Periductal Stromal Tumor of Breast: A Case Report and A Review of Literature
Article information
Periductal stromal tumor (PDST) is a rare biphasic tumor of the breast that exhibits low-grade malignancy and intermediate behavior. It is characterized by proliferation of atypical spindle cells surrounding benign mammary ducts and infiltrating adjacent adipose tissue. PDST is distinguished from phyllodes tumor by its lack of leaf-like architecture; however, it is still unclear whether PDST is a separate entity or a certain spectrum of phyllodes tumor [1,2]. Its therapeutic management is based on wide surgical excision with tumor-free margins.
We report the case of a 20-year-old female who presented with a lump in her left breast with clinical features mimicking fibroadenoma. Excisional biopsy was performed because the mass gradually enlarged and became painful. On histological examination a biphasic tumor composed of benign epithelial ducts surrounded by proliferation of spindle cells with dense hyperchromatic nuclei and mild-to-moderate atypia was found. Stromal overgrowth, variable stromal cellularity and myxoid background were evident. A diagnosis of PDST was established after extensive sampling and immunohistochemical analysis. Our patient was treated with surgical resection and has been recurrence-free for more than 38 months.
CASE REPORT
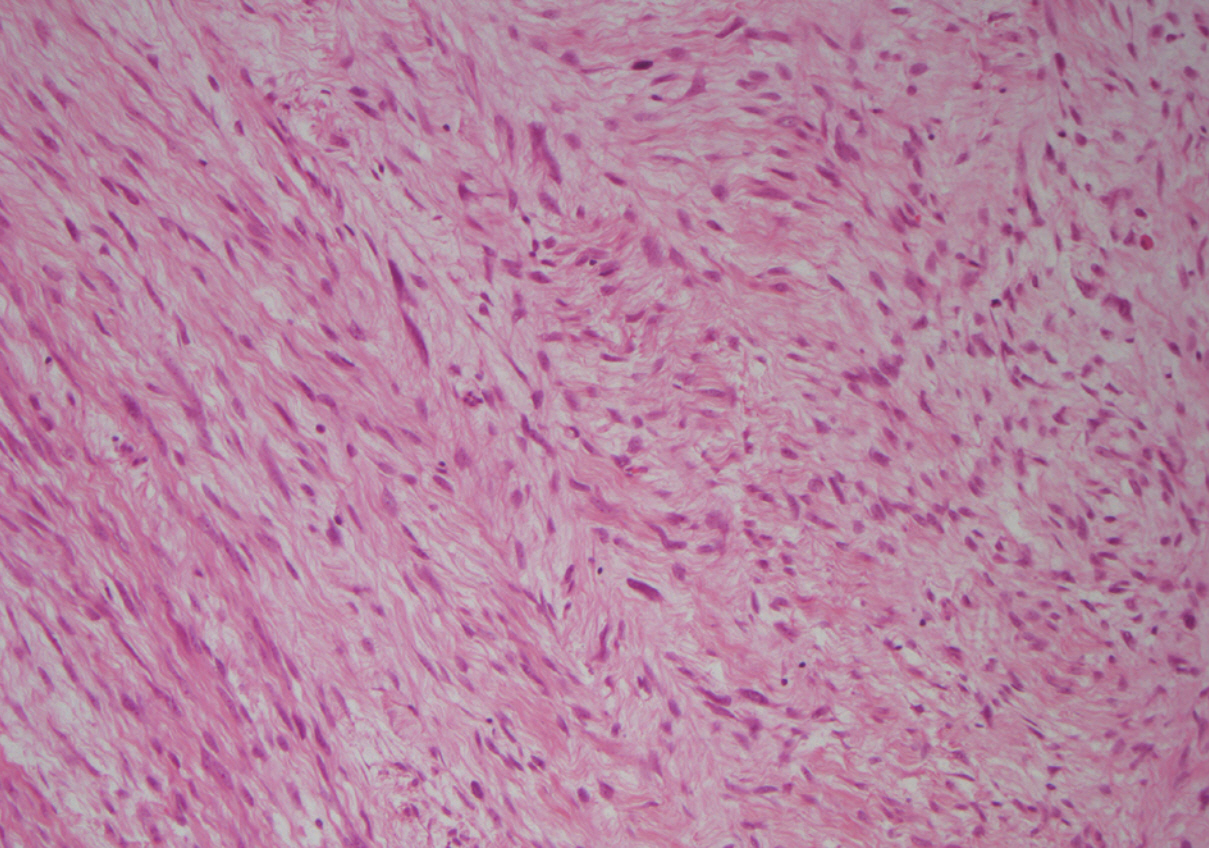
A 20-year-old female was referred to the breast unit due to a lump in her left breast that had increased in size. On physical examination the tumor was in the 3 o’clock position, well circumscribed, mobile, and non-tender. The patient had no previous history of breast disease herself, or within her family. She underwent an ultrasound scan (USS), which revealed a subtle hypoechoic lesion measuring 17×14×10 mm, and had an appearance consistent with fibroadenoma. Fine needle aspiration yielded a C2 benign result with no evidence of atypia. Due to the small size of the lesion and benign cytological diagnosis, the patient was discharged. Eighteen months later she presented again with the same lesion, which had now doubled in size and had become painful. A further USS was carried out which demonstrated that the lesion was still consistent with fibroadenoma and now measured 38×37×19 mm. Due to its rapid growth, excision of the lesion was performed. Macroscopically, the breast lump measured 45 mm in diameter and was poorly circumscribed, exhibiting a yellow and white cut surface. Microscopic examination revealed biphasic proliferation that comprised epithelial and mesenchymal components. The epithelial components composed of benign epithelial ducts but without a leaf-like configuration and were surrounded by mild-to-moderate atypical spindle cells, which infiltrated adjacent adipose tissue in a nodular pattern (Fig. 1). The tumor appeared to be focally present at the inked surgical margin. Stromal overgrowth, variable stromal cellularity, and myxoid background were observed, and the mitotic count was 3/10 high power field in stromal cells (Figs. 2, 3). There were foci of chronic inflammation along with lymphoid aggregates and germinal center formation. Immunocytochemical analysis showed that the neoplastic cells were CD34 positive (Fig. 2) and Ki-67 (a proliferative marker) was 5% to 10%. The tumor was negative for S100, estrogen receptor (ER), progesterone receptor (PR), MNF116, BCL2, β-catenin, AE1/AE3, cytokeratin 5/6, smooth muscle actin (SMA), desmin, and CD117. This immunoprofile together with histomorphology results confirmed the diagnosis of PDST. No adjuvant treatment was administered, but re-excision of the cavity was performed due to the positive surgical margin, and was free from tumor on subsequent histology examination. During a follow-up period of 12 months, USS showed a well-circumscribed lesion (U2) and a normal/benign (B1/B2) histological diagnosis was made after two further core biopsies. The patient has not shown any symptoms or signs of local or distant recurrence for approximately 38 months.
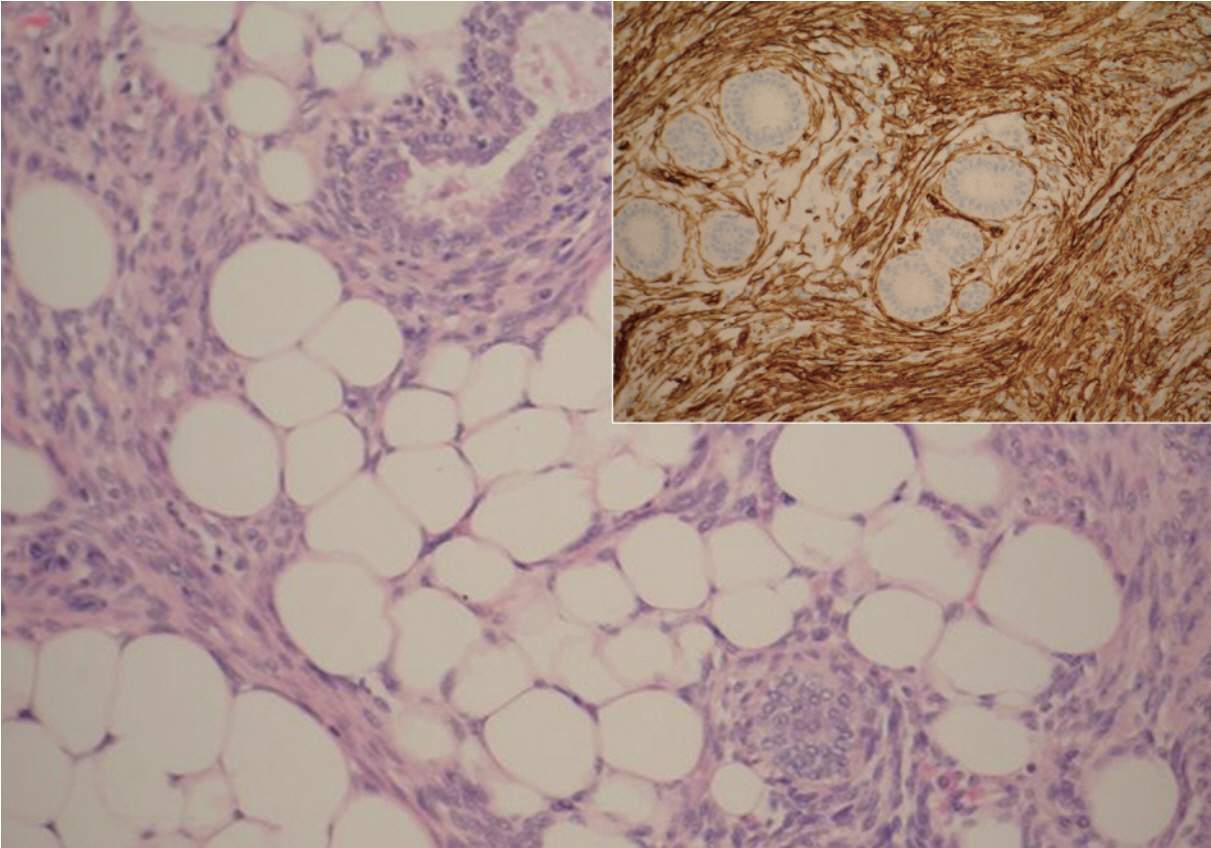
Periductal stromal tumor exhibiting benign ducts surrounded by atypical spindle cells, which appear to infiltrate adjacent adipose tissue. The spindle cells are diffusely positive for CD34 (inset).
DISCUSSION
PDST is a biphasic tumor of the breast characterized by proliferation of spindle cells surrounding benign ductal elements and infiltrating surrounding fatty tissue [1,2]. PDST is the preferred ‘neutral’ term for this entity [2], which most commonly occurs in adult women, usually perimenopausal or postmenopausal; however, it has also been described in a 14-year-old boy [3]. PDST on gross examination usually appears as an ill-defined white/grey or yellow mass. There may also be minute, scattered cysts. Our patient was a 20-year-old female with no previous history of breast disease. The breast lesion in the current case was poorly circumscribed with a solid, yellow/white cut surface, but no cystic changes were observed.
Microscopically PDST is composed of periductal spindle cell proliferation of variable cellularity and atypia around open ducts that lacks the leaf-like pattern typical of phyllodes tumors [1]. There are usually multiple nodules, often separated by fat. The main differential diagnosis includes phyllodes tumor, myxoid fibroadenoma, low-grade sarcoma, and fibromatosis. We used the criteria established by the Armed Forces Institute of Pathology (AFIP) of PDST: 1) a predominantly spindle-cell stromal proliferation of variable cellularity and atypia around open tubules and ducts devoid of a phyllodes pattern; 2) one or, more often, multiple nodules separated by adipose tissue; 3) stromal mitotic activity of ≥3/10 high-power fields; and 4) infiltration into surrounding mammary fibroadipose tissue [1]. The use of extensive tumor sampling and a broad panel of immunocytochemistry may help to establish the final diagnosis [4,5]. PDST is positive for CD34, some cases have been reported to be positive for CD117 and very few were positive for actin (HHF35), while it is negative for ER and PR. In the current case, stromal cells were diffusely positive for CD34, but negative for CD117, SMA, ER, and PR. The lack of the leaf-like architecture typical of phyllodes tumors is the main feature distinguishing PDST, as both lesions can have a similar immunoprofile. The characteristic pattern of atypical spindle cell proliferation around the open ducts can easily discriminate PDST from other lesions like myxoid fibroadenoma, low-grade sarcoma, and fibromatosis. β-Catenin is also often positive in fibromatosis and negative in PDST.
PDST is a low-grade malignancy of intermediate behavior that may progress to phyllodes tumor or another form of soft-tissue sarcoma [1,5]. The number of reported cases of PDST in the literature is limited; hence, the optimal means of management and prognosis have yet to be accurately established. The main therapeutic management of PDST is based on wide local excision with free surgical margins; neither axillary clearance nor adjuvant therapy is required [1]. In one series, 2 of 10 women experienced either recurrence or metastases within 12 months: one of these women had local recurrence with leaf-like formations, suggesting progression to a phyllodes tumor [6]. In our case the patient was treated with surgical resection and has been recurrence-free approximately 38 months. PDST may occasionally exhibit intraepithelial changes ranging from hyperplasia to intraductal carcinoma, and therefore close follow-up has been recommended [1,4]. The epithelial component in the current tumor demonstrated usual epithelial hyperplasia and there was no evidence of ductal carcinoma in situ or invasive carcinoma. Complete excision with tumor-free margins is the current recommended treatment; however, there has been little clinical experience of this disease and, hence, its management remains controversial. The prognosis in patients with PDST is unclear; thus, more studies with longer follow-up of patients are needed to determine the optimal management and the clinical behavior of this unusual breast neoplasm.
Notes
No potential conflict of interest relevant to this article was reported.