Intracranial Extracerebral Glioneuronal Heterotopia with Adipose Tissue and a Glioependymal Cyst: A Case Report and Review of the Literature
Article information
The presence of central nervous system (CNS) tissue outside the cranium is often referred to as "heterotopia," although technically this should be termed "ectopia," according to the dictionary definition. Glioneuronal heterotopia (GH) is a rare, mass-forming, malformative lesion. Ectopic glioneuronal tissue of the head and neck has been detected in the nasopharynx, oropharynx, tongue, palate, tonsils, soft tissue, eye, and orbit, and intracranial extracerebral glioneuronal heterotopia (IEGH) has also been reported, although less frequently.1,2 Since the first description of neuroglial heterotopia in the dorsal meninges of the cervical spinal cord by Wolbach in 1907,3 fewer than 20 cases of IEGH have been reported. Glioependymal cysts are rare, ependyma-lined, cystic lesions of the subarachnoid space, which have been referred to as epithelial or ependymal cysts. Histopathologically, they are lined with ependymal cells abutted on the glial layer and are commonly detected in the posterior fossa. The origin of glioependymal cysts of the posterior fossa is not clear, but these cysts may represent neuroglial heterotopia, persistent Blake's pouch (diverticulum of the roof of the fourth ventricle), or remnants of a tela chorioidea. We report here a case of IEGH that was predominantly composed of cerebellar tissue with some fat tissue and a large glioependymal cyst, and was initially misdiagnosed as a teratoma with a glioependymal cyst.
CASE REPORT
The patient was a 4-month-old female infant who was delivered spontaneously at 37 weeks of gestation. An atrial septal defect and patent foramen ovale of the heart were detected after birth. The patient also showed a low nasal bridge, a right-sided deviated nasal septum, ptosis of the left eye, and limited extra-ocular movement of the left eye.
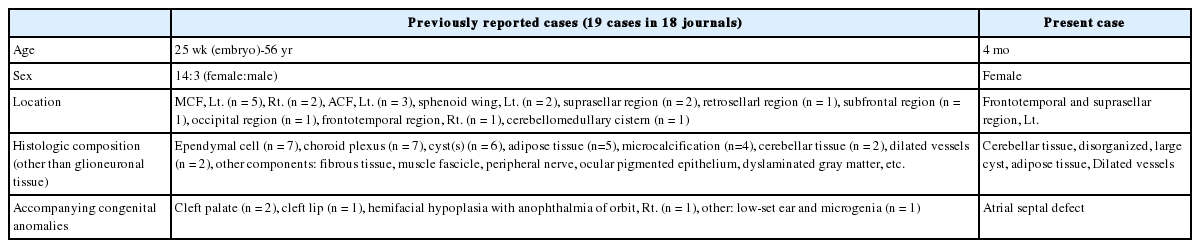
Brain magnetic resonance imaging (MRI) revealed a 5-cm mass in the left frontotemporal and suprasellar areas, with cystic changes at both the center and on the left side of the mass (Fig. 1). The peripheral portion of the mass had a component of fat tissue. A 5.6-cm arachnoid cyst was observed in the left middle cranial fossa (MCF). In addition, through the anterior skull base defect, we observed left paramedian herniation of the mass into 3 areas: the left nasal cavity, ethmoid sinus, and left medial extraconal orbital space.
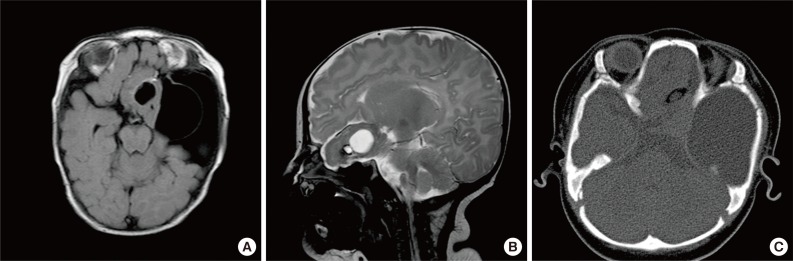
T2-weighted fluid-attenuated inversion recovery (A) and T2-weighted turbo-spin echo (B) brain magnetic resonance imaging showing a 5-cm mass with a cystic portion and a fatty component in the left frontotemporal and suprasellar areas. (C) Computed tomography of the ostiomeatal unit (non-contrast). A large cyst is present in the left middle cranial fossa. Left paramedian herniation of a solid cystic mass with a focal fat component into the left nasal cavity, ethmoid sinus, and left medial extraconal orbital space through the anterior skull base defect.
Following left temporal craniotomy and dural incision, an arachnoid cyst was found in the left sphenoid ridge. This arachnoid cyst was penetrated during surgery, and another cyst was detected under this arachnoid cyst. This second cyst was located in the middle of the mass, and pathological examination showed it to be a glioependymal cyst. Low vascular masses, which had a component of fat tissue, were present in the MCF and ethmoid sinus. All of these abnormal tissues were removed.
Follow-up MRI at 18 months after the operation, at the age of 25 months, revealed subdural fluid collection and a persistent enlarged left lateral ventricle. However, the patient's attainment of motor milestones was fair with no evident neurological deficit.
Histopathologically, the mass was composed of an admixture of well and poorly organized cerebellar and cerebral tissue, adipose tissue, and cysts lined with ciliated ependymal cells abutted on the glial and fibrous tissue (Fig. 2). The well-organized cerebellar tissue consisted of a molecular layer, a Purkinje cell layer, and inner and outer granule cell layers with well-formed white matter. However, in the poorly organized areas, these 3 layers were intermixed irregularly. In some areas, cerebellar deep gray-like organization was noted. Dilated vessels were observed in the fat and cerebral tissues. There were 2 types of cysts: an arachnoid cyst and a glioependymal cyst. The glioependymal cyst wall was also partly surrounded by atrophic cerebellar tissue. The ependymal lining cells were positive for S-100 protein, and the glial tissue underlying these cells was positive for glial fibrillary acidic protein.
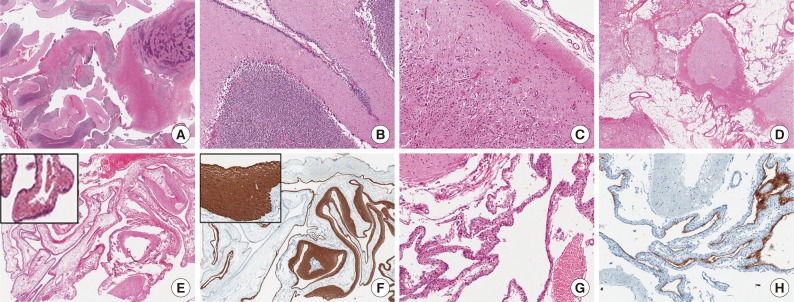
Sections of the frontotemporal mass (A-D) and the cysts (E-H). (A) The mass is composed of well-formed and disorganized cerebellar tissue. (B) Cerebellar tissue shows a Purkinje cell layer, a molecular layer, and external and inner granule cell layers. (C) Neocortical tissue has a focal cortical dysplasia-like appearance, with regard to subpial gliosis, a persistent granule cell layer, and dyslamination. (D) Adipose tissue is mixed with heterotopic brain tissue. (E) The glioependymal cyst is composed of ependymal cells lining variably thickened glial and fibrovascular tissues. (F) Glial tissue is positive for glial fibrillary acidic protein. (G) The arachnoid cyst, present alongside the glioependymal cyst, is lined with a cuboidal layer of meningothelial cells and fibrous tissue. (H) The meningothelial cells are positive for epithelial membrane antigen.
DISCUSSION
GH has been referred to as glioneuronal choristoma, glioneuronal hamartoma, brain heterotopia, and neuroglial heterotopia.1,4,5 It is a rare malformative lesion, and its precise incidence is unknown. Gyure et al.6 classified GH according to location and putative pathogenic mechanism as follows: intraparenchymal GH, dural and leptomeningeal GH, IEGH, distal GH of the lung and uterus, and midline nasal glioma. Nasal glioma is now thought to originate as a small sequestered encephalocele.2 According to Gyure's classification, IEGH is extremely rare. To date, only 19 cases of IEGH have been reported in the English language literature (Table 1).
We reviewed the previously reported cases of IEGH alongside our case. The most common location of IEGH was the MCF, predominantly involving the left side (left:right ratio=5:2). IEGH was located in the anterior cranial fossa (ACF) in only 3 cases. In 70% of the cases (7/10 cases) heterotopic glioneuronal tissues in the MCF or ACF extended through the cranial base foramina or the destructed skull base to extracranial sites such as the facial temporal region, the oral cavity, the region below the orbit,7 or the parapharyngeal space.2 In our case, the IEGH extended to the left nasal cavity, ethmoid sinus, and extraconal orbital space through the anterior skull base defect. Although IEGH is a clinically and pathologically benign lesion, if it extends to extracranial areas or herniates into the skull base it can be harmful. IEGH can distort the anatomy of the skull base, and if it extends inferiorly into the infratemporal fossa, it can displace the upper airway and cause orbital dysplasia.5 In any case, surgical removal is the treatment of choice.
The most striking component in our case was the prominent cerebellar tissue in the solid part of the mass. It was composed of fetal or neonatal stage cerebellum with all the cerebellar layers present; however, disorganized brain tissue was also noted. The radiological and intraoperative findings revealed an intact infratentorial cerebellum. Thus, we regarded the cerebellar tissue in the supratentorial extracerebral mass as a component of the IEGH. Nishio et al.8 also reported well-organized cerebellar tissue with Purkinje and granule cell layers in the IEGH, as in our case. Marubayashi and Matsukado4 also found cerebellar tissue with well-formed cerebellar dentate nuclei but with incomplete folding, indicating immaturity in their case of IEGH. The second remarkable component in our case was neocortical gray matter. As outlined in previous reports, an increase in the number of glial cells and neurons in the molecular layer of the heterotopic cerebral tissue or glioneuronal nodules was also observed in our case.6 The third component was intraparenchymal adipose tissue, which was admixed with the heterotopic brain tissue. Because of the presence of adipose tissue, the major differential diagnosis was teratoma; however, this was also observed in 5 of the previously reported cases.2,6,8,9,10 Gyure et al.6 suggested that the adipose tissue was simply an admixture of soft tissue around the IEGH, but Nishio et al.8 interpreted this tissue as embryonic ectomesenchymal elements (derivatives of the neural crest) that matured after aberrant migration during the formation of the neural tube. Skeletal muscle and salivary gland tissue have also been reported adjacent to the IEGH.2,10
Detection of tissue components such as mature neuroectodermal tissue, including cerebrum and cerebellum tissue, admixed with adipose tissue can lead to misdiagnoses of teratoma. However, the absence of tissues derived from other germ layers is the clue to distinguishing GH from mature teratoma. It has already been highlighted that GH may easily be misdiagnosed as a teratoma or a CNS tumor.6 However, some cases of GH are actually accompanied by nasopharyngeal teratoma.
Variably sized cysts in IEGH are not a rare finding, since they have been reported in 6 (31.6%) of the 19 cases (Table 1).1,2,4,5,8,9 Notably, 2 cysts were present in our case: one occupying the MCF and the other presenting around the tumor. Since, in our case, the glioependymal cyst was located inside the IEGH and some part of this cyst was surrounded by atrophic cerebellar tissue, the glioependymal cyst itself was thought be a main manifestation of the IEGH.
There are 3 major theories to explain the pathogenesis of IEGH: protrusion theory, accessory brain theory, and outpouching theory.9 Although many investigators favor a common origin for IEGHs and leptomeningeal and dural heterotopia, the protrusion theory of the brain into the subarachnoid space offers only a limited explanation for IEGHs6,9 because it cannot explain the presence of normal cerebellum tissue in its unusual position in combination with heterotopic adipose tissue. The accessory brain theory was introduced by Harris et al.7 because of the relatively large size of IEGHs. In the cases reported by Harris et al.,7 the sizes of the IEGHs were similar to the size of a normal brain. The authors therefore suggested the development of an accessory brain between the fifth and sixth weeks of embryogenesis. The third theory suggests that outpouching of CNS tissue may occur at the same time as outpouching of the developing telencephalon from the prosencephalon, at the base of the telencephalic vesicles. The outpouching theory would explain the reason that most IEGHs are located in an inferior position in the cranium, but it cannot explain the presence of cerebellar tissue. If the outpouching theory is applicable in our case, outpouching should occur from multiple brain vesicles, including telencephalic and metencephalic vesicles (cerebellar primordium), at the secondary 5-vesicle stage, or from the neural tube stage just before the primary 3-vesicle stage, and then differentiate into telencephalic and metencephalic brain tissues. Moreover, glioependymal cysts can also originate from the central canal (future ventricles) of the brain vesicles.
In addition to the 3 aforementioned major theories, the aberrant migration theory states that embryonic neuroepithelial tissue aberrantly migrates into the subarachnoid space and continues to grow, rather than undergoing degenerative changes, resulting in heterotopias.3,6 The aberrant migration theory can explain the histology of this GH, as it is composed of cerebral and cerebellar tissue in heterotopic loci. However, the mechanism by which this aberrantly migrated tissue becomes well organized in heterotopic loci is unknown.
In summary, we have described a rare case of IEGH that occurred as a congenital cystic mass in the MCF in a patient who presented with ptosis of the left eye and who had an atrial septal defect of the heart. Histologically, the IEGH was composed of well-formed cerebellar and cerebral tissue, adipose tissue, and a glioependymal cyst. The main differential diagnosis was mature teratoma. Further reports of IEGH cases would improve our comprehension of this lesion, and elucidation of the pathogenesis of IEGH may expand our understanding of the mal-development of the CNS.
Acknowledgments
This research was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A112005).
Notes
No potential conflict of interest relevant to this article was reported.