Ghost Cell Odontogenic Carcinoma Arising from Calcifying Cystic Odontogenic Tumor: A Case Report
Article information
Abstract
Ghost cell odontogenic carcinoma (GCOC) is an exceptionally rare and malignant odontogenic tumor with aggressive growth characteristics. We describe a case of GCOC which was considerably derived from a previously resected calcifying cystic odontogenic tumor (CCOT). Cellular atypia, mitotic activity, Ki-67 labeling index and matrix metalloprotease-9 positive expression rate were all increased in the currently resected specimen compared to the initial one. This is a rare case of malignant transformation of CCOT to GCOC with respect to its histopathological and immunohistochemical findings.
Primary malignant tumors of the jaws can arise from odontogenic epithelial remnants within the alveolar segment or from the transformation or degeneration of benign lesions.1 Ghost cell odontogenic carcinoma (GCOC) is a rare manifestation of such tumors, and may develop either as a de novo tumor or that which arise from a previously existing calcifying cystic odontogenic tumor (CCOT) or dentinogenic ghost cell tumor (DGCT).2 There have been very few reports about GCOCs arising from malignant transformation of benign lesions.3-5 Thus, more case reports serving as evidence are needed to better understand this tumor.
A 51-year-old man was diagnosed with maxillary GCOC, derived from a CCOT that had been removed by curettage a year ago. In this article, we describe and compare the clinical, pathological and immunohistochemical characteristics of the newly diagnosed GCOC and the previous CCOT, in order to understand the differences between these two tumors and especially, acquire more knowledge about GCOC.
CASE REPORT
The patient was referred to the Department of Oral and Maxillofacial Surgery, West China College of Stomatology, Sichuan University with a one-year history of a slowly growing, painful mass in the right maxillary region. Physical examination revealed a tender, soft, palpable mass measuring 3×3×1.5 cm with clear borders, adjacent to the right upper lip and nasal ala. Oral examination revealed a thickened vestibular groove between the right upper central incisor and the first molar, a swollen right maxilla and sensitivity of the adjacent teeth to percussion. Panoramic X-ray film revealed an oval, radiolucent lesion with clear borders located between the right upper central incisor and the first molar. Enlarged cervical lymph nodes were not found on physical examination, and both lungs were clear on chest X-ray.
Curettage of the cystic lesion was subsequently performed. The gross appearance of the resected specimen showed a cyst measuring 3×3×3 cm with a thin wall (0.2 cm). Histopathological examination (Fig. 1A) demonstrated the epithelial lining to be composed of a well-defined basal layer consisting of columnar or cubical cells, with nuclei in the barrier range situated away from the basilar membrane. An overlying layer of sparsely distributed polygonal or asteroid cells resembled a stellate reticulum. Sporadic or conglobate ghost cells were trapped in the epithelium. Immunohistochemistry showed that Ki-67 was sparsely expressed in the epithelial cells with a positive expression rate of 12.2% (Fig. 1B), whereas matrix metalloprotease-9 (MMP-9) was sporadically expressed in both cells and mesenchyma (Fig. 1C). Based on these findings, the tumor was diagnosed as a CCOT.
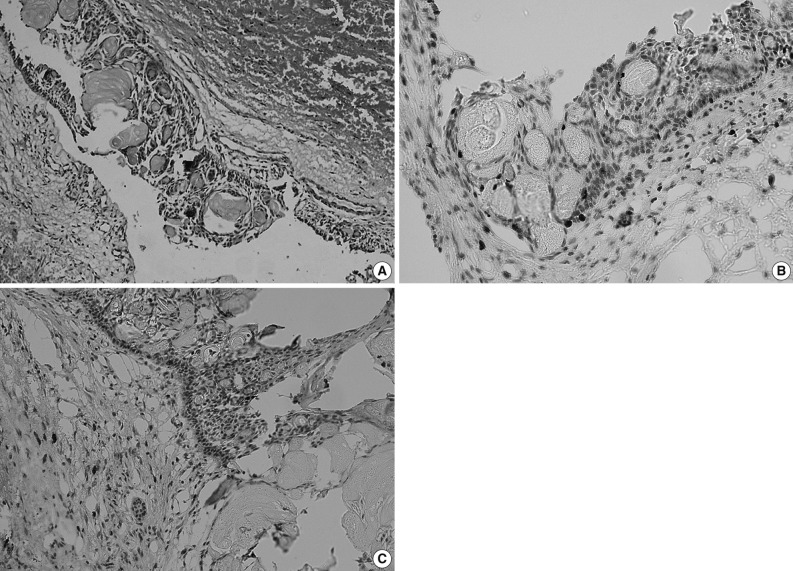
Calcifying cystic odontogenic tumor. (A) Histopathologic examination shows the epithelial lining is composed of columnar or cubical cells, and the nuclei of which are barrier-ranged. Sporadic or conglobate ghost cells are seen in the lining epithelium. (B) Immunohistochemistry shows the Ki-67 is sparsely expressed in tumor cells but negatively in ghost cells, and (C) matrix metalloprotease-9 (MMP-9) is sparsely expressed in tumor cells and interstitium but negatively in ghost cells (Ki-67 and MMP-9 marker).
One year after the operation, the patient returned to our hospital with a painful and rapidly growing mass in the formerly operated region of the right maxilla. Oral examination revealed a mass measuring 3×2.5×2 cm located on the inner surface between the cuspid teeth and the first molar of the right maxilla. The mass was solid and tender with a smooth surface and clear borders. Panoramic X-ray film revealed a nonopaque lesion with clear borders. Root apices of the involved teeth showed absorption (Fig. 2). Based on the patient's medical history, we suspected recurrence of CCOT.
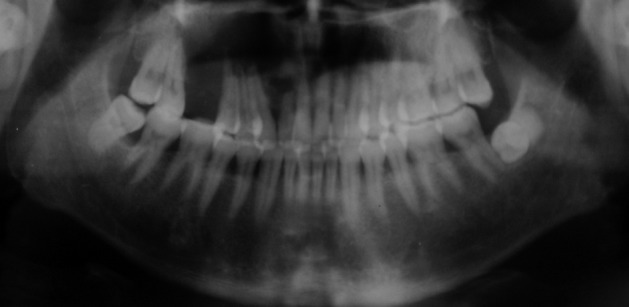
Panoramic X-ray film shows a nonopaque lesion located between the right upper lateral incisor and second premolar. The absorption of the root apex could be detected in the involved teeth.
Sub-total resection of the right maxilla was performed. The resected specimen was a solid tumor measuring 3×3×2.5 cm, with interior necrotic areas and devoid of an integrated envelope. Histopathological examination (Fig. 3A) showed that the tumor was composed of epithelial cell nests. The neoplastic cells showed cytological atypia, manifested mainly as hyperchromatic cells with variably sized nuclei, raised nuclear-cytoplasmic ratio and an increased number of mitotic figures (Fig. 3B). Clusters of ghost cells were diffusely distributed in the tumor nests. This tumor showed aggressive behaviour (Fig. 3C). Immunohistochemical staining revealed that Ki-67 was strongly expressed in the epithelial cells with a positive expression rate of 61.8% (Fig. 3D). MMP-9 was weakly expressed in the epithelial cells, but was strongly expressed in the tumor mesenchyma and was occasionally found in ghost cells (Fig. 3E). Pathologically, the tumor was diagnosed as GCOC.
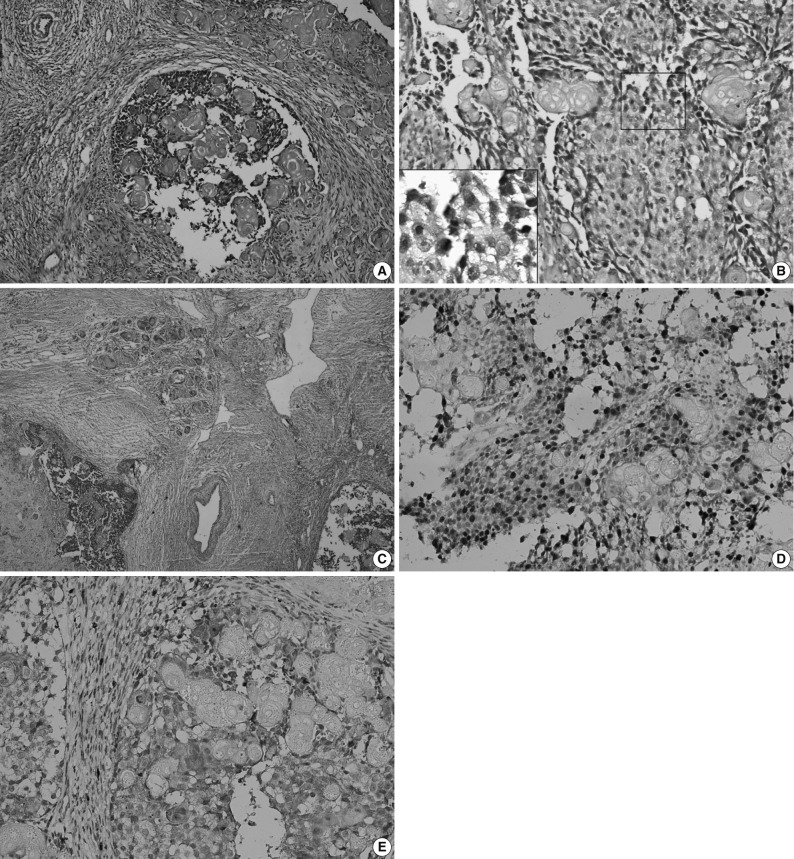
Ghost cell odontogenic carcinoma. (A) Histopathologic examination shows epithelial cell nests in tumor tissue. (B) Tumor cells are admixed with anucleate ghost cells. Inset: Several tumor cells show atypical mitoses. (C) The tumor cells invade the surrounding vessel. The tumor cells infiltrate into the adjacent fibro-vascular tissue. The clusters of ghost cells are diffusely distributed in the tumor nests. (D) Immunohistochemistry shows the Ki-67 is strongly expressed in the tumor cells but negatively in ghost cells, and (E) matrix metalloprotease-9 (MMP-9) is strongly expressed in interstitium, weakly in the tumor cells but sparsely in ghost cells (Ki-67 and MMP-9 marker).
Postoperatively, the patient survived and showed no evidence of recurrence or metastasis at the time of follow-up (i.e., a year after the second operation).
DISCUSSION
GCOC is a rare malignant tumor with no more than 30 cases reported in the English language medical literature. Among these cases nearly one-third of GCOCs were derived from a preexisting CCOT.6 In some cases, DGCT also occasionally combines with other odontogenic tumors, such as ameloblastoma to form GCOCs.7 With regard to newly developed tumors, most had certain similar characteristics such as rapid growth and infiltration or destruction of the surrounding tissues and structures. In our case, however, we observed only bony absorption of the teeth adjacent to the lesion due to early detection.
GCOC can occur at any age, with a predominance of male patients around 40 years old. More frequently, it occurs in the maxilla than the mandible.8 Jaw expansion with or without pain is the most common clinical presentation, which may also be associated with local paraesthesia, tooth mobility or displacement. Few patients had enlarged neck lymph nodes, but metastasis was extremely rare.3,9 With regard to CCOT, the tumor mainly manifested as gradual expansion of the jaw with occasional pain, probably on account of increased periosteal tension and compression of adjacent nerves and vessels by the expanding mass. Plain X-ray usually demonstrate CCOT manifesting as a single radiolucent shadow with clear borders, while GCOC is usually seen as a unicameral or multilocular radiolucent shadow without clear borders and associated with different degrees of bony destruction and infiltration of adjacent structures. As seen in our case, all tooth apices involved showed absorption.
Ward and Cohen10 suggested three possible explanations for the histogenesis of a cyst with lining epithelium and its associated carcinoma in jaws. Firstly, carcinomas and cysts have different origins, the former possibly originating from adjacent epithelium or by distant metastasis of a primary tumor. Secondly, the primary lesion was a carcinoma which partially underwent cystic degeneration. Thirdly, the primary lesion was a cyst, and the lining epithelium subsequently underwent malignant transformation. In our case, the two tumors occurred at the same site successively. The pathological characteristics of the first tumor corresponded with those of CCOT, and no malignant epithelium was found. However, the pathological characteristics of the second tumor corresponded with those of GCOC. Thus, the diagnosis of GCOC in our case agreed with the third theory mentioned above. We did not find any remnant of CCOT in the GCOC, which may imply that the earlier benign tumor had been completely removed.
According to the 2005 World Health Organization guidelines,11 GCOC usually exhibits prominent mitotic activity, nuclear atypia and cellular pleomorphism, groups of ghost cells and necrosis, and has an infiltrative growth pattern which presents locally aggressive and destructive behaviour. At times, metastatic deposits can also be found. Ki-67 is a sensitive biomarker of cell proliferation activity, which can be used to predict the severity and prognosis of tumor, while MMP-9 is another biomarker closely related to tumor invasion and metastasis.12 In our case, the positive expression rate of Ki-67 in CCOT and GCOC was 12.2% and 61.8%, respectively, which indicates that cell proliferation activity of GCOC was significantly higher than that of CCOT. In our study, whether tumor parenchyma or mesenchyma was involved, the positive expression rate of MMP-9 in GCOC was higher than that in CCOT. In both tumors, only a few ghost cells were positive for MMP-9 while all were negative for Ki-67.
GCOC has a low incidence. Most cases are sporadic, with different biological characteristics ranging from relatively indolent growth to an aggressive and potentially fatal course. However, distant metastases are uncommon. The recommended treatment for GCOC is wide surgical excision because of high recurrence rates in patients who had undergone local surgical resection or curettage. In this study, any other additional therapy was not performed after surgery because of the controversy regarding postoperative adjuvant irradiation with or without chemotherapy.1,3 With regard to the prognosis of GCOC, the overall five-year survival rate has been reported to be as high as 73%.13 However, in view of the fact that the biological behaviour of this tumor is unpredictable, long-term follow-up is highly recommended following therapy.14
In summary, the pathological characteristics of CCOT and GCOC show similarities but also significant differences which are helpful in predicting their malignant potential. Furthermore, measuring the expression of the biomarkers, Ki-67 and MMP-9, associated with tumor proliferation and invasion, is helpful in evaluating the prognosis of GCOC.
Notes
No potential conflict of interest relevant to this article was reported.