Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(4); 2013 > Article
-
Case Study
Sebaceous Carcinoma Arising in Mature Cystic Teratoma of Ovary - Hyo Jeong An, Yong Han Jung, Hye Kyoung Yoon, Soo Jin Jung
-
Korean Journal of Pathology 2013;47(4):383-387.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.4.383
Published online: August 26, 2013
Department of Pathology, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- Corresponding Author: Soo Jin Jung, M.D. Department of Pathology, Inje University Busan Paik Hospital, Inje University College of Medicine, 75 Bokji-ro, Busanjin-gu, Busan 614-735, Korea. Tel: +82-51-890-6642, Fax: +82-51-891-1834, soojinmd@hanmail.net
• Received: September 11, 2012 • Revised: October 30, 2012 • Accepted: October 31, 2012
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Roughly 1% of mature cystic teratomas undergo malignant transformation. In particular, cutaneous-type adnexal neoplasms may occur in mature cystic teratomas. Sebaceous carcinomas, which arise from mature cystic teratomas, have rarely been observed, with only seven cases previously reported. Here, we present a case of a 69-year-old female who had pelvic pain for two weeks and who subsequently underwent bilateral salpingo-oophorectomy and hysterectomy. Her left ovary showed a unilocular cyst, measuring 22.0 cm in diameter, filled with sebaceous material and a few hairs. A luminally-protruding solid mass measuring 4.0 cm in diameter was also noted. Microscopic findings revealed lobular or diffusely arranged basophilic, atypical sebaceous cells connected to a typical mature cystic teratoma. Tumor cells demonstrated positive immunoreactivity for high molecular weight cytokeratin, cytokeratin 7, cytokeratin 19, epithelial membrane antigen, and carcinoembryonic antigen. Here, we present a case of sebaceous carcinoma arising from a mature cystic teratoma along with a review of previously published reports.
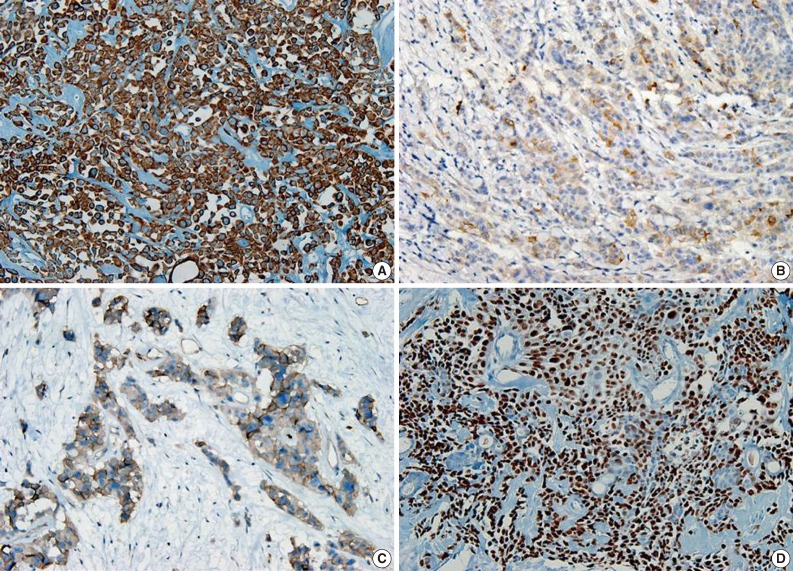
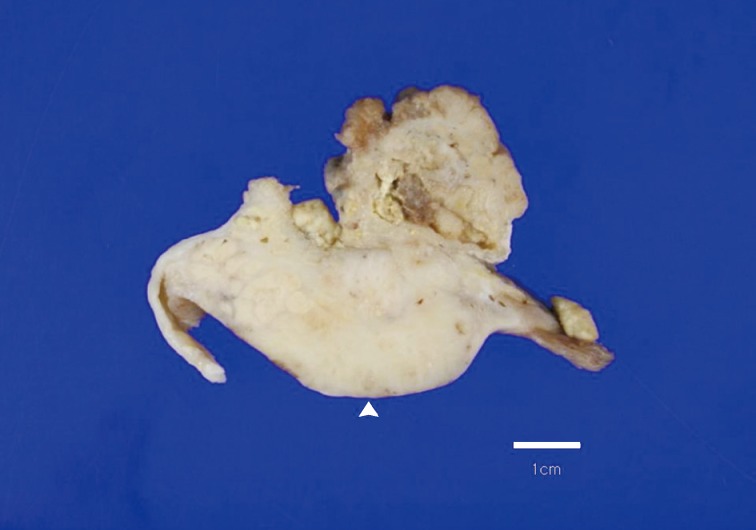
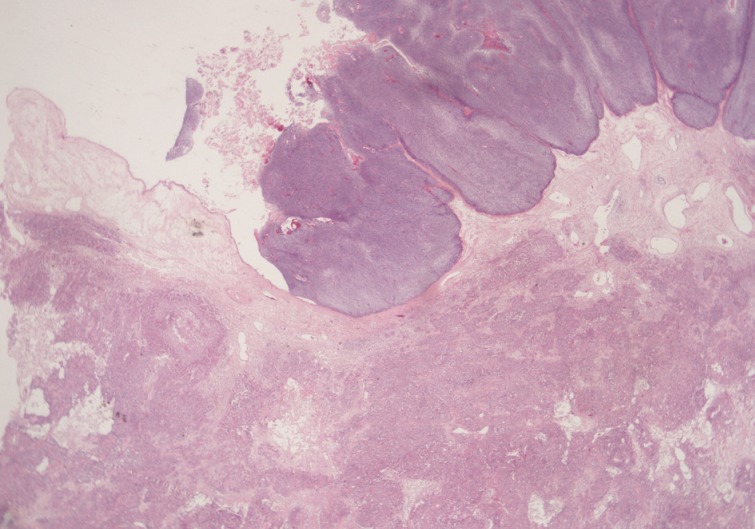
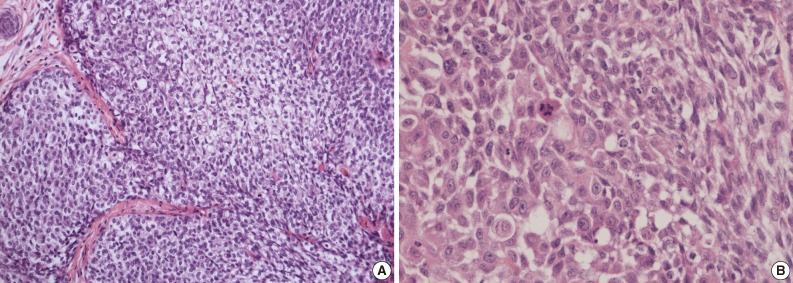
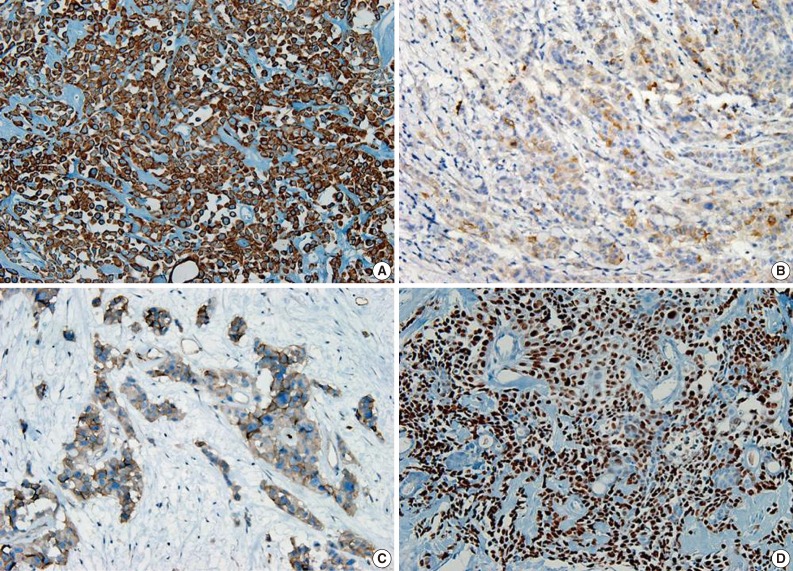
- A 69-year-old gravida 4, para 4 Korean woman visited our hospital with a two week history of pelvic pain. Ultrasonography and computed tomography of the abdomen demonstrated a large pelvic mass measuring 22.0 cm in maximal diameter and with a small amount of ascites. Her preoperative serum cancer antigen 125 level was elevated at 430.5 U/mL. Together, these findings were suggestive of malignancy. A total abdominal hysterectomy with bilateral salpingo-oophorectomy and partial omentectomy were performed. A massive left ovarian mass and omental cake were noted in the pelvic cavity, and numerous nodules (1-2 cm in size) were scattered in the peritoneum and along the intestinal serosal surface. A neoplastic implant measuring 1.5 cm in diameter was also observed at the posterior portion of the uterine body. The right ovary was unremarkable. The resected left ovary measured 22.0 cm in diameter and weighed 2,180 g. The outer, capsular surface of the ruptured left ovary appeared ragged with scattered tumor implants measuring up to 1.2 cm in diameter. On the cut section, the left ovary was replaced by a unilocular cyst filled with keratin-like material and brownish-serous fluid. The luminal surface of the cyst was smooth, but a luminally-protruding and outer expanding mass measuring 6.0 cm in diameter was noted. The cut surface of the mass was relatively grayish-white in color and firm in the luminal portion and was tan-colored and friable with hemorrhagic necrosis in the outer, expanding portion (Fig. 1). Microscopically, the smooth cystic wall was lined by stratified squamous epithelium with underlying sebaceous glands and other skin adnexal structures, findings consistent with a typical mature cystic teratoma. Benign squamous epithelium was abruptly replaced by a nodular arrangement of germinative cells with a pushing border, which protruded into the cyst lumen (Fig. 2). The nodular portion showed an alternatively dark and white area, which corresponded to generative cells (dark) and sebaceous cells (light) with cytoplasmic lipid vacuoles. There was no cytologic atypia or sparse mitosis. Taken together, these findings led to a diagnosis of sebaceous adenoma. Beneath the nodular portion, infiltrating trabeculae or nests of atypical cells were noted. The infiltrating portion was mostly separated but was focally contiguous with the sebaceous adenoma (Fig. 3A). Infiltrating cells exhibited conspicuous vacuoles in the cytoplasm and remarkable nuclear pleomorphism, prominent nucleoli, and frequent abnormal mitoses (Fig. 3B). There was no peripheral nuclear palisading or cleft-like spaces between the lobules. Tumor cells were immunohistochemically positive for cytokeratin (CK) 7 (Fig. 4A), CK19, high molecular weight CK, epithelial membrane antigen (EMA) (Fig. 4B), carcinoembryonic antigen (CEA) (Fig. 4C), and p63 (Fig. 4D), but stains were negative for CK20, p53, vimentin, human placental alkaline phosphatase, α-inhibin, S100 protein, c-erbB-2, estrogen receptor, and progesterone receptor. Periodic acid-Schiff (PAS) was negative in the large, cytoplasmic inclusion. The uterine myometrium showed direct infiltration of the sebaceous carcinoma, and the omental mass also revealed the same histology. The patient developed sepsis two weeks after diagnosis and was discharged home with palliative care.
CASE REPORT
- As the most common ovarian germ cell tumor, MCT undergoes malignant transformation in less than 2% of cases.2 MCTs with malignant change are usually larger than conventional MCT and have a grossly cauliflower-like appearance or a mural nodule protruding into the cyst lumen.11 Clinical presentation of malignant MCT depends on the extension and histological type of the secondary malignancy. Squamous cell carcinoma serves as the most common secondary tumor seen in 80% of malignantly-transformed MCT cases.1,3 Other malignancies have also been associated with MCT including adenocarcinoma, adenosquamous carcinoma, sarcoma, and melanoma.13,14 Normal skin adnexal structures, including sebaceous glands, are frequently observed in MCTs, but malignant, neoplastic transformation in such tissues is rare. Sebaceous cell carcinoma in association with MCT has been infrequently reported with only seven cases published.7,11
- Sebaceous carcinomas arising within MCTs have similar gross and microscopic findings with their cutaneous counterparts.15 However, tumor cells are frequently large and can have a squamoid appearance with occasional squamous pearls.10 In such cases, sebaceous carcinoma must be distinguished from squamous carcinoma with hydropic change. Occasionally, malignant squamous cells may accumulate glycogen with clear cytoplasm. This can be confused with large, foamy sebaceous cells. The present case also revealed squamoid differentiation. In contrast, the large, foamy sebaceous cells were PAS negative, a finding that confirmed the absence of glycogen, and the majority of tumor cell nests had sebaceous differentiation. Basal cell carcinoma was also considered in the differential diagnosis of sebaceous carcinoma with basaloid differentiation and inconspicuous lipidization. However, the lack of peripheral, palisading and peritumoral cleft along with the presence of obvious sebaceous differentiation throughout the tumor nest helped to exclude basal cell carcinoma. Basal cell carcinomas with sebaceous differentiation usually have vacuolated cells peripherally rather than centrally.8
- Sebaceous adenomas show nodular, lobulated growth with dark and light areas that correspond to generative cells (dark) and sebaceous cells (light) with cytoplasmic lipid vacuoles. In the present case, the luminally-protruding portion revealed a circumscribed sebaceous adenoma along with a contiguously-infiltrating, poorly-circumscribed sebaceous carcinoma. There are distinctive differences in cytology and pattern of invasion between sebaceous adenomas and sebaceous carcinomas. Cytologically, sebaceous adenomas lack striking nuclear atypia that was prominently seen in the sebaceous carcinoma area. Mitoses are also more frequent in carcinomas with atypical features.
- The pathogenesis of primary sebaceous carcinomas arising within a MCT has been debated. One hypothesis purports that carcinoma originates from pluripotent stem cells whereas other theories suggest that carcinoma arises from differentiated sebaceous cells in a MCT that then undergoes malignant transformation.11 The present case appears to support the malignant transformation hypothesis. However, the case here was also positive for the stem cell marker p63 in the squamoid area as well as positive for CK7 and CK19, findings suggestive of sebaceous differentiation. CEA and EMA are well-known markers for sweat gland differentiation in skin adnexal neoplasms. EMA is highly conserved in the neoplastic proliferation of adnexal structures with sebaceous differentiation16 and serves as a useful marker for discriminating from basal cell carcinoma. Our case exhibited focal positivity for EMA and CEA. Overexpression of c-erbB2 and hormonal receptors have been reported in sebaceous carcinomas,17 but both were negative in the current case.
- In general, prognosis for teratomas with malignant transformation is influenced by tumor extension and capsular rupture rather than degree of tumor extension and capsular and blood vessel invasion by the tumor.9 Prognosis of sebaceous carcinomas arising in an ovary remains unclear due to their rarity, although none of the patients in prior reports appear to have developed recurrence or metastases aside from one case. That patient was diagnosed as stage IIIB and required postoperative chemotherapy (cyclophosphamide and adriamycin) but subsequently died of urinary obstruction and sepsis,6 whereas the other six women were alive at 4-72 months of follow-up.7-12 Although our patient has been lost to follow-up, her general condition was poor with associated secondary sepsis. Her prognosis was therefore deemed poor.
- In summary, cases of sebaceous carcinoma arising within an ovarian MCT are extremely rare. The case presented here appears to be the first to report both a sebaceous carcinoma arising within an ovarian MCT combined with a sebaceous adenoma. The behavior of a sebaceous carcinoma arising from an ovarian MCT remains poorly understood due to their rarity. The present case was associated with metastasis and peritoneal seeding whereas previous reports mostly had uneventful outcomes.
DISCUSSION
- 1. Anteby EY, Ron M, Revel A, Shimonovitz S, Ariel I, Hurwitz A. Germ cell tumors of the ovary arising after dermoid cyst resection: a long-term follow-up study. Obstet Gynecol 1994; 83: 605-608. PubMed
- 2. Ayhan A, Bukulmez O, Genc C, Karamursel BS, Ayhan A. Mature cystic teratomas of the ovary: case series from one institution over 34 years. Eur J Obstet Gynecol Reprod Biol 2000; 88: 153-157. ArticlePubMed
- 3. Pins MR, Young RH, Daly WJ, Scully RE. Primary squamous cell carcinoma of the ovary: report of 37 cases. Am J Surg Pathol 1996; 20: 823-833. PubMed
- 4. Ueda Y, Kimura A, Kawahara E, Kitagawa H, Nakanishi I. Malignant melanoma arising in a dermoid cyst of the ovary. Cancer 1991; 67: 3141-3145. ArticlePubMed
- 5. Morimitsu Y, Nakashima O, Nakashima Y, Kojiro M, Shimokobe T. Apocrine adenocarcinoma arising in cystic teratoma of the ovary. Arch Pathol Lab Med 1993; 117: 647-649. PubMed
- 6. Betta PG, Cosimi MF. Sebaceous carcinoma arising in benign cystic teratoma of the ovary: case report. Eur J Gynaecol Oncol 1984; 5: 146-149. PubMed
- 7. Chumas JC, Scully RE. Sebaceous tumors arising in ovarian dermoid cysts. Int J Gynecol Pathol 1991; 10: 356-363. ArticlePubMed
- 8. Changchien CC, Chen L, Eng HL. Sebaceous carcinoma arising in a benign dermoid cyst of the ovary. Acta Obstet Gynecol Scand 1994; 73: 355-358. ArticlePubMed
- 9. Papadopoulos AJ, Ahmed H, Pakarian FB, Caldwell CJ, McNicholas J, Raju KS. Sebaceous carcinoma arising within an ovarian cystic mature teratoma. Int J Gynecol Cancer 1995; 5: 76-79. ArticlePubMed
- 10. Vartanian RK, McRae B, Hessler RB. Sebaceous carcinoma arising in a mature cystic teratoma of the ovary. Int J Gynecol Pathol 2002; 21: 418-421. ArticlePubMed
- 11. Ribeiro-Silva A, Chang D, Bisson FW, Ré LO. Clinicopathological and immunohistochemical features of a sebaceous carcinoma arising within a benign dermoid cyst of the ovary. Virchows Arch 2003; 443: 574-578. ArticlePubMedPDF
- 12. Venizelos ID, Tatsiou ZA, Roussos D, Karagiannis V. A case of sebaceous carcinoma arising within a benign ovarian cystic teratoma. Onkologie 2009; 32: 353-355. ArticlePubMed
- 13. Sumi T, Ishiko O, Maeda K, Haba T, Wakasa K, Ogita S. Adenocarcinoma arising from respiratory ciliated epithelium in mature ovarian cystic teratoma. Arch Gynecol Obstet 2002; 267: 107-109. ArticlePubMedPDF
- 14. Vimla N, Kumar L, Thulkar S, Bal S, Dawar R. Primary malignant melanoma in ovarian cystic teratoma. Gynecol Oncol 2001; 82: 380-383. ArticlePubMed
- 15. Cicchini C, Larcinese A, Ricci F, Aurello P, Mingazzini PL, Indinnimeo M. Monodermal highly specialized teratoma of the ovary: a sebaceous gland tumor. Eur J Gynaecol Oncol 2002; 23: 442-444. PubMed
- 16. Metze D, Soyer HP, Zelger B, et al. Expression of a glycoprotein of the carcinoembryonic antigen family in normal and neoplastic sebaceous glands: limited role of carcinoembryonic antigen as a sweat gland marker. J Am Acad Dermatol 1996; 34(5 Pt 1):735-744. PubMed
- 17. Cho KJ, Khang SK, Koh JS, Chung JH, Lee SS. Sebaceous carcinoma of the eyelids: frequent expression of c-erbB-2 oncoprotein. J Korean Med Sci 2000; 15: 545-550. ArticlePubMedPMC
REFERENCES
Fig. 1Overall findings of the left ovary demonstrate a unilocular cyst filled with keratin-like material. A luminally-protruding (arrow head) and outer, expanding mass measuring 6.0 cm is noted. The cut surface of the mass is relatively grayish-white and firm in the luminal portion and was tan-colored and friable with hemorrhagic necrosis in the outer, expanding region.
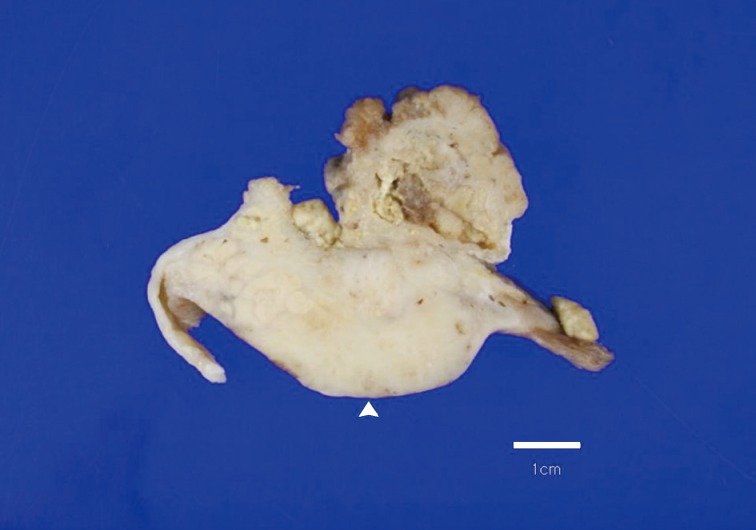

Fig. 2Microscopically, the luminally-protruding portion exhibits a nodular arrangement of germinative cells with a pushing border that is abruptly connected to a stratified squamous epithelium of a mature cystic teratoma. Beneath the nodular portion, sheets or nests of infiltrating atypical cells are noted.
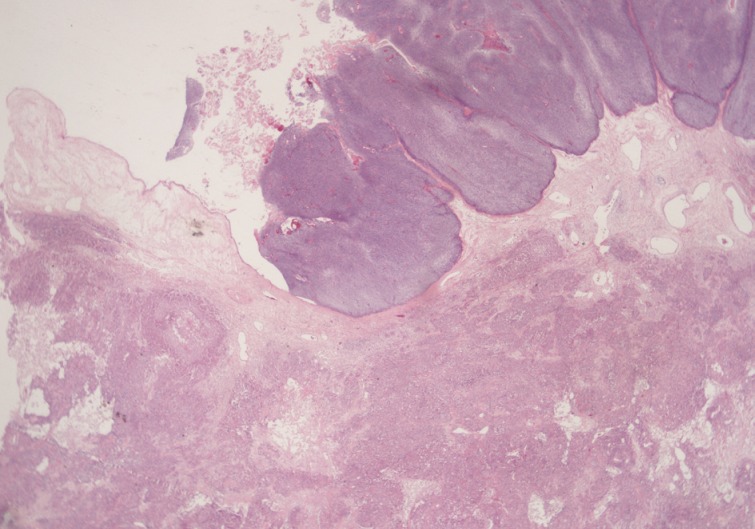

Fig. 3(A) Infiltrating cells arranged in trabeculae or nests that are focally contiguous to the inner, nodular portion with similar cytologic features. (B) Infiltrating cells show conspicuous vacuoles in the cytoplasm along with remarkable nuclear pleomorphism, prominent nucleoli, and frequent abnormal mitoses.
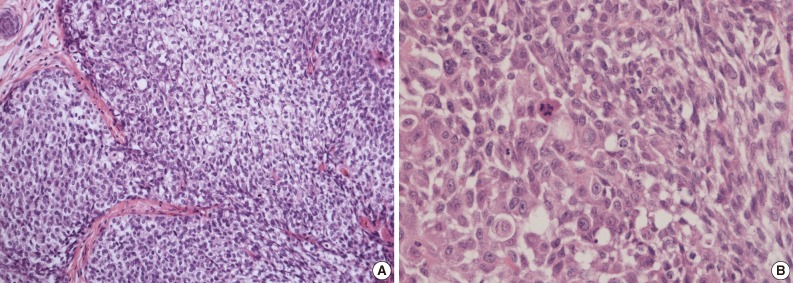

Figure & Data
References
Citations
Citations to this article as recorded by 

- How can we best manage ovarian sebaceous carcinomas arising from mature cystic teratomas?
Hong Min Shaye Peng, Sung Hock Chew, Yang Huang Grace Ng, Felicia Hui Xian Chin
BMJ Case Reports.2025; 18(2): e264651. CrossRef - Genetic Profiling of Sebaceous Carcinoma Arising from an Ovarian Mature Teratoma: A Case Report
Sumika Zaitsu, Yoko Aoyagi, Haruto Nishida, Kohei Nakamura, Mitsutake Yano, Eiji Kobayashi
International Journal of Molecular Sciences.2024; 25(12): 6351. CrossRef - Extraocular sebaceous carcinoma arising in a mature cystic teratoma of ovary: A case report and review of literature
Sara Pakbaz, Tanya Chawla, Marcus Q Bernardini, Liat Hogen, Marjan Rouzbahman
Human Pathology Reports.2022; 27: 300592. CrossRef - Sebaceous adenoma occurring within an intracranial dermoid cyst
Takashi Minamisaka, Johji Imura, Keitaro Shiraishi, Kohji Takagi, Takahiko Tomia, Sinichi Tanaka, Akira Noguchi, Takuya Akai, Kyo Noguchi, Satoshi Kuroda
Neuropathology.2022; 42(4): 289. CrossRef - Malignant transformation of mature cystic teratoma of the ovary
Doaa Atwi, Maria Kamal, Michael Quinton, Lewis A. Hassell
Journal of Obstetrics and Gynaecology Research.2022; 48(12): 3068. CrossRef - Sebaceous Carcinoma Arising in Ovarian Teratoma: First Report Associated With Germline Mismatch Repair Gene Mutation
Jacinta Murray, Patrick McIlwaine, Patrick J. Morrison, W. Glenn McCluggage
International Journal of Gynecological Pathology.2022; 41(6): 608. CrossRef - Impact of surgery and adjuvant treatment on the outcome of extraocular sebaceous carcinoma: a systematic review and individual patient's data analysis of 206 cases
Prashanth Giridhar, Lakhan Kashyap, Supriya Mallick, Ashish Dutt Upadhyay, Goura K. Rath
International Journal of Dermatology.2020; 59(4): 494. CrossRef - Mismatch repair deficiency is implicated in carcinoma arising from ovarian teratoma
Alvin Ho-Kwan Cheung, Chit Chow, Mei-Yung Yu, Wendy Wai-Tak Law, Peggy Pui-Ying Law, Paul Cheung-Lung Choi, Wei Kang, Ka-Fai To
Pathology.2019; 51(1): 67. CrossRef - Malignant transformation of an ovary mature cystic teratoma: case report and review of the literature
Elkin Fabián Dorado-Roncancio, Oscar Joel Carrillo-Garibaldi
Obstetrics & Gynecology International Journal.2019;[Epub] CrossRef - A case of ovarian clear cell carcinoma arising from ovarian mature cystic teratoma
Kazuya Maeda, Yoshito Terai, Shinichi Terada, Hiroshi Maruoka, Yuhei Kogata, Keisuke Ashihara, Yoshimichi Tanaka, Tomohito Tanaka, Hiroshi Sasaki, Satoshi Tsunetoh, Takashi Yamada, Masahide Ohmichi
Journal of Ovarian Research.2018;[Epub] CrossRef - Sebaceous carcinoma arising within an ovarian mature cystic teratoma: A case report with discussion of clinical management and genetic evaluation
Alyssa Wield, Melissa Hodeib, Mohammad Khan, Lindsay Gubernick, Andrew J. Li, Shivani Kandukuri
Gynecologic Oncology Reports.2018; 26: 37. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Sebaceous Carcinoma Arising in Mature Cystic Teratoma of Ovary




Fig. 1 Overall findings of the left ovary demonstrate a unilocular cyst filled with keratin-like material. A luminally-protruding (arrow head) and outer, expanding mass measuring 6.0 cm is noted. The cut surface of the mass is relatively grayish-white and firm in the luminal portion and was tan-colored and friable with hemorrhagic necrosis in the outer, expanding region.
Fig. 2 Microscopically, the luminally-protruding portion exhibits a nodular arrangement of germinative cells with a pushing border that is abruptly connected to a stratified squamous epithelium of a mature cystic teratoma. Beneath the nodular portion, sheets or nests of infiltrating atypical cells are noted.
Fig. 3 (A) Infiltrating cells arranged in trabeculae or nests that are focally contiguous to the inner, nodular portion with similar cytologic features. (B) Infiltrating cells show conspicuous vacuoles in the cytoplasm along with remarkable nuclear pleomorphism, prominent nucleoli, and frequent abnormal mitoses.
Fig. 4 Cytoplasm of tumor cells reveals immunohistochemically-positive reaction for cytokeratin 7 (A), epithelial membrane antigen (B), carcinoembryonic antigen (C), and nuclear positive reaction for p63 (D).
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Sebaceous Carcinoma Arising in Mature Cystic Teratoma of Ovary

 E-submission
E-submission