Guideline Recommendations for Testing of ALK Gene Rearrangement in Lung Cancer: A Proposal of the Korean Cardiopulmonary Pathology Study Group
Article information
Abstract
Rearrangement of anaplastic lymphoma kinase (ALK) gene is the best predictor of response to crizotinib, an ALK tyrosine kinase inhibitor. However, the prevalence of the ALK fusion is low, so accurate patient identification is crucial for successful treatment using ALK inhibitors. Furthermore, most patients with lung cancer present with advanced-stage disease at the time of diagnosis, so it is important for pathologists to detect ALK-rearranged patients while effectively maximizing small biopsy or cytology specimens. In this review, we propose a guideline recommendation for ALK testing approved by the Cardiopulmonary Pathology Study Group of the Korean Society of Pathologists.
Lung cancer remains the leading cause of cancer-related death throughout the world.1 Fortunately, outstanding development in cancer genomics and molecular targeted therapy has allowed targeted therapy that includes treatment optimization based on molecular testing and target mutation, especially in adenocarcinoma. Specifically, the great success of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) following the discovery of somatic EGFR gene mutation has raised hope that a targeted therapy will improve survival and quality of life for lung cancer patients.2-4 In this context, genetic analyses of various molecular markers in tumor tissue have become standard laboratory tests for the clinical management of lung cancers.
In 2007, the anaplastic lymphoma kinase (ALK) gene rearrangement creating an in-frame fusion protein between echinoderm microtubule-associated protein-like 4 (EML4) and ALK was described in non-small cell lung carcinoma (NSCLC) by Soda et al.5 The presence of ALK gene rearrangement in lung adenocarcinomas is the best predictor of response to crizotinib, an ALK tyrosine kinase inhibitor.6-8 The Food and Drug Administration (FDA) approved crizotinib with a companion diagnostic fluorescence in situ hybridization (FISH) test (Vysis, Abbott Molecular Inc., Des Plaines, IL, USA) for ALK-rearranged NSCLC. However, the prevalence of the ALK fusion is low, at around 5% in various previous studies.6,8-13 Thus, accurate patient identification is crucial for successful treatment using the ALK inhibitor and the proposal of a standard guideline suitable for the Korean medical community is also needed.
Herein, we reviewed several publications related with consensus opinions and recommendations for molecular testing in lung cancer, including ALK testing, and propose a guideline recommendation for ALK testing approved by the Cardiopulmonary Pathology Study Group of the Korean Society of Pathologists (Table 1).
PATIENT ELIGIBILITY
Clinical characteristics associated with the ALK gene rearrangement are known to be adenocarcinoma histology, never/light smoking history, and younger age.6,13-15 However, not all ALK-rearranged patients demonstrate these characteristics. ALK fusion has also been detected in older patients (over 70 years old) with a smoking history, and in patients with squamous cell carcinoma.12,16 Therefore, clinical characteristics alone are not the determinant of ALK testing. Guidelines from the College of American Pathologists (CAP), the International Association for the Study of Lung Cancer (IASLC), and the Association for Molecular Pathology (AMP) also state that clinical characteristics (age, sex, ethnicity, smoking history, etc.) are not sufficiently sensitive or specific to be used to select or exclude patients for treatment or testing for ALK gene rearrangement.17
Recent published guidelines recommend histological type as the most important factor for determining whether ALK testing should be performed. Patients who are diagnosed with adenocarcinoma, large cell carcinoma, or non-small cell carcinoma with an adenocarcinoma component are recommended for ALK testing.17,18 Thus, accurate histological diagnosis is the first step for molecular testing. Pathologists should try to further classify poorly differentiated NSCLCs into more specific types using immunohistochemistry (IHC), such as thyroid transcription factor-1, napsin A, p63, p40, and cytokeratin5/6.19-24 There have been a few reports about squamous cell carcinoma harboring ALK gene rearrangement, but the frequency is very low.6,16 Thus, the CAP-IASLC-AMP guideline does not recommend ALK testing in lung cancer cases without any adenocarcinoma component, such as pure squamous cell carcinomas, pure small cell carcinomas, or large cell carcinomas lacking any IHC evidence of adenocarcinoma differentiation.17
Actually, about two-thirds of lung cancer patients present with advanced stage at the time of diagnosis, and small biopsies or cytology specimens are the only available samples for diagnosis and molecular testing. In these cases, histological sub-typing may not be always feasible, the biopsy or cytology specimens may not be representative of the whole tumor, and any adenocarcinoma component cannot be completely excluded. For these cases, ALK testing is recommended and the clinical features, such as young age and/or lack of smoking history, may be used to select patients for testing.17
SPECIMEN TYPE AND PROCESSING FOR ALK TESTING
Specimen type
Various biopsy specimens obtained by different techniques including endoscopic biopsies, core-needle biopsies, biopsies guided by endobronchial ultrasound (EBUS) or endoscopic esophageal ultrasound, mediastinoscopy, and thoracotomy can be used for ALK testing. Recent studies have shown that cytology specimens are suitable for ALK testing, and that the results are highly concordant with those of corresponding histological specimens, thus cytology specimens such as EBUS-guided fine-needle aspiration (FNA), transthoracic FNA, bronchial secretions or brushes, bronchoalveolar lavages, and pleural effusions can be used for ALK testing.25-28 Thus, both histological and cytological specimens are acceptable for ALK testing, if appropriately processed and validated.
Tumor tissues from either primary tumors or metastatic lesions are equally suitable for ALK testing according to biopsy accessibility. Although discordance in ALK status between primary and metastatic disease has been reported,29 data are insufficient regarding which one is better for ALK testing.17 For patients with multiple, synchronous primary lung adenocarcinomas, each tumor may be tested. However, testing of multiple different areas within a single tumor is not necessary, because heterogeneity of ALK gene status does not seem to be related to the presence of a different histological pattern or biology, but is more likely due to technical problems.17
Sample selection
A sufficient number of tumor cells are crucial for successful molecular testing. The number of tumor cells required for IHC assessment of ALK protein remains undefined, as IHC can be performed as long as there are at least a few clusters of viable tumor cells. Regarding ALK FISH, a minimum of 50 to 100 assessable tumor cells are required. Unlike EGFR mutation testing, cells are analyzed individually, so tumor percentage is not as critical. However, it is necessary to choose slides or regions of slides in which the tumor cells do not overlap, and to distinguish them from the adjacent non-neoplastic cells. If the tumor component is very focal within the sample, it is recommended that the area examined be marked on the slide so that it may be readily identifiable under a dark field fluorescence microscope. In this step, the pathologist has the responsibility to determine if the selected sample contains a sufficient number and quality of tumor cells to ensure the quality of the analysis.
Sample processing
Formalin-fixed, paraffin-embedded (FFPE) tissues are routinely used for molecular testing. The diagnostic kits for ALK testing were developed and validated only on FFPE histological samples.18 The first and most important step in tissue processing is immediate fixation, preferably within one hour after the sample is removed from the patient.30,31 It is widely recognized that 10% neutral-buffered formalin is ideal for preparing FFPE samples, whereas the optimal fixation time ranges from six to 48 hours.18,31-33 Specimens treated with decalcifying solution (e.g., bone biopsy) are usually suboptimal for molecular studies, because the solution may interfere with IHC and can frequently compromise FISH testing, so the reliability of molecular testing is reduced.
Regarding cytological specimens, most sample types, including conventional smears, cytospins, or liquid-based preparations (e.g., ThinPrep, Hologic, or SurePath, BD Diagnostics) regardless of fixation type (air-dried and alcohol-based fixatives) can be used for ALK testing. Cytology specimens should be fixed immediately by the usual alcohol-based methods. For FISH analysis, the use of adhesive-coated or positively charged slides in lung cytology is recommended, as these slides improve the adherence of the cells. FISH works equally well on unstained specimens as well as those processed with Papanicolaou, hematoxylin, or a modified Giemsa stain, and a separate procedure is not usually required. However, in case of a modified Giemsa stain, de-staining with an acid-alcohol technique is recommended before FISH analysis.25 Cell blocks are regarded as appropriate for molecular testing and can be handled in the same way as histological FFPE specimens.
DIAGNOSTIC METHODS FOR ALK GENE REARRANGEMENT
Several methods are currently available to assess ALK gene rearrangement, including FISH, IHC, and reverse transcriptase-polymerase chain reaction (RT-PCR).
Fluorescence in situ hybridization
FISH is currently the standard method to detect ALK rearrangements because it was used previously in clinical trials, and is the only test correlated with clinical response. The FDA has approved crizotinib with a companion diagnostic FISH test for ALK-rearranged NSCLC using the certified commercial kit, Abbott Vysis (ALK Break Apart FISH Probe Kit, Abbott Molecular Inc.). The kit includes red (3') and green (5')-colored break-apart probes, which overlap in a fused signal without ALK gene rearrangement (Fig. 1A). Although the break-apart FISH assay requires experience for interpretation, patience, high cost, and technical expertise, this assay is highly sensitive and specific for the detection of ALK gene rearrangement regardless of ALK fusion partners.
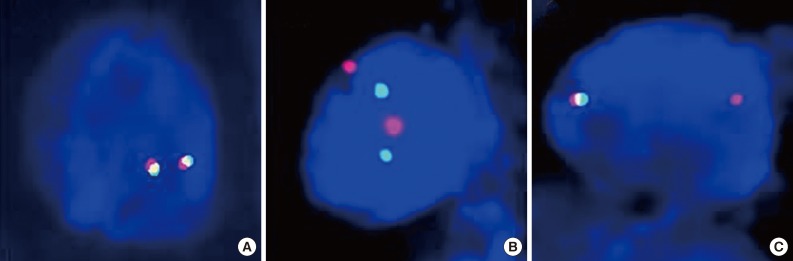
Break-apart fluorescence in situ hybridization (FISH) patterns. Representative FISH images of anaplastic lymphoma kinase (ALK)-negative (A) and ALK-rearranged tumors (B, C) are demonstrated. Tumors are considered ALK-positive when green and red signals are separated by at least two signal diameters (B) or a single red signal without a corresponding green signal, in addition to fused and/or break-apart signals (C).
Interpretation should be performed in areas of the slide with good signal, in which at least 50% of all nuclei are easily analyzable, with minimal background or nuclear fluorescent "noise." Areas where the borders of individual nuclei are not clearly identifiable and/or high cell density causes excessive nuclear overlap are easily misinterpreted, and should be avoided. Criteria that must be met for a break-apart FISH assay to be considered positive for ALK rearrangement include: at least 50 cells counted and at least 15% of the counted cells demonstrating separated green and red signals by at least two signal diameters (Fig. 1B), and/or an isolated red signal (Fig. 1C).34 There have been several reports in which a few cases showed "borderline" ALK FISH positivity that approached the 15% cutoff point or less than two signal diameter distances. These cases were regarded as negative with the current criteria and suggest alternative ALK diagnostic techniques and/or an assessment of their response to ALK inhibitors would be required. There are also rare instances when FISH showed atypical signal patterns, such as for an isolated 5' signal that does not fulfill current positive criteria. Such unusual patterns require confirmation of ALK status using a different secondary assay or examination of the effects of crizotinib therapy.
Immunohistochemistry
ALK protein represents a potential marker for indicating ALK gene rearrangement, and immunohistochemical detection of ALK protein can be a rapid screening method with low cost for detection of ALK-rearranged NSCLC. When the ALK1 antibody, which has been used for the diagnosis of anaplastic large cell lymphoma (ALCL) is applied, detection of ALK protein in NSCLCs is difficult because the protein expression level is usually lower in NSCLCs than in ALCL.35 Therefore, several factors including antigen retrieval, primary antibodies with high affinity and sufficient concentration, incubation time and temperature, and strong amplification of the signal should be applied to improve the sensitivity of ALK IHC. There are three ALK antibodies (ALK1, 5A4, and D5F3) that have been studied in depth for NSCLC. D5F3 and 5A4 appeared to be both more sensitive and more specific than the ALK1 antibody.35-38 For a detection system, there are various systems with a substantial degree of signal amplification (Leica/Novocastra Novolink, Dako Advance, Tyramide, Envision+, Ventana i-view). Using highly sensitive detection methods in combination with high affinity antibodies, IHC can effectively detect ALK fusion protein with high sensitivity and specificity. However, so far, there is no qualified guideline for the selection of primary antibodies or the detection system.
There are still several considerations regarding the use of ALK IHC as a screening method for ALK rearrangement. First of all, there is no accepted standard criterion for IHC interpretation. IHC scoring has been proposed by several publications, including a representatively binary system (positive/negative)9,37,39,40 and a scoring system in the range of 0 to 3 by signal intensity and percentage (Fig. 2).11,35,36,38,41-44 As a screening tool, IHC score is an important criterion for the selection of patients for ALK FISH testing or crizotinib therapy. Paik et al.11 showed good correlations between IHC score and FISH results: cases assigned an ALK IHC score of 3 showed FISH-positivity, while ALK IHC scores of 0 and 1 showed FISH-negativity. However, there are arguments that a four-tiered scoring system creates higher inter-observer variability, so a binary system is more than clear cut for the selection of ALK-positive patients.44 Thus, we recommend that tumors that are positive for ALK IHC, either weakly or strongly, should still be referred to FISH analysis for confirmation of a rearrangement, regardless of primary antibody or detection system.

Anaplastic lymphoma kinase protein expression by immunohistochemistry with a 0-3 intensity-based scoring system. Representative images of score 3 (A), 2 (B), 1 (C), and 0 (D) are displayed.
Another consideration is the lack of clinical validation for crizotinib treatment in ALK IHC positive and FISH negative cases. Although there was a report that showed a FISH negative, IHC positive patient who showed better response to crizotinib,45 this single instance is insufficient to develop a specific recommendation regarding the use of ALK IHC as a sole determinant of ALK TKI therapy.
If the IHC method is eventually proven to be accurate in detecting ALK rearrangements and variability, and interpretation can be standardized, IHC assays hold the potential to facilitate the routine identification of ALK-rearranged lung adenocarcinoma. Many studies have investigated and reported the high concordance between IHC and FISH results.11,35,39-44,46,47 The CAP-IASLC-AMP guideline also recommends that a properly validated IHC method be used as a screening modality, and that tumors that fail to demonstrate ALK immunoreactivity with a sensitive IHC method need not be tested for ALK rearrangement by FISH.17
Reverse transcriptase-polymerase chain reaction
ALK rearrangement generates a unique sequence and the PCR primer that hybridizes with the fusion transcript is responsible for the high sensitivity of the RT-PCR test.48-50 However, RT-PCR requires ALK fusion variants to be known so that primers to all variants should be included in the reaction.51-53 Although, despite an ever-expanding list of ALK fusion variants, all the reported variants require skillful application. In addition, the majority of current ALK fusion variants were detected by RT-PCR in fresh frozen tumor tissue. However, in daily clinical practice, most of the tumor tissues available for molecular profiling are FFPE samples,53 where the integrity of RNAs is likely to be greatly compromised compared with fresh frozen tissue. Thus, at the present time, RT-PCR is not recommended as a first-line diagnostic method for determining ALK fusion status.
DIAGNOSTIC ALGORITHM
Based on the advantages and characteristics of individual methods for detecting ALK gene status, several investigator groups have proposed diagnostic algorithms for ALK testing.11,18,36,54,55 Considering the trends that the histological types are usually determined by only small biopsy materials or cytological specimens in advanced NSCLC patients, we recommend that ALK rearrangement testing should be done simultaneously with EGFR/K-ras mutation testing for eligible patients (Fig. 3). We also propose that validated IHC can be used as a screening method to detect the ALK rearrangement and ALK FISH can be a confirmative method for all cases showing positive ALK IHC results. If the consensus between pathologists and institutional clinicians can be established, 'reflex molecular testing' is recommended to minimize the exhaustion of small biopsy tissue samples for the necessary molecular tests, and to provide a rapid determination of the genetic characteristics of NSCLC patients.
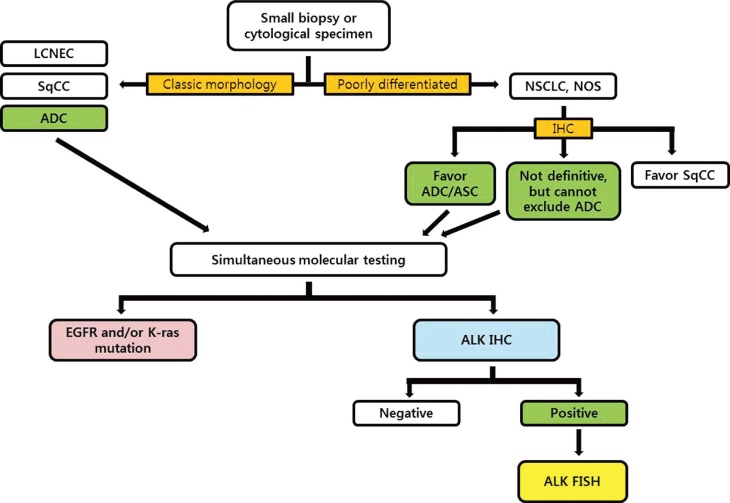
Diagnostic algorithm for molecular testing of small biopsy or cytological specimens in non-small cell lung cancer patients. LCNEC, large cell neuroendocrine carcinoma; SqCC, squamous cell carcinoma; ADC, adenocarcinoma; NSCLC, non-small cell lung carcinoma; NOS, not otherwise specified; ASC, adenosquamous carcinoma; IHC, immunohistochemistry; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; FISH, fluorescence in situ hybridization.
REPORTING THE RESULTS
Molecular testing reports should contain the following information: 1) identification of the patient including pathologic number, age, sex, hospital unit number, and requesting physician/department, 2) material used for the analysis including biopsy site and sample source, 3) methodology used for analysis and the type of commercial test used, and 4) test results, expressed in terms of negativity or positivity for the rearrangement of the ALK gene. We recommend reporting the following details: total number of counted nuclei, percentage of the nuclei showing gene rearrangements (break-apart and isolated red signal), as well as atypical pattern (e.g., isolated green signal), and copy number gain if observed, 5) receipt day and report day, 6) comments, and 7) names of testing technician and corresponding pathologist.
PROPOSAL FOR AN EXTERNAL QUALITY ASSESSMENT PROGRAM
For optimal ALK testing in NSCLC, the quality of the sample, validation status of the analytical procedure, and reliable reporting of the test results are crucial. There are several accessible external quality assessment (EQA) programs for the quality assessment of molecular testing (e.g., http://lung.eqascheme.org; http://kras.eqascheme.org; http://www.ukneqas.org.uk; http://www.emqn.org/emqn/schemes; http://www.quip-ringversuche.de). At this time, no external quality control program for ALK testing exists. To improve the reliability of assays for detecting ALK positivity, as well as optimal information regarding patient selection for ALK inhibitors, further studies should be performed to compare and validate these different diagnostic assays. Quality control programs used in HER-2 testing in Spain, the United Kingdom, and Scandinavia would be a good role model.33
CONCLUSION: ROLE OF PATHOLOGISTS AND FUTURE PERSPECTIVES
Molecular testing for targetable mutation has emerged as the standard of care in the management of patients with lung adenocarcinoma. Standardization and optimization of diagnostic molecular testing methods using clinical samples have become increasingly important. However, because most lung cancer patients present with an advanced tumor stage at the time of diagnosis, the diagnosis of lung cancer is often based on small specimens from a biopsy or cytology alone. Therefore, it is important that the pathologists handle specimens carefully for further molecular profiling, including EGFR and ALK tests, and do their best to obtain rapid and accurate determination of the patients who are candidates for targeted therapy. Each pathology department must fully validate the detection methods and develop a strategy to manage clinical samples and closely collaborate with clinicians. As a professional group, pathologists should take the lead in determining laboratory references, based on a balance between patient care and resource availability.
Notes
No potential conflict of interest relevant to this article was reported.