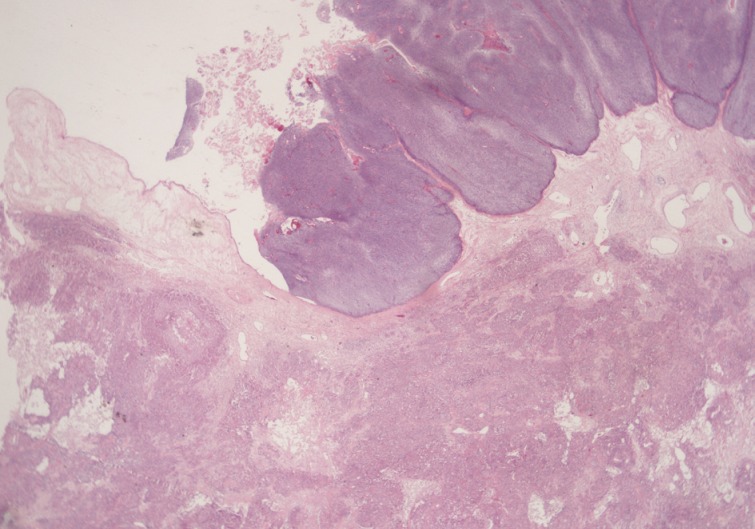
Microscopically, the luminally-protruding portion exhibits a nodular arrangement of germinative cells with a pushing border that is abruptly connected to a stratified squamous epithelium of a mature cystic teratoma. Beneath the nodular portion, sheets or nests of infiltrating atypical cells are noted.
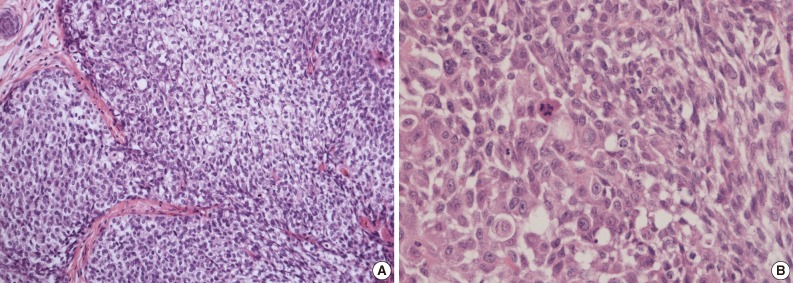
(A) Infiltrating cells arranged in trabeculae or nests that are focally contiguous to the inner, nodular portion with similar cytologic features. (B) Infiltrating cells show conspicuous vacuoles in the cytoplasm along with remarkable nuclear pleomorphism, prominent nucleoli, and frequent abnormal mitoses.
DISCUSSION
As the most common ovarian germ cell tumor, MCT undergoes malignant transformation in less than 2% of cases.2 MCTs with malignant change are usually larger than conventional MCT and have a grossly cauliflower-like appearance or a mural nodule protruding into the cyst lumen.11 Clinical presentation of malignant MCT depends on the extension and histological type of the secondary malignancy. Squamous cell carcinoma serves as the most common secondary tumor seen in 80% of malignantly-transformed MCT cases.1,3 Other malignancies have also been associated with MCT including adenocarcinoma, adenosquamous carcinoma, sarcoma, and melanoma.13,14 Normal skin adnexal structures, including sebaceous glands, are frequently observed in MCTs, but malignant, neoplastic transformation in such tissues is rare. Sebaceous cell carcinoma in association with MCT has been infrequently reported with only seven cases published.7,11
Sebaceous carcinomas arising within MCTs have similar gross and microscopic findings with their cutaneous counterparts.15 However, tumor cells are frequently large and can have a squamoid appearance with occasional squamous pearls.10 In such cases, sebaceous carcinoma must be distinguished from squamous carcinoma with hydropic change. Occasionally, malignant squamous cells may accumulate glycogen with clear cytoplasm. This can be confused with large, foamy sebaceous cells. The present case also revealed squamoid differentiation. In contrast, the large, foamy sebaceous cells were PAS negative, a finding that confirmed the absence of glycogen, and the majority of tumor cell nests had sebaceous differentiation. Basal cell carcinoma was also considered in the differential diagnosis of sebaceous carcinoma with basaloid differentiation and inconspicuous lipidization. However, the lack of peripheral, palisading and peritumoral cleft along with the presence of obvious sebaceous differentiation throughout the tumor nest helped to exclude basal cell carcinoma. Basal cell carcinomas with sebaceous differentiation usually have vacuolated cells peripherally rather than centrally.8
Sebaceous adenomas show nodular, lobulated growth with dark and light areas that correspond to generative cells (dark) and sebaceous cells (light) with cytoplasmic lipid vacuoles. In the present case, the luminally-protruding portion revealed a circumscribed sebaceous adenoma along with a contiguously-infiltrating, poorly-circumscribed sebaceous carcinoma. There are distinctive differences in cytology and pattern of invasion between sebaceous adenomas and sebaceous carcinomas. Cytologically, sebaceous adenomas lack striking nuclear atypia that was prominently seen in the sebaceous carcinoma area. Mitoses are also more frequent in carcinomas with atypical features.
The pathogenesis of primary sebaceous carcinomas arising within a MCT has been debated. One hypothesis purports that carcinoma originates from pluripotent stem cells whereas other theories suggest that carcinoma arises from differentiated sebaceous cells in a MCT that then undergoes malignant transformation.11 The present case appears to support the malignant transformation hypothesis. However, the case here was also positive for the stem cell marker p63 in the squamoid area as well as positive for CK7 and CK19, findings suggestive of sebaceous differentiation. CEA and EMA are well-known markers for sweat gland differentiation in skin adnexal neoplasms. EMA is highly conserved in the neoplastic proliferation of adnexal structures with sebaceous differentiation16 and serves as a useful marker for discriminating from basal cell carcinoma. Our case exhibited focal positivity for EMA and CEA. Overexpression of c-erbB2 and hormonal receptors have been reported in sebaceous carcinomas,17 but both were negative in the current case.
In general, prognosis for teratomas with malignant transformation is influenced by tumor extension and capsular rupture rather than degree of tumor extension and capsular and blood vessel invasion by the tumor.9 Prognosis of sebaceous carcinomas arising in an ovary remains unclear due to their rarity, although none of the patients in prior reports appear to have developed recurrence or metastases aside from one case. That patient was diagnosed as stage IIIB and required postoperative chemotherapy (cyclophosphamide and adriamycin) but subsequently died of urinary obstruction and sepsis,6 whereas the other six women were alive at 4-72 months of follow-up.7-12 Although our patient has been lost to follow-up, her general condition was poor with associated secondary sepsis. Her prognosis was therefore deemed poor.
In summary, cases of sebaceous carcinoma arising within an ovarian MCT are extremely rare. The case presented here appears to be the first to report both a sebaceous carcinoma arising within an ovarian MCT combined with a sebaceous adenoma. The behavior of a sebaceous carcinoma arising from an ovarian MCT remains poorly understood due to their rarity. The present case was associated with metastasis and peritoneal seeding whereas previous reports mostly had uneventful outcomes.
Notes
No potential conflict of interest relevant to this article was reported.