Classic Papillary Thyroid Carcinoma with Tall Cell Features and Tall Cell Variant Have Similar Clinicopathologic Features
Article information
Abstract
Background
The tall cell variant of papillary thyroid carcinoma (TCVPTC) is more aggressive than classic papillary thyroid carcinoma (PTC), but the percentage of tall cells needed to diagnose TCVPTC remains controversial. In addition, little is known about the clinicopathologic features of classic PTC with tall cell features (TCF).
Methods
We retrospectively selected and reviewed the clinicopathologic features and presence of the BRAF mutation in 203 cases of classic PTC, 149 cases of classic PTC with TCF, and 95 cases of TCVPTCs, which were defined as PTCs having <10%, 10-50%, and ≥50% tall cells, respectively.
Results
TCVPTCs and classic PTCs with TCF did not vary significantly in clinicopathologic characteristics such as pathologic (p) T stage, extrathyroidal extension, pN stage, lateral lymph node metastasis, or BRAF mutations; however, these features differed significantly in TCVPTCs and classic PTCs with TCF in comparison to classic PTCs. Similar results were obtained in a subanalysis of patients with microcarcinomas (≤1.0 cm in size).
Conclusions
Classic PTCs with TCF showed a similar BRAF mutation rate and clinicopathologic features to TCVPTCs, but more aggressive characteristics than classic PTCs.
Papillary thyroid carcinoma (PTC) is a disease with an indolent course, excellent overall prognosis, and a long-term survival rate close to that of the general population;1 however, some variants of PTC have been associated with an increased risk of recurrent disease and aggressive behavior.2 The tall cell variant of PTC (TCVPTC) is the most common aggressive variant of PTC.2,3,4 The incidence of TCVPTC ranges from 4% to 17% of all PTCs, and its disease-free 10-year survival rate is believed to be 10% to 15% lower than that of classic PTC.5,6 The incidence of this disease in Korea has been reported to be up to 5%, although there are limited existing data.7,8 Some studies suggest that TCV PTC is still under-diagnosed worldwide.3,4,5,9,10 In fact, 1% to 13% of tumors originally diagnosed as classic PTC can be reclassified as TCV by thyroid expertise.11,12 TCVPTC was originally described by Hawk and Hazard9 in 1976 as a distinctive subtype of PTC. Tall tumor cells are at least three times as tall as they are wide and have moderate to abundant eosinophilic cytoplasm, basally oriented nuclei and nuclear features typical of classic PTC.2,10 TCVPTCs present later in life and have more aggressive pathologic features such as a larger size and higher frequency of extrathyroidal extension, lymph node metastasis, vascular invasion, and distant metastasis, when compared to classic PTC.5,9,11,13,14 However, some studies have shown that the worse prognosis of TCVPTC is related to prognostic factors such as old age, tumor size, and extrathyroidal extension rather than the histologic type itself.4
There is still controversy with regard to what percentage of tall cells defines a TCVPTC.2,9,15 Thresholds ranging from 10 to 75% have been suggested by various studies.2,5,15,16,17,18 PTCs harboring fewer tall cells than the required cutoff percentage are diagnosed as classic PTC with tall cell features (TCF). Therefore, the incidence and clinical results of TCVPTC may vary according to different diagnostic criteria and the pathologist's level of experience.15,19
The goal of this study was to investigate the prevalence of TCVPTC in Korea and possible differences in clinicopathologic features between TCVPTCs and classic PTCs with TCF according to the percentage of tall cells.
MATERIALS AND METHODS
Patients
We performed a retrospective review of a prospectively maintained database of patients with PTC under approval by the Institutional Review Board of The Catholic University of Korea Seoul St. Mary's Hospital (KC13RISI0917). A total of 2,139 patients underwent surgery for PTC St. Mary's Hospital between January 2012 and December 2013. We enrolled a total of 244 consecutive patients with TCVPTC (n=95) and classic PTC with TCF (n=149) and also selected 203 consecutive patients with classic PTC as a control group. The age of the patients at the time of diagnosis ranged from 22 to 79 years (mean 47.6 years for classic PTC, mean 51.0 years for classic PTC with TCF, and mean 47.1 years for TCVPTC).
Histopathologic review
All classic PTC, classic PTC with TCF and TCVPTC cases were reviewed by three board-certified surgical pathologists (C.K.J, G.S.P, and Y.S.L) with special interest in thyroid to identify clinicopathologic characteristics and the percentage of tall cells. Tall cells were defined as cells with a height at least three times their width, abundant eosinophilic cytoplasm and nuclear features characteristic of classic PTCs such as enlarged, irregular, clear nuclei with grooves and pseudo-inclusions (Fig. 1).2,5,13,19 A tumor was classified as a classic PTC if it had any well-formed papillary structure and contained <10% tall cells. A tumor was further defined as classic PTC with TCF if it harbored between 10% and 50% tall cells (Fig. 2) and as a TCVPTC if it contained 50% or more tall cells. The 50% criterion for TCVPTC was based on the World Health Organization classification and previous studies on TCVPTC.5,12,13,15,18,19,20,21 To determine whether samples reached the cutoff values of 10% and 50% of tall cells in the whole sections of tumor tissue, we used a digital training set which has the known percentage calculated from digitization of whole slide imaging. Discrepancies between the observers were found in less than 10% of the reviewed slides. A consensus was reached when there was discrepancy between the observers. We excluded any cases with tumor necrosis or marked mitotic activity (≥3 mitoses/10 high power field, 400×) because these pathologic features themselves could be related to tumor aggressiveness.5,6,19,20 In cases with multiple tumor foci, the largest tumors were selected as the primary lesions. TNM staging was performed according to the American Joint Committee's Cancer staging manual 7th edition.
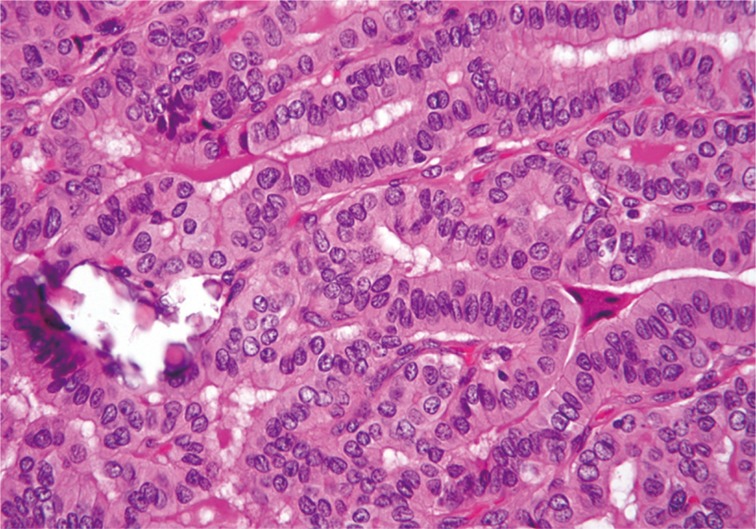
Tall cell variant of papillary thyroid carcinoma exhibits elongated follicular and closely packed papillary growth patterns. Tall cells display prominent cell membranes, dense eosinophilic cytoplasm, and nuclear features typical of papillary carcinoma.
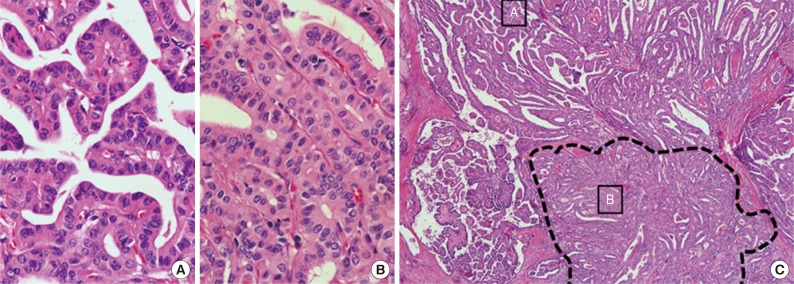
Classic papillary thyroid carcinomas with tall cell features contain 10% to 50% of cells with tall cell characteristics. (C) Dotted lines show the area containing tall cells. Tall cells are arranged back-to-back in a parallel pattern. Two small boxes indicate regions enlarged images (A, classic papillary; B, tall cells).
BRAF mutation analysis
Of a total of 447 PTC cases, 417 underwent BRAF mutation testing. Genomic DNA was extracted from two or three 10-µm thick paraffin tissue sections using the QIAamp DNA Mini kit (Qiagen, Hilden, Germany). The tissue sections were manually microdissected to enrich for tumor cells. We screened for mutations in exon 15 of the BRAF gene using polymerase chain reaction amplification and direct DNA sequencing, as described previously.8,22
Statistical analysis
We used Student's t-test to compare two different groups of continuous parametric data with a normal distribution. Pearson's chi-square test was used to assess the relationship between categorical variables. For the multivariate analysis, we included all variables with a univariate probability (p) value of <.10 in a binary logistic regression test. Two-sided p-values <.05 were considered to be statistically significant. Statistical analysis was performed with SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Out of a total of 2,139 patients with PTCs, 149 (7.0%) had classic PTC with TCF and 95 (4.4%) had TCVPTC.
Comparison of clinicopathologic characteristics among histopathologic types
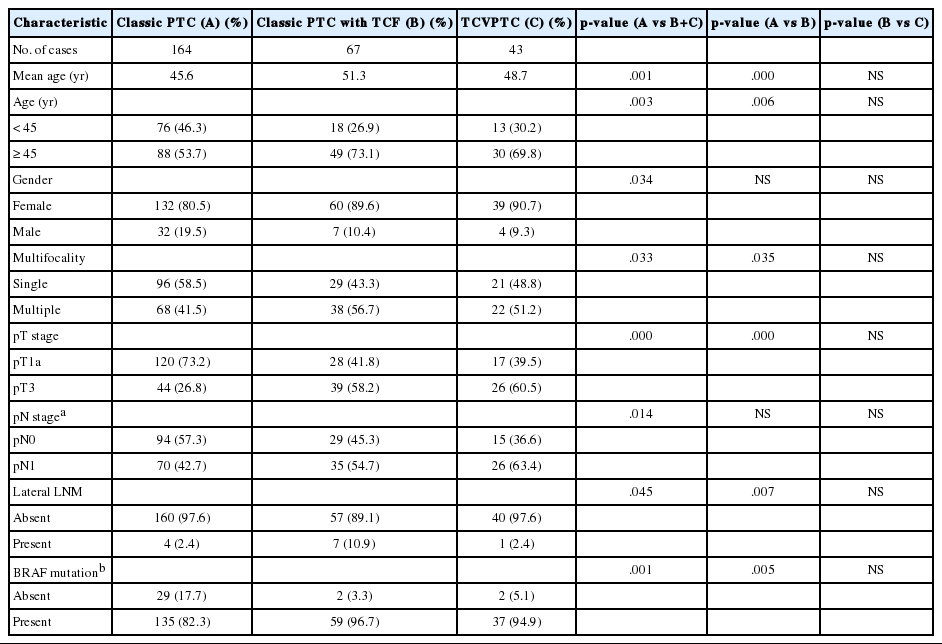
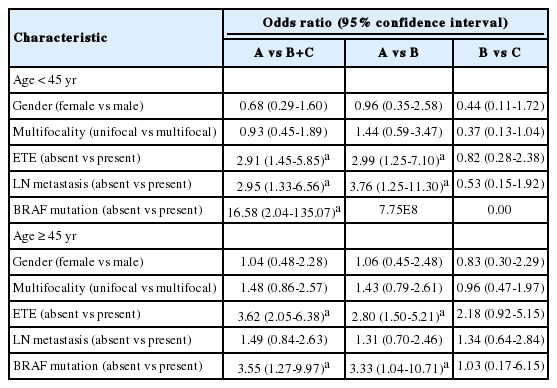
When the clinicopathologic features of classic PTCs were compared with those of TCVPTCs and classic PTCs with TCF, patients with classic PTCs were younger at the time of surgery and showed lower pT and pN stages, a lower rate of extrathyroidal extension, a lower rate of lateral lymph node metastasis, and a lower frequency of BRAF mutations (Table 1). All BRAF mutations were V600E mutations. There were no statistically significant differences between TCVPTCs and classic PTCs with TCF with regard to mean age and clinicopathologic features such as pT, extrathyroidal extension, pN, lateral lymph node metastasis, or rate of BRAF mutation (Table 1). In multivariate analysis (Table 2), age group, extrathyroidal extension, lymph node metastasis, and BRAF mutations of classic PTC were significantly different from those of TCVPTCs and classic PTCs with TCF. The clinicopathologic features of TCVPTCs were not significantly different from those of classic PTCs with TCF.
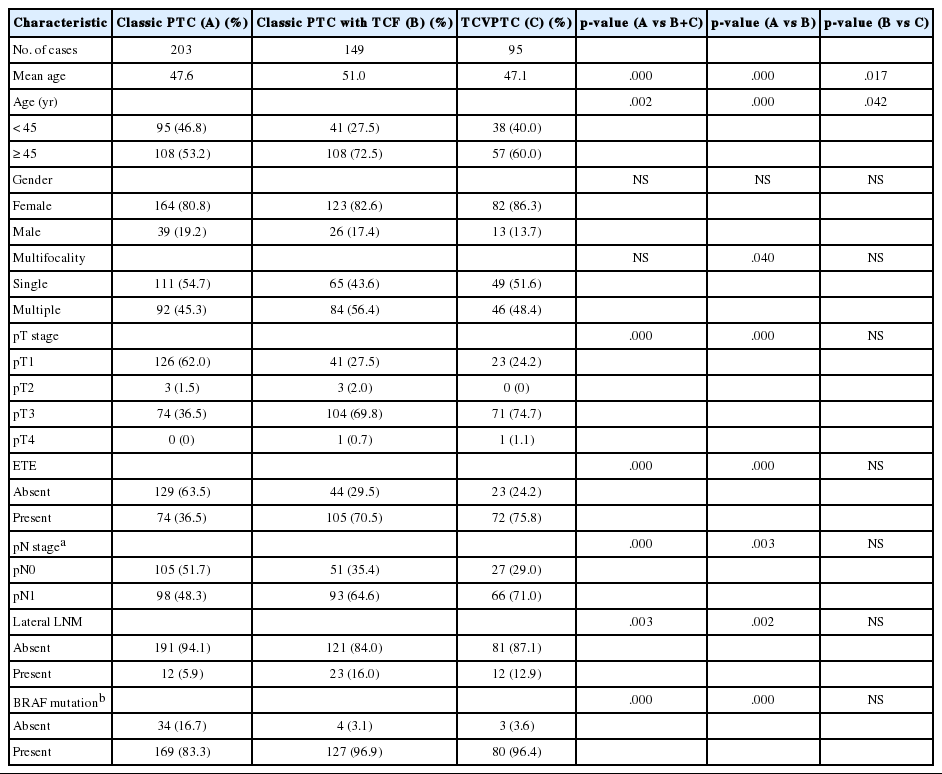
Clinicopathologic characteristics of papillary thyroid carcinoma (PTC) according to histopathologic types
Subanalysis of microcarcinomas (≤1.0 cm in size) according to histologic subtype
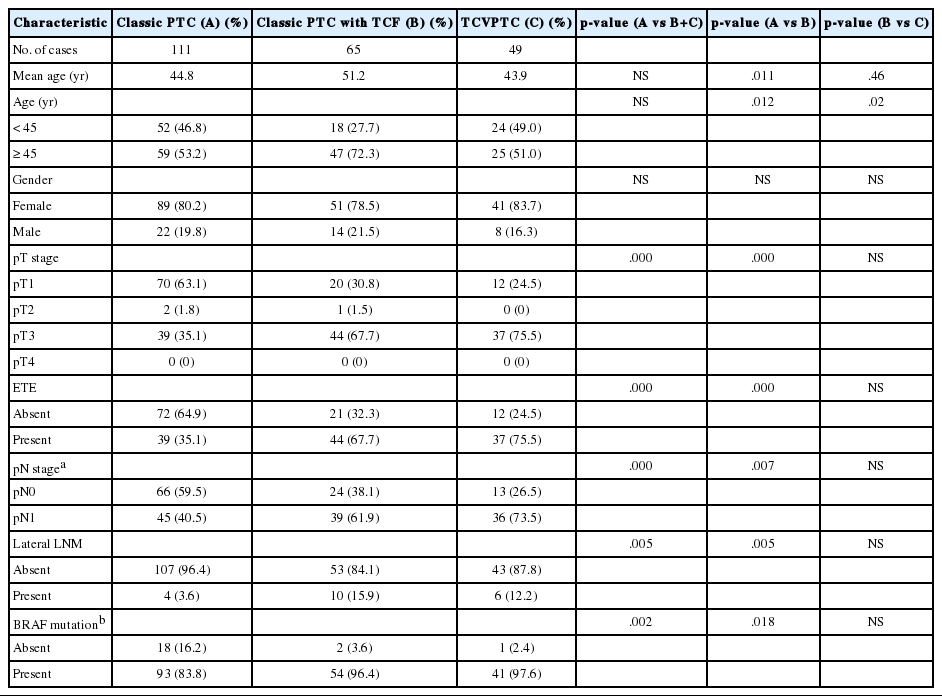
Patients with classic PTCs were younger at surgery and showed a higher frequency of single lesions, lower pT and pN stages, a lower rate of extrathyroidal extension, and a lower frequency of BRAF mutations, compared to patients with classic PTCs with TCF or TCVPTCs (Table 3). There were no statistically significant differences in clinicopathologic characteristics between TCVPTCs and classic PTCs with TCF (Table 3).
Subanalysis of unifocal tumors according to histologic subtype
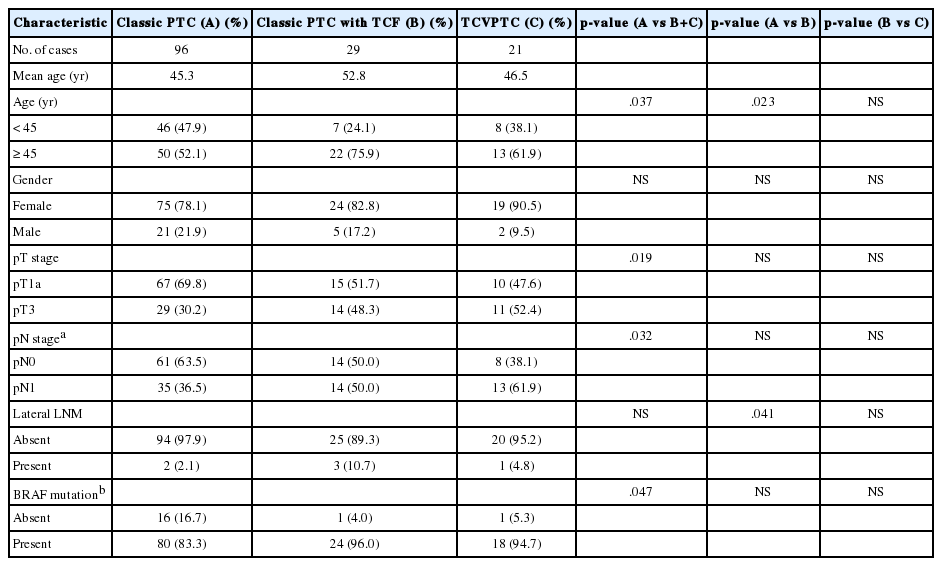
We only included unifocal cancers to eliminate any possible bias from tumor multifocality. We further analyzed the clinicopathologic differences between classic PTCs, classic PTCs with TCF or TCVPTCs. The results for unifocal cancers were in the same range: clinicopathologic features of classic PTCs were significantly different from those of classic PTCs with TCF or TCVPTCs, but no significant differences were observed in clinicopathologic characteristics between TCVPTCs and classic PTCs with TCF (Appendices 1, 2).
Subanalysis of histologic subtypes according to patient age groups (<45 years vs ≥45 years)
In a multivariate subanalysis by patient age group (Table 4), there were no significant differences in clinicopathologic variables between patients under or over the age of 45 in the classic PTC with TCF and TCVPTC groups. The clinicopathologic features of classic PTCs were significantly different from those of classic PTCs with TCF or TCVPTCs across age groups.
DISCUSSION
We demonstrated that classic PTC with TCF (PTC with 10% to 50% tall cells) had the same demographic and clinicopathologic characteristics as TCVPTC, and these tumors showed more aggressive features than classic PTC.
TCVPTC typically affects older patients and has a larger size, higher stage at presentation, higher rate of extrathyroidal extension, greater risk of recurrence, and poorer disease-specific survival compared to classic PTC.3,4,9,16,18,19,23 Bernstein et al.24 indicated that despite controlling for size, TCVs of papillary thyroid microcarcinomas were still associated with higher pT and, pN stage and aggressive pathologic features such as extrathyroidal extension. In our study, we also demonstrated that microcarcinomas with a tall cell component were more aggressive than those with classic PTC morphology.
There is controversy regarding the high incidence of lymph node metastasis in TCVPTC. Some authors have demonstrated that patients with TCVPTC have a higher rate of nodal metastases than patients with classic PTC.4,16 Bernstein et al.24 showed that microcarcinomas with TCVPTC have a higher rate of central lymph node metastasis than microcarcinomas with classic PTC (39% vs 13%). However, other authors did not find significant differences in the lymph node positivity rate between classic PTCs and TCVPTCs.3,17,19 For example, Ganly et al.19 demonstrated no differences in nodal metastases among TCVPTCs, classic PTCs with TCF and classic PTCs. Such discrepancies might be caused by different patient ages or different criteria regarding the percentage of tall cells necessary for a diagnosis of TCVPTC. In our multivariate analysis according to the age groups, there were significant differences in the rates of lymph node metastasis between classic PTC and classic PTC with TCF in patients under the age of 45 years. However, the differences in lymph node metastasis rate disappeared in patients over the age of 45 years. Johnson et al.16 and Bernstein et al.24 used a 30% threshold of tall cells and showed a higher rate of nodal positivity in TCVPTCs. Investigators who used a 50% to 75% threshold of tall cells for TCVPTC diagnosis did not find a significant differences in nodal positivity between TCVPTCs and classic PTCs.17,19
Various investigators have used different thresholds of 10%,15 30%,11,15,24 50%,5,12,13,15,18,19,20,21 70%,3 or 75%2,14,17 tall cells to define TCVPTC. Ghossein and LiVolsi5 and Regalbuto et al.18 suggested a cut-off of at least 50% tall cells to classify TCVPTC.
In our study, we used a 10% threshold for classic PTCs with TCF and a 50% threshold for TCVPTCs. Using this definition, lymph node metastases were significantly more frequent in classic PTCs with TCF and TCVPTCs than in classic PTCs. We also demonstrated a statistically significant difference between classic PTCs and classic PTCs with TCF (10% to 50% tall cells) with regard to lymph node status such as pN and lateral lymph node metastasis (Table 1).
Beninato et al.15 reported that patients with classic PTC with TCF (tumors with ≥10% tall cells) have more aggressive tumor features such as older age at onset, higher stage, more extrathyroidal extension (3% to 14%), more lymph node metastases (40% to 68%), increased lymphovascular invasion (2% to 17%), and a higher frequency of positive surgical margins, compared with classic PTC. These aggressive features have been shown to be associated with a greater risk of recurrence and were maintained with increasing tall cell percentage (≥10%, ≥30%, ≥50%) within PTCs with tall cells.15 Other authors have suggested that classic PTCs with TCF may be significantly associated with older age at presentation, larger tumor size, higher frequency of extrathyroidal extension, and BRAF mutations regardless of the percentage of tall cells.13 We also demonstrated a statistically significant difference in clinicopathologic features and BRAF mutations between classic PTCs and classic PTCs with TCF (10% to 50% tall cells) (Table 1).
The more aggressive clinicopathologic features of classic PTCs with TCF and TCVPTCs at presentation translate into worse outcomes.19 Recent clinical guidelines also suggest that TCVPTCs require more aggressive surgical resection.25 The aggressive nature of TCVPTC may be related to a higher prevalence of BRAF mutations.26 BRAF mutations in PTC have been correlated with aggressive tumor behavior, including extrathyroidal extension, tumor recurrence, advanced tumor stage at presentation and lymph node metastasis,27 even in microcarcinomas.28 The BRAF oncogene is a strong activator of the mitogen-activated protein kinase signaling pathway, which leads to uncontrolled cell proliferation and transformation into malignancy.29 BRAF mutation also plays a role in extracellular matrix remodeling and is associated with an increase in matrix metalloproteinases, a desmoplastic stromal reaction, and invasiveness.30 Finkelstein et al.21 demonstrated a significant association between the presence of the BRAF mutation and fibrosis, desmoplastic stromal reaction, and infiltrating tumor borders. The BRAF mutation occurs commonly in Korean patients with PTC, ranging in frequency from 52% to 87% of all cases.8 The prevalence of the BRAF mutation worldwide is reported to be as high as 80% to 100% in TCVPTCs.29 For example, Bernstein et al.24 demonstrated that the BRAF mutation was detected in 93% of all microcarcinomas with TCVPTC. In our study, the prevalence of the BRAF mutation was 96.9% and 96.4% in classic PTCs with TCF and TCVPTCs, respectively.
Age at presentation is the single most important prognostic factor in thyroid cancer.1 In our study, we found that classic PTCs with TCF and TCVPTCs were more frequent in patients over the age of 45. Interestingly, PTCs with ≥10% tall cells were pathologically more aggressive than classic PTCs regardless of age groups (<45 years and ≥45 years).
Our study has some limitations, such as a retrospective design, relatively small sample size, and the fact that the data were collected by one institution. Furthermore, we did not analyze the outcomes and prognosis of patients because the follow-up period was too short.
In conclusion, PTCs with more than 10% tall cells show more aggressive clinicopathologic features than classic PTCs regardless of tumor size and age. Classic PTCs with TCF have a similar BRAF mutation rate and clinicopathologic characteristics to those of TCVPTCs. Therefore, we suggest that the presence of any tall cells should be noted in pathologic reports because of their clinical significance.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future planning (2013R1A2A2A01068570).
Notes
No potential conflict of interest relevant to this article was reported.