Search
- Page Path
- HOME > Search
Original Articles
- National quality assurance program using digital cytopathology: a 5-year digital transformation experience by the Korean Society for Cytopathology
- Yosep Chong, Hyeong Ju Kwon, Soon Auck Hong, Sung Soon Kim, Bo-Sung Kim, Younghee Choi, Yoon Jung Choi, Jung-Soo Pyo, Ji Yun Jeong, Soo Jin Jung, Hoon Kyu Oh, Seung-Sook Lee
- J Pathol Transl Med. 2025;59(5):320-333. Published online September 15, 2025
- DOI: https://doi.org/10.4132/jptm.2025.06.27
- 2,707 View
- 102 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
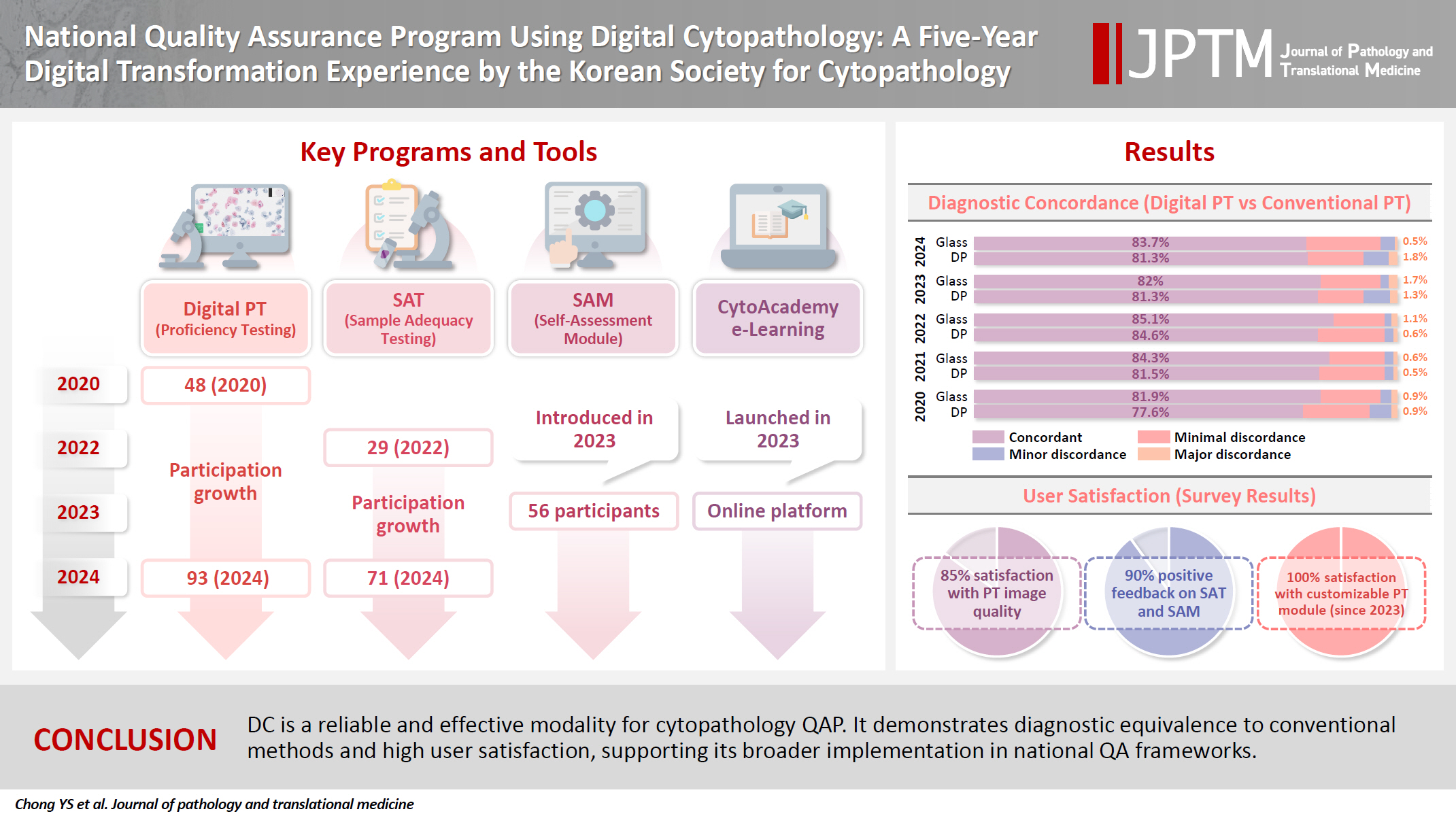
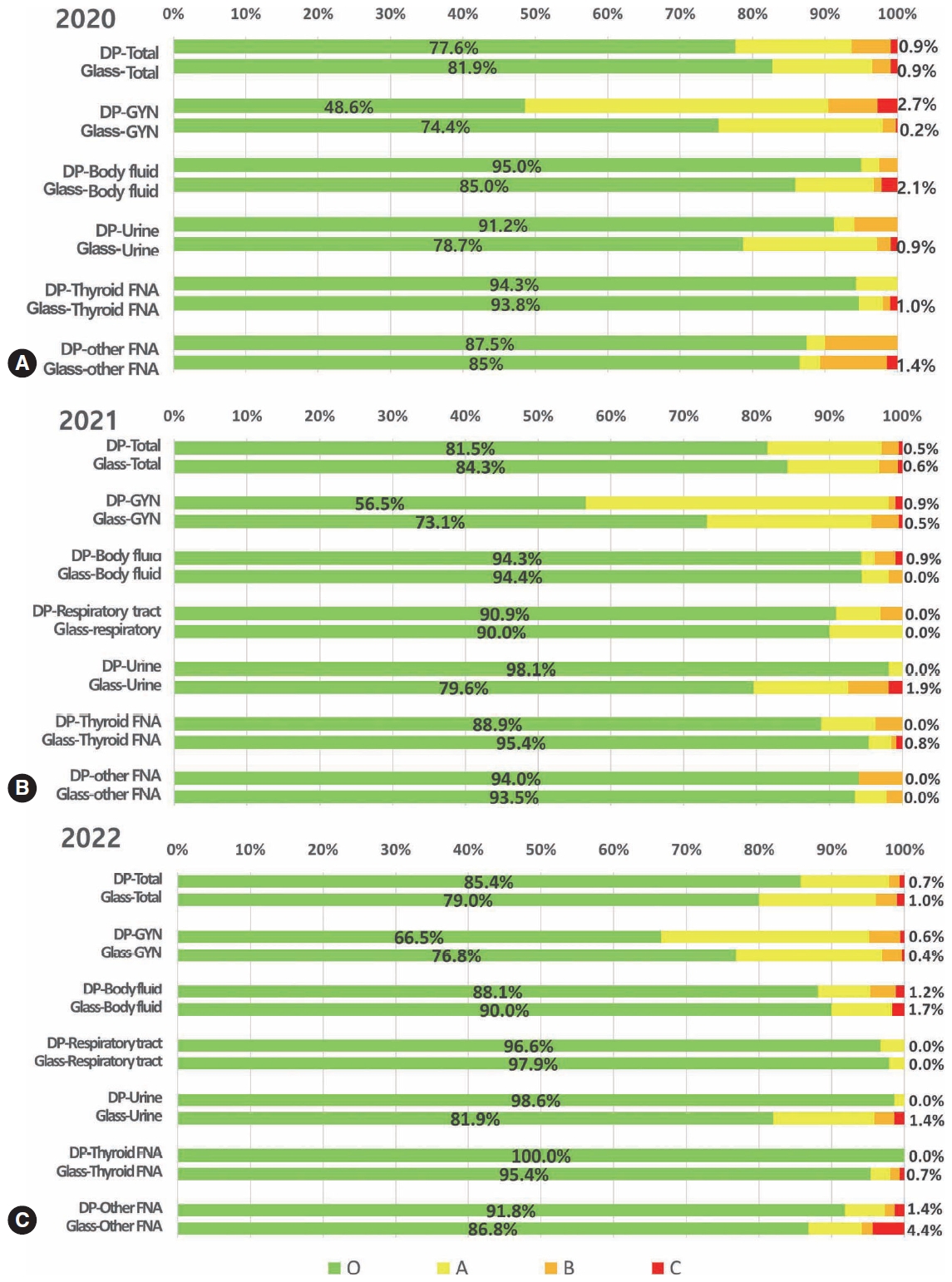
Digital cytopathology (DC) is emerging as a transformative approach in quality assurance programs (QAP), though its comprehensive evaluation remains limited. Since 2020, the Korean Society for Cytopathology has progressively incorporated DC into its national QAP, including digital proficiency testing (PT), sample adequacy testing (SAT), a customizable PT module, and a self-assessment module (SAM), aiming for full digital implementation by 2026. Methods: This 5-year study assessed diagnostic concordance between conventional and digital PT formats and analyzed participant feedback on service quality and digital image usability across PT, SAT, and SAM. Parallel testing was conducted during the transitional phase, and satisfaction was measured through structured surveys. Results: Participation in digital PT increased from 48 institutions in 2020 to 93 in 2024, while digital SAT participation rose from 29 to 71 between 2022 and 2024. In 2023, 56 institutions joined SAM. Diagnostic concordance rates were comparable between digital and conventional PTs (78.6%–84.6% vs. 82.0%–85.1%), including similar category C (major discordance) rates. Satisfaction with digital PT services and image quality exceeded 85%, and over 90% of institutions reported positive feedback on SAT and SAM. Over 80% were satisfied with the customizable PT module. Conclusions: DC is a reliable and effective modality for cytopathology QAP. It demonstrates diagnostic equivalence to conventional methods and high user satisfaction, supporting its broader implementation in national quality assurance frameworks. -
Citations
Citations to this article as recorded by- Practice of Cytopathology in Korea: A 40‐Year Evolution Through Standardization, Digital Transformation, and Global Partnership
Yosep Chong, Ran Hong, Hyeong Ju Kwon, Haeryoung Kim, Lucia Kim, Soon Jae Kim, Yoon Jung Choi
Diagnostic Cytopathology.2025;[Epub] CrossRef
- Practice of Cytopathology in Korea: A 40‐Year Evolution Through Standardization, Digital Transformation, and Global Partnership
- Diagnostic proficiency test using digital cytopathology and comparative assessment of whole slide images of cytologic samples for quality assurance program in Korea
- Yosep Chong, Soon Auck Hong, Hoon Kyu Oh, Soo Jin Jung, Bo-Sung Kim, Ji Yun Jeong, Ho-Chang Lee, Gyungyub Gong
- J Pathol Transl Med. 2023;57(5):251-264. Published online August 24, 2023
- DOI: https://doi.org/10.4132/jptm.2023.07.17
- 7,685 View
- 339 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
The Korean Society for Cytopathology introduced a digital proficiency test (PT) in 2021. However, many doubtful opinions remain on whether digitally scanned images can satisfactorily present subtle differences in the nuclear features and chromatin patterns of cytological samples.
Methods
We prepared 30 whole-slide images (WSIs) from the conventional PT archive by a selection process for digital PT. Digital and conventional PT were performed in parallel for volunteer institutes, and the results were compared using feedback. To assess the quality of cytological assessment WSIs, 12 slides were collected and scanned using five different scanners, with four cytopathologists evaluating image quality through a questionnaire.
Results
Among the 215 institutes, 108 and 107 participated in glass and digital PT, respectively. No significant difference was noted in category C (major discordance), although the number of discordant cases was slightly higher in the digital PT group. Leica, 3DHistech Pannoramic 250 Flash, and Hamamatsu NanoZoomer 360 systems showed comparable results in terms of image quality, feature presentation, and error rates for most cytological samples. Overall satisfaction was observed with the general convenience and image quality of digital PT.
Conclusions
As three-dimensional clusters are common and nuclear/chromatin features are critical for cytological interpretation, careful selection of scanners and optimal conditions are mandatory for the successful establishment of digital quality assurance programs in cytology. -
Citations
Citations to this article as recorded by- Sensitivity, Specificity, and Cost–Benefit Effect Between Primary Human Papillomavirus Testing, Primary Liquid‐Based Cytology, and Co‐Testing Algorithms for Cervical Lesions
Chang Gok Woo, Seung‐Myoung Son, Hye‐Kyung Hwang, Jung‐Sil Bae, Ok‐Jun Lee, Ho‐Chang Lee
Diagnostic Cytopathology.2025; 53(1): 35. CrossRef - Integration of AI‐Assisted in Digital Cervical Cytology Training: A Comparative Study
Yihui Yang, Dongyi Xian, Lihua Yu, Yanqing Kong, Huaisheng Lv, Liujing Huang, Kai Liu, Hao Zhang, Weiwei Wei, Hongping Tang
Cytopathology.2025; 36(2): 156. CrossRef - National quality assurance program using digital cytopathology: a 5-year digital transformation experience by the Korean Society for Cytopathology
Yosep Chong, Hyeong Ju Kwon, Soon Auck Hong, Sung Soon Kim, Bo-Sung Kim, Younghee Choi, Yoon Jung Choi, Jung-Soo Pyo, Ji Yun Jeong, Soo Jin Jung, Hoon Kyu Oh, Seung-Sook Lee
Journal of Pathology and Translational Medicine.2025; 59(5): 320. CrossRef - Integration of Digital Cytology in Quality Assurance Programs for Cytopathology
Yosep Chong, Maria Jesús Fernández Aceñero, Zaibo Li, Andrey Bychkov
Acta Cytologica.2025; : 1. CrossRef - Practice of Cytopathology in Korea: A 40‐Year Evolution Through Standardization, Digital Transformation, and Global Partnership
Yosep Chong, Ran Hong, Hyeong Ju Kwon, Haeryoung Kim, Lucia Kim, Soon Jae Kim, Yoon Jung Choi
Diagnostic Cytopathology.2025;[Epub] CrossRef - Quantitative Assessment of Focus Quality in Whole-Slide Imaging of Thyroid Liquid-Based Cytology Using Laplacian Variance
Chan Kwon Jung, Chankyung Kim, Sora Jeon, Andrey Bychkov
Endocrine Pathology.2025;[Epub] CrossRef - Validation of digital image slides for diagnosis in cervico-vaginal cytology
Francisco Tresserra, Gemma Fabra, Olga Luque, Miriam Castélla, Carla Gómez, Carmen Fernández-Cid, Ignacio Rodríguez
Revista Española de Patología.2024; 57(3): 182. CrossRef - Improved Diagnostic Accuracy of Thyroid Fine-Needle Aspiration Cytology with Artificial Intelligence Technology
Yujin Lee, Mohammad Rizwan Alam, Hongsik Park, Kwangil Yim, Kyung Jin Seo, Gisu Hwang, Dahyeon Kim, Yeonsoo Chung, Gyungyub Gong, Nam Hoon Cho, Chong Woo Yoo, Yosep Chong, Hyun Joo Choi
Thyroid®.2024; 34(6): 723. CrossRef
- Sensitivity, Specificity, and Cost–Benefit Effect Between Primary Human Papillomavirus Testing, Primary Liquid‐Based Cytology, and Co‐Testing Algorithms for Cervical Lesions
- Continuous quality improvement program and its results of Korean Society for Cytopathology
- Yoo-Duk Choi, Hoon-Kyu Oh, Su-Jin Kim, Kyung-Hee Kim, Yun-Kyung Lee, Bo-Sung Kim, Eun-Jeong Jang, Yoon-Jung Choi, Eun-Kyung Han, Dong-Hoon Kim, Younghee Choi, Chan-Kwon Jung, Sung-Nam Kim, Kyueng-Whan Min, Seok-Jin Yoon, Hun-Kyung Lee, Kyung Un Choi, Hye Kyoung Yoon
- J Pathol Transl Med. 2020;54(3):246-252. Published online April 15, 2020
- DOI: https://doi.org/10.4132/jptm.2020.02.22
- 7,044 View
- 131 Download
- 6 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Since 1995, the Korean Society for Cytopathology has overseen the Continuous Quality Improvement program for cytopathology laboratories. The Committee of Quality Improvement has carried out an annual survey of cytology data for each laboratory and set standards for proficiency tests. Methods: Evaluations were conducted four times per year from 2008 to 2018 and comprised statistics regarding cytology diagnoses of previous years, proficiency tests using cytology slides provided by the committee, assessment of adequacy of gynecology (GYN) cytology slides, and submission of cytology slides for proficiency tests. Results: A total of 206 institutes participated in 2017, and the results were as follows. The number of cytology tests increased from year to year. The ratio of liquid-based cytology in GYN gradually decreased, as most of the GYN cytology had been performed at commercial laboratories. The distribution of GYN diagnoses demonstrated nearly 3.0% as atypical squamous cells. The rate for squamous cell carcinoma was less than 0.02%. The atypical squamous cell/squamous intraepithelial lesion ratio was about 3:1 and showed an upward trend. The major discordant rate of cytology-histology in GYN cytology was less than 1%. The proficiency test maintained a major discordant rate less than 2%. The rate of inappropriate specimens for GYN cytology slides gradually decreased. Conclusions: The Continuous Quality Improvement program should be included in quality assurance programs. Moreover, these data can contribute to development of national cancer examination guidelines and facilitate cancer prevention and treatment. -
Citations
Citations to this article as recorded by- Practice of Cytopathology in Korea: A 40‐Year Evolution Through Standardization, Digital Transformation, and Global Partnership
Yosep Chong, Ran Hong, Hyeong Ju Kwon, Haeryoung Kim, Lucia Kim, Soon Jae Kim, Yoon Jung Choi
Diagnostic Cytopathology.2026; 54(2): 146. CrossRef - A Study on the Workload of Cytotechnologists: Focus on Commercial Laboratories
Eun-Suk PARK
Korean Journal of Clinical Laboratory Science.2025; 57(2): 228. CrossRef - Integration of Digital Cytology in Quality Assurance Programs for Cytopathology
Yosep Chong, Maria Jesús Fernández Aceñero, Zaibo Li, Andrey Bychkov
Acta Cytologica.2025; : 1. CrossRef - National quality assurance program using digital cytopathology: a 5-year digital transformation experience by the Korean Society for Cytopathology
Yosep Chong, Hyeong Ju Kwon, Soon Auck Hong, Sung Soon Kim, Bo-Sung Kim, Younghee Choi, Yoon Jung Choi, Jung-Soo Pyo, Ji Yun Jeong, Soo Jin Jung, Hoon Kyu Oh, Seung-Sook Lee
Journal of Pathology and Translational Medicine.2025; 59(5): 320. CrossRef - Diagnostic proficiency test using digital cytopathology and comparative assessment of whole slide images of cytologic samples for quality assurance program in Korea
Yosep Chong, Soon Auck Hong, Hoon Kyu Oh, Soo Jin Jung, Bo-Sung Kim, Ji Yun Jeong, Ho-Chang Lee, Gyungyub Gong
Journal of Pathology and Translational Medicine.2023; 57(5): 251. CrossRef - Re-Increasing Trends in Thyroid Cancer Incidence after a Short Period of Decrease in Korea: Reigniting the Debate on Ultrasound Screening
Chan Kwon Jung, Ja Seong Bae, Young Joo Park
Endocrinology and Metabolism.2022; 37(5): 816. CrossRef - Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice
Soon Auck Hong, Haeyoen Jung, Sung Sun Kim, Min-Sun Jin, Jung-Soo Pyo, Ji Yun Jeong, Younghee Choi, Gyungyub Gong, Yosep Chong
Journal of Pathology and Translational Medicine.2022; 56(6): 361. CrossRef
- Practice of Cytopathology in Korea: A 40‐Year Evolution Through Standardization, Digital Transformation, and Global Partnership

 E-submission
E-submission


 First
First Prev
Prev



