Search
- Page Path
- HOME > Search
Reviews
- HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation
- Soomin Ahn, Ji Won Woo, Kyoungyul Lee, So Yeon Park
- J Pathol Transl Med. 2020;54(1):34-44. Published online November 6, 2019
- DOI: https://doi.org/10.4132/jptm.2019.11.03
- 38,013 View
- 1,323 Download
- 189 Web of Science
- 187 Crossref
-
 Abstract
Abstract
 PDF
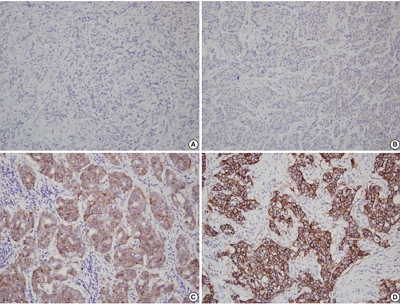
PDF - Human epidermal growth factor receptor 2 (HER2) protein overexpression and/or HER2 gene amplification is found in about 20% of invasive breast cancers. It is a sole predictive marker for treatment benefits from HER2 targeted therapy and thus, HER2 testing is a routine practice for newly diagnosed breast cancer in pathology. Currently, HER2 immunohistochemistry (IHC) is used for a screening test, and in situ hybridization is used as a confirmation test for HER2 IHC equivocal cases. Since the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines on HER2 testing was first released in 2007, it has been updated to provide clear instructions for HER2 testing and accurate determination of HER2 status in breast cancer. During HER2 interpretation, some pitfalls such as intratumoral HER2 heterogeneity and increase in chromosome enumeration probe 17 signals may lead to inaccurate assessment of HER2 status. Moreover, HER2 status can be altered after neoadjuvant chemotherapy or during metastatic progression, due to biologic or methodologic issues. This review addresses recent updates of ASCO/CAP guidelines and factors complicating in the interpretation of HER2 status in breast cancers.
-
Citations
Citations to this article as recorded by- A Comprehensive Model Outperformed the Single Radiomics Model in Noninvasively Predicting the HER2 Status in Patients with Breast Cancer
Weimin Liu, Yiqing Yang, Xiaohong Wang, Chao Li, Chen Liu, Xiaolei Li, Junzhe Wen, Xue Lin, Jie Qin
Academic Radiology.2025; 32(1): 24. CrossRef - Multiparametric MRI Radiomics With Machine Learning for Differentiating HER2-Zero, -Low, and -Positive Breast Cancer: Model Development, Testing, and Interpretability Analysis
Yongxin Chen, Siyi Chen, Wenjie Tang, Qingcong Kong, Zhidan Zhong, Xiaomeng Yu, Yi Sui, Wenke Hu, Xinqing Jiang, Yuan Guo
American Journal of Roentgenology.2025;[Epub] CrossRef - Cervical Lymph Node Metastasis of Unknown Origin and Remote Primary at a Tertiary Cancer Centre in North India: Case Series with Review of Literature
Kriti Grover, Siddharth Arora, Mansi Dey, Deepti Awasthi, Harshad Sharma, Bibhu Prasad Mishra, Nitesh Mohan, Cheena Garg, Arjun Agarwal
Indian Journal of Otolaryngology and Head & Neck Surgery.2025; 77(1): 424. CrossRef - Pooled clinical trial analyses evaluating outcomes of HER2-low vs HER2-0 expression in patients with metastatic breast cancer following chemotherapy
Elizabeth B. Lamont, Emily Stein, Paolo Tarantino, Sara M. Tolaney, Corinne Ahlberg, Krishna Chinnathambu, Jiezhi Qi, Jackie Bilan, Ruthie Davi, Lisa Ensign
Breast Cancer Research and Treatment.2025; 210(1): 11. CrossRef - Insights into AI advances in immunohistochemistry for effective breast cancer treatment: a literature review of ER, PR, and HER2 scoring
Genevieve Chyrmang, Kangkana Bora, Anup Kr. Das, Gazi N. Ahmed, Lopamudra Kakoti
Current Medical Research and Opinion.2025; 41(1): 115. CrossRef - Prediction of Lung Adenocarcinoma Driver Genes Through Protein–Protein Interaction Networks Utilizing GenePlexus
Fei Yuan, Yu‐Hang Zhang, FeiMing Huang, Xiaoyu Cao, Lei Chen, JiaBo Li, WenFeng Shen, KaiYan Feng, YuSheng Bao, Tao Huang, Yu‐Dong Cai
PROTEOMICS.2025;[Epub] CrossRef - Quantitative expression of estrogen, progesterone and human epidermal growth factor receptor-2 and their correlation with immunohistochemistry in breast cancer at Uganda Cancer Institute
Henry Wannume, Nixon Niyonzima, Sam Kalungi, Julius Boniface Okuni, Tonny Okecha, Edward Kakungulu, Steven Mpungu Kiwuwa, Geoffrey Waiswa, Sylvester Kadhumbula, Monica Namayanja, Martin Nabwana, Jackson Orem, Kenji Fujiwara
PLOS ONE.2025; 20(1): e0311185. CrossRef - Automated Quantification of HER2 Amplification Levels Using Deep Learning
Ching-Wei Wang, Kai-Lin Chu, Ting-Sheng Su, Keng-Wei Liu, Yi-Jia Lin, Tai-Kuang Chao
IEEE Journal of Biomedical and Health Informatics.2025; 29(1): 333. CrossRef - CAR-macrophage therapy for HER2-overexpressing advanced solid tumors: a phase 1 trial
Kim A. Reiss, Mathew G. Angelos, E. Claire Dees, Yuan Yuan, Naoto T. Ueno, Paula R. Pohlmann, Melissa L. Johnson, Joseph Chao, Olga Shestova, Jonathan S. Serody, Maggie Schmierer, Madison Kremp, Michael Ball, Rehman Qureshi, Benjamin H. Schott, Poonam Son
Nature Medicine.2025; 31(4): 1171. CrossRef - Human Cytomegalovirus Infection and Breast Cancer: A Literature Review of Clinical and Experimental Data
Rancés Blanco, Juan P. Muñoz
Biology.2025; 14(2): 174. CrossRef - An antibody-photosensitiser bioconjugate overcomes trastuzumab resistance in HER2-positive breast cancer
Mireia Jordà-Redondo, Ana Piqueras, Ana Castillo, Pedro Luis Fernández, Roger Bresolí-Obach, Lidia Blay, Joan Francesc Julián Ibáñez, Santi Nonell
European Journal of Medicinal Chemistry.2025; 290: 117511. CrossRef - Dynamic HER2-low status among patients with triple negative breast cancer (TNBC) and the impact of repeat biopsies
Yael Bar, Geoffrey Fell, Aylin Dedeoglu, Natalie Moffett, Neelima Vidula, Laura Spring, Seth A. Wander, Aditya Bardia, Naomi Ko, Beverly Moy, Leif W. Ellisen, Steven J. Isakoff
npj Breast Cancer.2025;[Epub] CrossRef - Clinical significances of RPL15 gene expression in circulating tumor cells of patients with breast cancer
Ying Zhuang, Keli Su, Shushu Liu, Wei Fan, Huijuan Lv, Wei Zhong
Biomedical Reports.2025; 22(5): 1. CrossRef - Advancing Breast Cancer Treatment: The Role of Immunotherapy and Cancer Vaccines in Overcoming Therapeutic Challenges
Marco Palma
Vaccines.2025; 13(4): 344. CrossRef - Complete preclinical evaluation of the novel antibody mimetic Nanofitin-IRDye800CW for diverse non-invasive diagnostic applications in the management of HER-2 positive tumors
Margherita Iaboni, Federico Crivellin, Francesca Arena, Francesca La Cava, Alessia Cordaro, Francesco Stummo, Daniele Faletto, Simon Huet, Leo Candela, Jessy Pedrault, Eugenia R. Zanella, Andrea Bertotti, Francesco Blasi, Alessandro Maiocchi, Luisa Poggi,
Scientific Reports.2025;[Epub] CrossRef - New insights on Galectin-9 expression in cancer prognosis: An updated systemic review and meta-analysis
Chun Yan So, Yusong Li, Kwan Ting Chow, Xiaosheng Tan
PLOS ONE.2025; 20(3): e0320441. CrossRef - Role of artificial intelligence –based machine learning model in predicting HER2/neu gene status in breast cancer
Ghada Mohamed, Omar Hamdy, Anwar Alkallas, Youssef Tahoun, Mohammed Mohammed Gomaa, Inas Moaz, Ahmed Orabi, Yasmine Hany elzohery, AL-Shimaa Zakaria, Mahitab Ibrahim Eltohamy
Pathology - Research and Practice.2025; 270: 155927. CrossRef - HER2 mRNA Score From Quantitative ERBB2 mRNA Expression of Oncotype Dx
Hyunwoo Lee, Jai Min Ryu, Se Kyung Lee, Byung Joo Chae, Jonghan Yu, Jeong Eon Lee, Seok Won Kim, Seok Jin Nam, Yoon Ah Cho, Eun Yoon Cho
American Journal of Surgical Pathology.2025; 49(8): 807. CrossRef - Neratinib and metformin: A novel therapeutic approach against HER2-Positive Breast Cancer
Hadeel Kheraldine, Arij Fouzat Hassan, Sumayyah Saeed, Maysaloun Merhi, Jericha Miles Mateo, Monika Ulamec, Melita Peric-Balja, Semir Vranic, Hamda Al-Thawadi, Ala-Eddin Al Moustafa
Biomedicine & Pharmacotherapy.2025; 187: 118034. CrossRef - Machine learning prediction of HER2-low expression in breast cancers based on hematoxylin–eosin-stained slides
Jun Du, Jun Shi, Dongdong Sun, Yifei Wang, Guanfeng Liu, Jingru Chen, Wei Wang, Wenchao Zhou, Yushan Zheng, Haibo Wu
Breast Cancer Research.2025;[Epub] CrossRef - Predicting sentinel lymph node metastatic burden with intravoxel incoherent motion diffusion-weighted imaging and dynamic contrast-enhanced magnetic resonance imaging in clinical early-stage breast cancer patients
Mingli Jin, Fang Xiao, Qi Zhao, Ying Jiang, Zhihua Pan, Zhicai Duan, Juxi Jiang, Miaoqi Zhang, Jian Shu
Magnetic Resonance Imaging.2025; 121: 110397. CrossRef - HbA1c levels and breast cancer prognosis in women without diabetes
Jonas Busk Holm, Jens Meldgaard Bruun, Peer Christiansen, Reimar Wernich Thomsen, Jan Frystyk, Deirdre Cronin-Fenton, Signe Borgquist
BMC Cancer.2025;[Epub] CrossRef - Clinical Outcomes in Early-Stage HER2-Low and HER2-Zero Breast Cancer: Single-Center Experience
Jamshid Hamdard, Mehmet Haluk Yücel, Harun Muğlu, Özgür Açıkgöz, Aslı Çakır, Ahmet Bilici, Ömer Fatih Ölmez
Journal of Clinical Medicine.2025; 14(9): 2937. CrossRef - Molecular Mechanism of Breast Cancer and Predisposition of Mouse Mammary Tumor Virus Propagation Cycle
Arya Ghosh, Subash C.B. Gopinath
Current Medicinal Chemistry.2025; 32(12): 2330. CrossRef - ASSESSMENT OF RECEPTOR CONVERSION ROLE FOR ADVANCED BREAST CANCER ON THE CHEMORESISTANCE OCCURRENCE
Oleksii V. Movchan, Ivan I. Smolanka, Andriy O. Lyashenko, Anton D. Loboda, Iryna V. Dosenko, Oksana M. Ivankova
Clinical and Preventive Medicine.2025; (3): 56. CrossRef - Combining conventional magnetic resonance imaging (MRI) parameters with clinicopathologic data for differentiation of the three-tiered human epidermal growth factor receptor 2 (HER2) status in breast cancer
W. Liu, C. Liu, Y. Yang, Y. Chen, A. Muhetaier, Z. Lin, Z. Weng, X. Wang, P. Zhang, J. Qin
Clinical Radiology.2025; 86: 106955. CrossRef - IN SILICO STUDY: THE POTENTIAL OF KILEMO (Litsea cubeba) ENDEMIC PLANT FROM KALIMANTAN AS ANTI-BREAST CANCER THROUGH HER2 INHIBITION
Dwi Utami, Tarisha Elmaningtyas Zahro, Khairun Nisa, Muhammad Farid
JIIS (Jurnal Ilmiah Ibnu Sina): Ilmu Farmasi dan Kesehatan.2025; 10(1): 166. CrossRef - Trastuzumab Deruxtecan in Previously Treated HER2-Low Metastatic Breast Cancer: Real-World Multicentric Study in the Portuguese Population
Luísa Soares Miranda, Maria João Sousa, Miguel Martins Braga, Marisa Couto, Isabel Vieira Fernandes, Francisca Abreu, Inês Eiriz, Catarina Lopes Fernandes, Alice Fonseca Marques, Maria Teresa Marques, Raquel Romão, Fernando Gonçalves, Joana Simões, Antóni
Cancers.2025; 17(12): 1911. CrossRef - Factors influencing pathological complete response to neoadjuvant chemotherapy in resectable breast cancer: A retrospective study
Jian Kang, Huifen Xiong, Xiaoliu Jiang, Zhaohui Huang, Yonghong Guo, Yali Cao, Jingxian Ding
Oncology Letters.2025; 30(1): 1. CrossRef - Association of HER2-low with clinicopathological features in patients with early invasive lobular breast cancer: an international multicentric study
Karen Van Baelen, Ha-Linh Nguyen, François Richard, Gitte Zels, Maria Margarete Karsten, Guilherme Nader-Marta, Peter Vermeulen, Luc Dirix, Adam David Dordevic, Evandro de Azambuja, Denis Larsimont, Marion Maetens, Elia Biganzoli, Hans Wildiers, Ann Smeet
Breast Cancer Research.2025;[Epub] CrossRef - Collagenase type IV improves the quality of HER2 fluorescence in situ hybridization for breast cancers
Shang-En Lee, Sheng-Chi Hsu, Tsai-Hsien Hung, Kwai-Fong Ng, Tse-Ching Chen
American Journal of Clinical Pathology.2025; 164(3): 342. CrossRef - Resonant multi-focal scanning super-resolution microscopy with extended depth and field-of-view
Kidan Tadesse, Keyi Han, Wenhao Liu, Oliver S. Lee, Shu Jia
Cell Reports Physical Science.2025; 6(7): 102680. CrossRef - Scalable Nuclei Detection in HER2-SISH Whole Slide Images via Fine-Tuned Stardist with Expert-Annotated Regions of Interest
Zaka Ur Rehman, Mohammad Faizal Ahmad Fauzi, Wan Siti Halimatul Munirah Wan Ahmad, Fazly Salleh Abas, Phaik-Leng Cheah, Seow-Fan Chiew, Lai-Meng Looi
Diagnostics.2025; 15(13): 1584. CrossRef - Molecular landscape of HER2-mutated non-small cell lung cancer in Northeastern Brazil: Clinical, histopathological, and genomic insights
Cleto Dantas Nogueira, Samuel Frota, Huylmer Lucena Chaves, Juliana Cordeiro de Sousa, Guilherme de Sousa Veloso, Francisco Jonathan dos Santos Araujo, Gabriel Barbosa Silva, Samuel Silva Ferreira, Marclesson Santos Alves, Fabio Nasser, Ezequiel Rangel, F
Oncotarget.2025; 16(1): 467. CrossRef - Correlation between CTMP expression levels and resistance to trastuzumab in HER2 + metastatic breast cancer
Mania Makhoul, Maher Saifo, Fariz Ahmad, Jumana Saleh
Discover Oncology.2025;[Epub] CrossRef - The association between body mass index and neoadjuvant chemotherapy response in patients with breast cancer
Jonas Busk Holm, Stine Blaabjerg Skovbjerg, Hanne Melgaard Nielsen, Peer Christiansen, Jens Meldgaard Bruun, Jan Alsner, Deirdre Cronin-Fenton, Signe Borgquist
Breast Cancer Research.2025;[Epub] CrossRef - An immunohistochemical characterization of basal cell carcinoma in patients below 40 years of age
Vincent Waller, Myriam Boeschen, Thomas Lingscheidt, Mathias Stiller, Lena Eickenscheidt, Thomas Wilhelm, Sonja Grunewald, Mirjana Ziemer, Jan‐Christoph Simon, Hendrik Bläker, Maximilian von Laffert
JDDG: Journal der Deutschen Dermatologischen Gesellschaft.2025; 23(11): 1414. CrossRef - Clinical Relationship Between Serum ApoB, HER2, and Myocardial Ischemia Risk in Breast Cancer Patients
Yeyan Lei, Dongmei Li, Shuang Bai, Xing Zeng, Rongyuan Yang, Qing Liu
Cancer Reports.2025;[Epub] CrossRef - Dual-targeting and steric hindrance resolution in HER2 IHC: a novel approach to improve diagnostic sensitivity
Li Luo, Xi Zhang, Linqiong Chen, Zhuohan Chen, Yuchen Wang, Kaihao Huang, Xiaoyun Lin, Hongxiang Zhu, Wangqi Du
BMC Cancer.2025;[Epub] CrossRef - Time‐Dependent Diffusion MRI‐Based Microstructural Mapping for Characterizing HER2‐Zero, ‐Low, ‐Ultra‐Low, and ‐Positive Breast Cancer
Xiaoxia Wang, Yao Huang, Ying Cao, Huifang Chen, Xueqin Gong, Xiaosong Lan, Jiuquan Zhang, Zhaoxiang Ye
Journal of Magnetic Resonance Imaging.2025; 62(6): 1754. CrossRef - Digital pathology enabling lean management of HER2/neu testing in breast Cancer
Aishwarya Sharma, Prarthna Shah, Manali Ranade, Trupti Pai, Ayushi Sahay, Asawari Patil, Tanuja Shet, Heena Gupta, Devika Chauhan, Puneet Somal, Sankalp Sancheti, Sangeeta Desai
Journal of Pathology Informatics.2025; 19: 100515. CrossRef - Enhancing HER2-low breast cancer detection with quantitative transcriptomics
Maria-Anna Misiakou, Maj-Britt Jensen, Maj-Lis Talman, Bent Ejlertsen, Maria Rossing
npj Breast Cancer.2025;[Epub] CrossRef - Caracterización inmunohistoquímica del cáncer de mama correlacionado con histopatología,estudio realizado en un hospital de Ecuador
María Fernanda Calderón León, Diego Mauricio Cabrera Moyano, Jorge Daniel Cárdenas Rodríguez, Paula Andrea Vásquez Jaramillo, Andrea Alexandra Saltos Román, Maryoli González Sánchez
Journal of the Selva Andina Research Society.2025; 16(2): 116. CrossRef - Relationship of vitamin D receptor expression with hormone receptors and other clinicopathological features in primary breast carcinomas: A retrospective cross-sectional study
Yaşar Ünlü, Ethem Ömeroğlu, Abdülhalim Serden Ay, Meryem İlkay Eren Karanis, Kilinç Ayşe Nur Uğur, Esra Yilmaz
Medicine.2025; 104(35): e44222. CrossRef - Improving HER2 Diagnostics with Digital Real‐Time PCR for Ultrafast, Precise Prediction of Anti‐HER2 Therapy Response in Patients with Breast Cancer
Hee‐Joo Choi, Soo Young Park, Minsik Song, Jinhyuk Chang, YoonSik Kim, Hosub Park, Chihwan David Cha, Sohyeon Yang, Nam Hun Heo, Min Ji Song, Da Sol Kim, Hayeon Kim, Minuk Kim, Jae Eun Park, Yesung Lee, EunChae Ji, Heekyoung Chung, Ilecheon Jeong, Mineui
Small Methods.2025;[Epub] CrossRef - Unveiling Metabolic Signatures as Potential Biomarkers in Common Cancers: Insights from Lung, Breast, Colorectal, Liver, and Gastric Tumours
Kha Wai Hon, Rakesh Naidu
Biomolecules.2025; 15(10): 1376. CrossRef - Validation of ancillary procedures on formalin liquid fixed aspiration cytologic samples: from minimum to maximum
Orsolya Rideg, Tímea Dergez, Arnold Tóth, Tamás Tornóczky, Gábor Pavlovics, Endre Kálmán
American Journal of Clinical Pathology.2025; 164(6): 924. CrossRef - Challenges & recommendations for identification of human epidermal growth factor receptor -2 (HER2)-low metastatic breast cancer in India: Expert opinion statement
Neeraj Arora, Jyoti Bajpai, Amanjit Bal, Atul Batra, Anurag Gupta, Deepak Kumar Mishra, Geetashree Mukherjee, Trupti Pai, Mayur Parihar, Geeta V. Patil Okaly, Shilpa Prabhudesai, Milap Shah, Somashekhar S.P.
The Indian Journal of Medical Research.2025; 162: 279. CrossRef - Eine immunhistologische Charakterisierung des Basalzellkarzinoms bei Patienten unter 40 Jahren
Vincent Waller, Myriam Boeschen, Thomas Lingscheidt, Mathias Stiller, Lena Eickenscheidt, Thomas Wilhelm, Sonja Grunewald, Mirjana Ziemer, Jan‐Christoph Simon, Hendrik Bläker, Maximilian von Laffert
JDDG: Journal der Deutschen Dermatologischen Gesellschaft.2025; 23(11): 1414. CrossRef - Comparison of Immunohistochemical 2 (+) HER2 Gene Status with SISH in Invasive Breast Carcinoma
Alper Sayiner, Hatice Karaman, Fatma Şenel, Arzu Tasdemir, Merve Doğan, İpek Özer
Sakarya Medical Journal.2025; 15(4): 338. CrossRef - Concurrent genomic assessment of circulating tumour cells and ctDNA to guide therapy in metastatic breast cancer
Rebecca C. Allsopp, Karen Page, Evie Wren, Georgios Nteliopoulos, Kelly L. T. Gleason, Gurdeep Matharu Lall, Shradha Bhagani, Emmanuel Acheampong, Marc K. Wadsley, Raoul Charles Coombes, Jacqueline A. Shaw
BMC Cancer.2025;[Epub] CrossRef - Management Strategies and Outcomes in HR+/HER2− Metastatic Breast Cancer Receiving CDK4/6 Inhibitors and Subsequent Therapies
Katarzyna Pogoda, Hubert Pawlik, Anna Balata, Magdalena Czopowicz, Agata Bak, Iwona Twardowska, Malgorzata Meluch, Maria Wojda, Agnieszka Mlodzinska, Ewa Szombara, Renata Sienkiewicz, Aleksandra Konieczna, Elzbieta Brewczynska, Izabela Lemanska, Anna Majs
Breast Cancer: Targets and Therapy.2025; Volume 17: 1307. CrossRef - Identification of novel chemical scaffolds against kinase domain of cancer causing human epidermal growth factor receptor 2: a systemic chemoinformatic approach
Faris Alrumaihi
Journal of Biomolecular Structure and Dynamics.2024; 42(12): 6269. CrossRef - Standardized pathology report for HER2 testing in compliance with 2023 ASCO/CAP updates and 2023 ESMO consensus statements on HER2-low breast cancer
Mariia Ivanova, Francesca Maria Porta, Marianna D’Ercole, Carlo Pescia, Elham Sajjadi, Giulia Cursano, Elisa De Camilli, Oriana Pala, Giovanni Mazzarol, Konstantinos Venetis, Elena Guerini-Rocco, Giuseppe Curigliano, Giuseppe Viale, Nicola Fusco
Virchows Archiv.2024; 484(1): 3. CrossRef - Analytical Performance Evaluation of a 523-Gene Circulating Tumor DNA Assay for Next-Generation Sequencing–Based Comprehensive Tumor Profiling in Liquid Biopsy Samples
Johannes Harter, Eleonora Buth, Janina Johaenning, Florian Battke, Maria Kopp, Henning Zelba, Martin Schulze, Jiri Koedding, Saskia Biskup
The Journal of Molecular Diagnostics.2024; 26(1): 61. CrossRef - Are There More HER2 FISH in the Sea? An Institution’s Experience in Identifying HER2 Positivity Using Fluorescent In Situ Hybridization in Patients with HER2 Negative Immunohistochemistry
Camille Suydam, Fairouz Chibane, Nicole Brown, Madeleine Schlafly, Alicia H. Arnold, Intisar Ghleilib, Melissa Easley, Joseph White
Annals of Surgical Oncology.2024; 31(1): 376. CrossRef - HER2 copy number determination in breast cancer using the highly sensitive droplet digital PCR method
Beate Alinger-Scharinger, Cornelia Kronberger, Georg Hutarew, Wolfgang Hitzl, Roland Reitsamer, Klaassen-Federspiel Frederike, Martina Hager, Thorsten Fischer, Karl Sotlar, Heidi Jaksch-Bogensperger
Virchows Archiv.2024; 485(1): 53. CrossRef - Impact of HER2-low status for patients with early-stage breast cancer and non-pCR after neoadjuvant chemotherapy: a National Cancer Database Analysis
Huiyue Li, Jennifer K. Plichta, Kan Li, Yizi Jin, Samantha M. Thomas, Fei Ma, Li Tang, Qingyi Wei, You-Wen He, Qichen Chen, Yuanyuan Guo, Yueping Liu, Jian Zhang, Sheng Luo
Breast Cancer Research and Treatment.2024; 204(1): 89. CrossRef - Qualification of a multiplexed tissue imaging assay and detection of novel patterns of HER2 heterogeneity in breast cancer
Jennifer L. Guerriero, Jia-Ren Lin, Ricardo G. Pastorello, Ziming Du, Yu-An Chen, Madeline G. Townsend, Kenichi Shimada, Melissa E. Hughes, Siyang Ren, Nabihah Tayob, Kelly Zheng, Shaolin Mei, Alyssa Patterson, Krishan L. Taneja, Otto Metzger, Sara M. Tol
npj Breast Cancer.2024;[Epub] CrossRef - Clinical Utility and Benefits of Comprehensive Genomic Profiling in Cancer
Melissa Yuwono Tjota, Jeremy P Segal, Peng Wang
The Journal of Applied Laboratory Medicine.2024; 9(1): 76. CrossRef - High contrast breast cancer biomarker semi-quantification and immunohistochemistry imaging using upconverting nanoparticles
Sanathana Konugolu Venkata Sekar, Hui Ma, Katarzyna Komolibus, Gokhan Dumlupinar, Matthias J. Mickert, Krzysztof Krawczyk, Stefan Andersson-Engels
Biomedical Optics Express.2024; 15(2): 900. CrossRef - HER2-low breast cancer and response to neoadjuvant chemotherapy: a population-based cohort study
Ximena Baez-Navarro, Mieke R. van Bockstal, Agnes Jager, Carolien H.M. van Deurzen
Pathology.2024; 56(3): 334. CrossRef - Fast-tracking drug development with biomarkers and companion diagnostics
Noreen McBrearty, Devika Bahal, Suso Platero
Journal of Cancer Metastasis and Treatment.2024;[Epub] CrossRef - Emerging Landscape of Targeted Therapy of Breast Cancers With Low Human Epidermal Growth Factor Receptor 2 Protein Expression
Gary Tozbikian, Savitri Krishnamurthy, Marilyn M. Bui, Michael Feldman, David G. Hicks, Shabnam Jaffer, Thaer Khoury, Shi Wei, Hannah Wen, Paula Pohlmann
Archives of Pathology & Laboratory Medicine.2024; 148(2): 242. CrossRef - Treatment Patterns and Health Outcomes among Patients with HER2 IHC0/-Low Metastatic or Recurrent Breast Cancer
Eliya Farah, Chantelle Carbonell, Devon J. Boyne, Darren R. Brenner, Jan-Willem Henning, Daniel Moldaver, Simran Shokar, Winson Y. Cheung
Cancers.2024; 16(3): 518. CrossRef - Clinical Internal Dosimetry and Biodistribution of 177Lu-DOTA-Trastuzumab in HER2-Positive Metastatic and Locally Advanced Breast Carcinoma
Yoga S. Narwadkar, Rahul V. Parghane, Sudeep Sahu, Sangita Lad, Kamal Deep, Gaurav Wanage, Tejal Suralkar, Sharmila Banerjee, Sudeep Gupta, Sandip Basu, Rajendra A. Badwe
Clinical Nuclear Medicine.2024; 49(4): e149. CrossRef - Molecular Classifications in Gastric Cancer: A Call for Interdisciplinary Collaboration
Cristina Díaz del Arco, María Jesús Fernández Aceñero, Luis Ortega Medina
International Journal of Molecular Sciences.2024; 25(5): 2649. CrossRef - The combined immunohistochemical expression of AMBRA1 and SQSTM1 identifies patients with poorly differentiated cutaneous squamous cell carcinoma at risk of metastasis: A proof of concept study
Michael H. Alexander, William J. Cousins, Tom Ewen, Andrew P. South, Penny Lovat, Niki Stefanos
Journal of Cutaneous Pathology.2024; 51(6): 450. CrossRef - Concordance between pathologists and between specimen types in detection of HER2-low breast carcinoma by immunohistochemistry
Jing Wang, Esther Yoon, Savitri Krishnamurthy
Annals of Diagnostic Pathology.2024; 70: 152288. CrossRef - Detection of HER2 expression using 99mTc-NM-02 nanobody in patients with breast cancer: a non-randomized, non-blinded clinical trial
Lingzhou Zhao, Yan Xing, Changcun Liu, Shaofei Ma, Wenhua Huang, Zhen Cheng, Jinhua Zhao
Breast Cancer Research.2024;[Epub] CrossRef - Pyrotinib and Trastuzumab Plus Chemotherapy Serve as an Acceptable Neoadjuvant Regimen Exhibiting Good Efficacy and Tolerance in HER2-Positive Breast Cancer Patients
Yibo Chen, Tianyi Zhang, Rui Zhang, Xuchen Cao
Cancer Biotherapy and Radiopharmaceuticals.2024; 39(6): 435. CrossRef - Clinicopathological and prognostic significance of VEGF, PDGF-B, and HER2/neu expression in gallbladder cancer
Pooja Shukla, Kumudesh Mishra, Ratnakar Shukla, Ruchira Vishwakarma, Niraj Kumari, Narendra Krishnani, Anu Behari, Vinay K. Kapoor
Journal of Cancer Research and Therapeutics.2024; 20(1): 349. CrossRef - Open questions, current challenges, and future perspectives in targeting human epidermal growth factor receptor 2-low breast cancer
G. Curigliano, R. Dent, H. Earle, S. Modi, P. Tarantino, G. Viale, S.M. Tolaney
ESMO Open.2024; 9(4): 102989. CrossRef - Concordance of HER2 status between core needle biopsy and surgical resection specimens of breast cancer: an analysis focusing on the HER2-low status
Sei Na, Milim Kim, Yujun Park, Hyun Jung Kwon, Hee-Chul Shin, Eun-Kyu Kim, Mijung Jang, Sun Mi Kim, So Yeon Park
Breast Cancer.2024; 31(4): 705. CrossRef - Impact of Neoadjuvant Chemotherapy (NAC) on Biomarker Expression in Breast Cancer
Suji Lee, Jee Yeon Kim, So Jeong Lee, Chung Su Hwang, Hyun Jung Lee, Kyung Bin Kim, Jung Hee Lee, Dong Hoon Shin, Kyung Un Choi, Chang Hun Lee, Gi Yeong Huh, Ahrong Kim
Medicina.2024; 60(5): 737. CrossRef - Clinical Use of Molecular Biomarkers in Canine and Feline Oncology: Current and Future
Heike Aupperle-Lellbach, Alexandra Kehl, Simone de Brot, Louise van der Weyden
Veterinary Sciences.2024; 11(5): 199. CrossRef - Clinical Validation of Artificial Intelligence–Powered PD-L1 Tumor Proportion Score Interpretation for Immune Checkpoint Inhibitor Response Prediction in Non–Small Cell Lung Cancer
Hyojin Kim, Seokhwi Kim, Sangjoon Choi, Changhee Park, Seonwook Park, Sergio Pereira, Minuk Ma, Donggeun Yoo, Kyunghyun Paeng, Wonkyung Jung, Sehhoon Park, Chan-Young Ock, Se-Hoon Lee, Yoon-La Choi, Jin-Haeng Chung
JCO Precision Oncology.2024;[Epub] CrossRef - Mesenchymal-epithelial transition factor amplification correlates with adverse pathological features and poor clinical outcome in colorectal cancer
Qiu-Xiao Yu, Ping-Ying Fu, Chi Zhang, Li Li, Wen-Ting Huang
World Journal of Gastrointestinal Surgery.2024; 16(5): 1395. CrossRef - Lipid nanoparticles-based RNA therapies for breast cancer treatment
Luigia Serpico, Yuewen Zhu, Renata Faria Maia, Sumedha Sumedha, Mohammad-Ali Shahbazi, Hélder A. Santos
Drug Delivery and Translational Research.2024; 14(10): 2823. CrossRef - Human epidermal growth factor receptor 2 (HER2) status in breast cancer: practice points and challenges
Natthawadee Laokulrath, Mihir Gudi, Syed Ahmed Salahuddin, Angela Phek Yoon Chong, Cristine Ding, Jabed Iqbal, Wei Qiang Leow, Benjamin Yongcheng Tan, Gary Tse, Emad Rakha, Puay Hoon Tan
Histopathology.2024; 85(3): 371. CrossRef - Comprehensive Immunohistochemical Analysis of Epithelial–Mesenchymal Transition Biomarkers in the Invasive Micropapillary Cancer of the Breast
Ozden Oz, Funda Alkan Tasli, Resmiye Irmak Yuzuguldu, Baha Zengel, Demet Kocatepe Cavdar, Merih Guray Durak, Raika Durusoy, Pranshu Sahgal
International Journal of Breast Cancer.2024;[Epub] CrossRef - Advancing HER2-low breast cancer management: enhancing diagnosis and treatment strategies
Simona Borstnar, Ivana Bozovic-Spasojevic, Ana Cvetanovic, Natalija Dedic Plavetic, Assia Konsoulova, Erika Matos, Lazar Popovic, Savelina Popovska, Snjezana Tomic, Eduard Vrdoljak
Radiology and Oncology.2024; 58(2): 258. CrossRef - Peer‐to‐peer validation of Ki‐67 scoring in a pathology quality circle as a tool to assess interobserver variability: are we better than we thought?
Marit Bernhardt, Leonie Weinhold, Christine Sanders, Oliver Hommerding, Jan‐Frederic Lau, Marieta Toma, Verena Tischler, Matthias Schmid, Tomasz Zienkiewicz, Ralf Hildenbrand, Peter Gerlach, Hui Zhou, Martin Braun, Gunnar Müller, Erich Sieber, Christian M
APMIS.2024; 132(10): 718. CrossRef - Immunohistochemical Expression of Cyclin D1 and p16 in Invasive Breast Carcinoma and Its Association with Clinicopathological Parameters
Shaivy Malik, Shakthivel V., Sana Ahuja, Charanjeet Ahluwalia
Indian Journal of Surgical Oncology.2024; 15(4): 864. CrossRef - Novel engineered HER2 specific recombinant protein nanocages for targeted drug delivery
Javad Kheshti, Mohammad Ahmadyousefi, Meysam Soleimani
Molecular Biology Reports.2024;[Epub] CrossRef - Circulating C-reactive protein levels as a prognostic biomarker in breast cancer across body mass index groups
J. B. Holm, E. Baggesen, D. Cronin-Fenton, J. Frystyk, J. M. Bruun, P. Christiansen, S. Borgquist
Scientific Reports.2024;[Epub] CrossRef - Standardized molecular pathology workflow for ctDNA-based ESR1 testing in HR+/HER2- metastatic breast cancer
Elena Guerini-Rocco, Konstantinos Venetis, Giulia Cursano, Eltjona Mane, Chiara Frascarelli, Francesco Pepe, Mariachiara Negrelli, Edoardo Olmeda, Davide Vacirca, Alberto Ranghiero, Dario Trapani, Carmen Criscitiello, Giuseppe Curigliano, Christian Rolfo,
Critical Reviews in Oncology/Hematology.2024; 201: 104427. CrossRef - The differences between pure and mixed invasive micropapillary breast cancer: the epithelial–mesenchymal transition molecules and prognosis
Ozden Oz, Resmiye Irmak Yuzuguldu, Ayse Yazici, Demet Kocatepe Cavdar, Cengiz Yilmaz, Mucteba Ozturk, Hilal Duzel, Duygu Gurel
Breast Cancer Research and Treatment.2024; 208(1): 41. CrossRef - Best practices for achieving consensus in HER2‐low expression in breast cancer: current perspectives from practising pathologists
Gary Tozbikian, Marilyn M. Bui, David G Hicks, Shabnam Jaffer, Thaer Khoury, Hannah Y Wen, Savitri Krishnamurthy, Shi Wei
Histopathology.2024; 85(3): 489. CrossRef - Consensus Guidelines on Human Epidermal Growth Factor Receptor 2 (HER2)-Low Testing in Breast Cancer in Malaysia
Pathmanathan Rajadurai, Sarala Ravindran, Bang Rom Lee, Suria Hayati Md Pauzi, Seow Fan Chiew, Kean Hooi Teoh, Navarasi S. Raja Gopal, Mastura Md Yusof, Cheng Har Yip
Cancers.2024; 16(13): 2325. CrossRef - Revolutionizing Pathology with Artificial Intelligence: Innovations in Immunohistochemistry
Diana Gina Poalelungi, Anca Iulia Neagu, Ana Fulga, Marius Neagu, Dana Tutunaru, Aurel Nechita, Iuliu Fulga
Journal of Personalized Medicine.2024; 14(7): 693. CrossRef - Interobserver Variability in HER‐2 Immunostaining Interpretation of Metastatic HER2 Low Breast Cancers in Cytology Specimens
Niyati Desai, Courtney F. Connelly, Simon Sung, Adela Cimic, Swikrity U. Baskota
Diagnostic Cytopathology.2024; 52(12): 722. CrossRef - Weakly-supervised deep learning models enable HER2-low prediction from H &E stained slides
Renan Valieris, Luan Martins, Alexandre Defelicibus, Adriana Passos Bueno, Cynthia Aparecida Bueno de Toledo Osorio, Dirce Carraro, Emmanuel Dias-Neto, Rafael A. Rosales, Jose Marcio Barros de Figueiredo, Israel Tojal da Silva
Breast Cancer Research.2024;[Epub] CrossRef - Understanding the spectrum of HER2 status in breast cancer: From HER2-positive to ultra-low HER2
Sana Ahuja, Adil Aziz Khan, Sufian Zaheer
Pathology - Research and Practice.2024; 262: 155550. CrossRef - Systematic review of added immunotherapy in traditional treatment for HER2 positive breast cancer patients
Rohan Choudhari
Innovative Practice in Breast Health.2024; 3-4: 100013. CrossRef - Detecting early-stage breast cancer with GATA3-positive circulating tumor cells
Chun-Hsin Hsieh, Ya-Herng Chang, Pei-Ying Ling, Ying-Tai Jin, Pei-Hsuan Lo, Hei-Jen Jou
Taiwanese Journal of Obstetrics and Gynecology.2024; 63(5): 745. CrossRef - First-in-human study of DP303c, a HER2-targeted antibody-drug conjugate in patients with HER2 positive solid tumors
Jian Zhang, Yiqun Du, Yanchun Meng, Xiaojun Liu, Yuxin Mu, Yunpeng Liu, Yehui Shi, Jufeng Wang, Aimin Zang, Shanzhi Gu, Tianshu Liu, Huan Zhou, Hongqian Guo, Silong Xiang, Xialu Zhang, Suqiong Wu, Huanhuan Qi, Mengke Li, Xichun Hu
npj Precision Oncology.2024;[Epub] CrossRef - Targeting CD276 for T cell-based immunotherapy of breast cancer
Ilona Hagelstein, Laura Wessling, Alexander Rochwarger, Latifa Zekri, Boris Klimovich, Christian M. Tegeler, Gundram Jung, Christian M. Schürch, Helmut R. Salih, Martina S. Lutz
Journal of Translational Medicine.2024;[Epub] CrossRef - Correlation of Androgen Receptor Expression With Ki67 Proliferative Index and Other Clinicopathological Characteristics in Invasive Mammary Carcinomas
D Keerthana Devi, V Pavithra, Leena D Joseph, Chithra Bhanu Challa
Cureus.2024;[Epub] CrossRef - CD81-guided heterologous EVs present heterogeneous interactions with breast cancer cells
Elena Gurrieri, Giulia Carradori, Michela Roccuzzo, Michael Pancher, Daniele Peroni, Romina Belli, Caterina Trevisan, Michela Notarangelo, Wen-Qiu Huang, Agata S. A. Carreira, Alessandro Quattrone, Guido Jenster, Timo L. M. Ten Hagen, Vito Giuseppe D’Agos
Journal of Biomedical Science.2024;[Epub] CrossRef - Deciphering HER2-low breast cancer (BC): insights from real-world data in early stage breast cancer
Anna Pous, Adrià Bernat-Peguera, Assumpció López-Paradís, Beatriz Cirauqui, Vanesa Quiroga, Iris Teruel, Eudald Felip, Angelica Ferrando-Díez, Milana Bergamino, Laia Boronat, Margarita Romeo, Gemma Soler, Christian Mariño, Paula Rodríguez-Martínez, Laura
Therapeutic Advances in Medical Oncology.2024;[Epub] CrossRef - Liquid biopsy-based technologies: a promising tool for biomarker identification in her2-low breast cancer patients for improved therapeutic outcomes
Aldo D’Alessandro, Anca Florentina Deaconu, Sandro Mandolesi, Federico Pio Fabrizio, Massimo Lombardi, Giovanna Liguori, Giovanni Pepe, Nicola Marino, Aureliano Stingi, Alessandro D’Alessandro, Antonio Giordano
Journal of Cancer Metastasis and Treatment.2024;[Epub] CrossRef - SPP1 mRNA Expression Is Associated with M2 Macrophage Infiltration and Poor Prognosis in Triple-Negative Breast Cancer
Yu-Chia Chen, Chia-Ching Chen, Rong-Fu Chen, Hsin-Hung Chen, Po-Ming Chen
Current Issues in Molecular Biology.2024; 46(12): 13499. CrossRef - MicroRNAs and their role in breast cancer metabolism (Review)
Wen Lee, Bann Yeo, Rozi Mahmud, Geok Tan, Mohamed Wahid, Yoke Cheah
International Journal of Oncology.2024;[Epub] CrossRef - CDH1 methylation and expression of E-cadherin and other markers in breast cancer
Luiz Fernando de Queiroz, Marcelo Soares da Mota e Silva, Fernando Colonna Rosman, Siane Lopes Bittencourt Rosas, Heitor Siffert Pereira de Souza, Maria da Glória da Costa Carvalho
Mastology.2024;[Epub] CrossRef - Surgical Management of the Axilla in HR+/HER2– Breast Cancer in the Z1071 Era: A Propensity Score-Matched Analysis of the National Cancer Database
Vayda R. Barker, Samer A. Naffouje, Melissa A. Mallory, Susan A. Hoover, Christine Laronga
Annals of Surgical Oncology.2023; 30(13): 8371. CrossRef - Noninvasive identification of HER2-low-positive status by MRI-based deep learning radiomics predicts the disease-free survival of patients with breast cancer
Yuan Guo, Xiaotong Xie, Wenjie Tang, Siyi Chen, Mingyu Wang, Yaheng Fan, Chuxuan Lin, Wenke Hu, Jing Yang, Jialin Xiang, Kuiming Jiang, Xinhua Wei, Bingsheng Huang, Xinqing Jiang
European Radiology.2023; 34(2): 899. CrossRef - Systemic investigation of inetetamab in combination with small molecules to treat HER2-overexpressing breast and gastric cancers
Lan Deng, Le Zhao, Lifen Liu, Haomin Huang
Open Life Sciences.2023;[Epub] CrossRef - HER2-low expression in patients with advanced or metastatic solid tumors
B. Uzunparmak, C. Haymaker, G. Raso, S. Masciari, L. Wang, H. Lin, A. Gorur, B. Kirby, A.-M. Cimo, A. Kennon, Q. Ding, G. Urschel, Y. Yuan, G. Feng, Y. Rizvi, A. Hussain, C. Zhu, P. Kim, G. Abbadessa, V. Subbiah, T.A. Yap, J. Rodon, S.A. Piha-Paul, F. Mer
Annals of Oncology.2023; 34(11): 1035. CrossRef - Data on 2D culture characterisation of potential markers in human HER2-positive breast cancer cell lines
Son H. Pham, Lyn R. Griffiths, Rachel K. Okolicsanyi, Larisa M. Haupt
Data in Brief.2023; 46: 108880. CrossRef - Determining HER2 Status by Artificial Intelligence: An Investigation of Primary, Metastatic, and HER2 Low Breast Tumors
Christiane Palm, Catherine E. Connolly, Regina Masser, Barbara Padberg Sgier, Eva Karamitopoulou, Quentin Simon, Beata Bode, Marianne Tinguely
Diagnostics.2023; 13(1): 168. CrossRef - Gene amplification mutations originate prior to selective stress in Acinetobacter baylyi
Jennifer A Herrmann, Agata Koprowska, Tesa J Winters, Nancy Villanueva, Victoria D Nikityuk, Feini Pek, Elizabeth M Reis, Constancia Z Dominguez, Daniel Davis, Eric McPherson, Staci R Rocco, Cynthia Recendez, Shyla M Difuntorum, Kelly Faeth, Mario D Lopez
G3.2023;[Epub] CrossRef - Flow Rate-Independent Multiscale Liquid Biopsy for Precision Oncology
Jie Wang, Robert Dallmann, Renquan Lu, Jing Yan, Jérôme Charmet
ACS Sensors.2023; 8(3): 1200. CrossRef - Single-cell HER2 quantification via instant signal amplification in microdroplets
Xiaoxian Liu, Yifan Zhu, Caoxin Li, Yanyun Fang, Jinna Chen, Fei Xu, Yanqing Lu, Perry Ping Shum, Ying Liu, Guanghui Wang
Analytica Chimica Acta.2023; 1251: 340976. CrossRef - Molecular imaging of HER2 receptor: Targeting HER2 for imaging and therapy in nuclear medicine
Daniela Miladinova
Frontiers in Molecular Biosciences.2023;[Epub] CrossRef - CORRELATION BETWEEN DIFFERENT CLINICOPATHOLOGICAL PARAMETERS AND MOLECULAR SUBTYPES OF FEMALE BREAST CARCINOMA IN SOUTH REGION OF IRAQ
Yassir Alaa Muhammed Hassan Shubbar
Wiadomości Lekarskie.2023; 76(1): 97. CrossRef - Pathological identification of HER2-low breast cancer: Tips, tricks, and troubleshooting for the optimal test
Elham Sajjadi, Elena Guerini-Rocco, Elisa De Camilli, Oriana Pala, Giovanni Mazzarol, Konstantinos Venetis, Mariia Ivanova, Nicola Fusco
Frontiers in Molecular Biosciences.2023;[Epub] CrossRef - Protective Effect of HER2 Gene Polymorphism rs24537331 in the Outcome of Canine Mammary Tumors
Ana Canadas-Sousa, Marta Santos, Patrícia Dias-Pereira
Animals.2023; 13(8): 1384. CrossRef - Can Patients with HER2-Low Breast Cancer Benefit from Anti-HER2 Therapies? A Review
Jin Wang, Dongying Liao, Xuemin Zhang, Changhong Miao, Kuang Chen
Breast Cancer: Targets and Therapy.2023; Volume 15: 281. CrossRef - HER2 Low Breast Cancer: A New Subtype or a Trojan for Cytotoxic Drug Delivery?
Marina Popović, Tajana Silovski, Marija Križić, Natalija Dedić Plavetić
International Journal of Molecular Sciences.2023; 24(9): 8206. CrossRef - HER2-Low Breast Cancer—Diagnostic Challenges and Opportunities for Insights from Ongoing Studies: A Podcast
Aditya Bardia, Giuseppe Viale
Targeted Oncology.2023; 18(3): 313. CrossRef - Histopathological and immunohistochemical analysis of predictive and prognostic markers in spontaneous canine mammary cancer
Vladimír Tancoš, Marcel Kovalik, Martin Levkut, Martina Bobrovská, Petra Kolenčíková, Ľubomír Straka, Zuzana Ševčíková, Ondřej Škor, Martina Antošová, Lukáš Plank, Keith L. Thoday
Acta Veterinaria Brno.2023; 92(2): 143. CrossRef - Dissecting sources of variability in patient response to targeted therapy: anti-HER2 therapies as a case study
Timothy Qi, Yanguang Cao
European Journal of Pharmaceutical Sciences.2023; 186: 106467. CrossRef - The Effect of HER2-Low Status on Pathological Complete Response and Survival in Triple-Negative Breast Cancer: A Systemic Review and Meta-Analysis
Yakup Ergun, Baran Akagunduz, Cengiz Karacin, Sema Turker, Gokhan Ucar
Clinical Breast Cancer.2023; 23(6): 567. CrossRef - Efficacy, toxicity and prognostic factors of pyrotinib‑involved neoadjuvant therapy in HER2‑positive breast cancer: A retrospective study
Hao Wang, Hailing Cao, Zhiyun Guo
Oncology Letters.2023;[Epub] CrossRef - Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer
Feng Ye, Saikat Dewanjee, Yuehua Li, Niraj Kumar Jha, Zhe-Sheng Chen, Ankush Kumar, Vishakha, Tapan Behl, Saurabh Kumar Jha, Hailin Tang
Molecular Cancer.2023;[Epub] CrossRef - Weakly supervised bilayer convolutional network in segmentation of HER2 related cells to guide HER2 targeted therapies
Ching-Wei Wang, Kun-Lin Lin, Hikam Muzakky, Yi-Jia Lin, Tai-Kuang Chao
Computerized Medical Imaging and Graphics.2023; 108: 102270. CrossRef - Immune Biomarkers in Triple-Negative Breast Cancer: Improving the Predictivity of Current Testing Methods
Francesca Maria Porta, Elham Sajjadi, Konstantinos Venetis, Chiara Frascarelli, Giulia Cursano, Elena Guerini-Rocco, Nicola Fusco, Mariia Ivanova
Journal of Personalized Medicine.2023; 13(7): 1176. CrossRef - HER2 Equivocal (Score = 2+) Breast Carcinoma Cases Identified by Immunohistochemistry at a South African Hospital. What is the Impact of Fluorescent In Situ Hybridization Testing?
Reena Dhansukh Mohanlal, Nikki Bouwer, Pascale Willem
Applied Immunohistochemistry & Molecular Morphology.2023; 31(8): 555. CrossRef - Discordance of HER2 Expression and/or Amplification on Repeat Testing
Timothy P. DiPeri, Kathleen Kong, Kaushik Varadarajan, Daniel D. Karp, Jaffer A. Ajani, Shubham Pant, Michael F. Press, Sarina A. Piha-Paul, Ecaterina E. Dumbrava, Funda Meric-Bernstam
Molecular Cancer Therapeutics.2023; 22(8): 976. CrossRef - Low and Ultra-Low HER2 in Human Breast Cancer: An Effort to Define New Neoplastic Subtypes
Mariausilia Franchina, Cristina Pizzimenti, Vincenzo Fiorentino, Maurizio Martini, Giuseppina Rosaria Rita Ricciardi, Nicola Silvestris, Antonio Ieni, Giovanni Tuccari
International Journal of Molecular Sciences.2023; 24(16): 12795. CrossRef - Genetic analysis of oligo-recurrence breast cancer: correlation with clinical outcomes
Kuikui Jiang, Danyang Zhou, Fei Xu, Wen Xia, Qiufan Zheng, Qianyi Lu, Rongzhen Luo, Ruoxi Hong, Shusen Wang
BMC Cancer.2023;[Epub] CrossRef - Single domain antibodies specific for HER2 dimerization domain effectively disrupts HER2 dimerization
Ahmad Najafi, Reza Valadan, Hossein Asgarian-Omran, Alireza Rafiei, Mohsen Tehrani
International Immunopharmacology.2023; 124: 110999. CrossRef - Récepteur du facteur de croissance épidermique HER2, tests utilisés pour rechercher son amplification dans le cancer du sein : principes et limites
Imane Eliahiai, Mohammed Eljiar, Sanae Chaib, Jinane KHarmoum, Mariame Chraïbi
Bulletin du Cancer.2023; 110(12): 1301. CrossRef - Integrated Molecular Characterization of HER2-Low Breast Cancer Using Next Generation Sequencing (NGS)
Jean-Louis Merlin, Marie Husson, Nassim Sahki, Pauline Gilson, Vincent Massard, Alexandre Harlé, Agnès Leroux
Biomedicines.2023; 11(12): 3164. CrossRef - GLUCOSE LEVELS OF PLEURAL EFFUSION FLUID AND HER2 STATUS IN PLEURAL-METASTATIC BREAST CANCER
Muhammad Dhanny Irawan, Desak Gede Agung Suprabawati, Heru Purwanto
Majalah Biomorfologi.2023; 33(2): 75. CrossRef - Design of a Ratiometric Plasmonic Biosensor for Herceptin Detection in HER2-Positive Breast Cancer
Neda Shahbazi, Rouholah Zare-Dorabei, Seyed Morteza Naghib
ACS Biomaterials Science & Engineering.2022; 8(2): 871. CrossRef - A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
Mahdi Sadeghi, Soheila Kashanian, Seyed Morteza Naghib, Esfandyar Askari, Fateme Haghiralsadat, Davood Tofighi
Nanotechnology Reviews.2022; 11(1): 793. CrossRef - Anti-HER2 therapy in metastatic breast cancer: many choices and future directions
Carrie S. Wynn, Shou-Ching Tang
Cancer and Metastasis Reviews.2022; 41(1): 193. CrossRef - Electroanalytical overview: screen-printed electrochemical sensing platforms for the detection of vital cardiac, cancer and inflammatory biomarkers
Robert D. Crapnell, Alejandro Garcia-Miranda Ferrari, Nina C. Dempsey, Craig E. Banks
Sensors & Diagnostics.2022; 1(3): 405. CrossRef - FTO genotype was associated with breast cancer in HER2 negative patients
Fateme Montazeri, Hossein Hatami, Soroor Fathi, Naeemeh Hasanpour Ardekanizadeh, Fatemeh Bourbour, Samira Rastgoo, Fatemeh Shafiee, Mohammad Esmail Akbari, Maryam Gholamalizadeh, Seyed Alireza Mosavi Jarrahi, Saeid Doaei
Clinical Nutrition ESPEN.2022; 49: 495. CrossRef - Breast Cancer Human Epidermal Growth Factor Receptor 2 mRNA Molecular Testing Compared to Immunohistochemistry with Correlation to Neoadjuvant Therapy Response
Mahmoud Behairy, Samia Mohamed Gabal, Mohamed Sherif Negm
Open Access Macedonian Journal of Medical Sciences.2022; 10(A): 352. CrossRef - Validity and utility of HER2/ERBB2 copy number variation assessed in liquid biopsies from breast cancer patients: A systematic review
Noortje Verschoor, Teoman Deger, Agnes Jager, Stefan Sleijfer, Saskia M. Wilting, John W.M. Martens
Cancer Treatment Reviews.2022; 106: 102384. CrossRef - RETRACTED: Longitude Variation of the microRNA-497/FGF-23 Axis during Treatment and Its Linkage with Neoadjuvant/Adjuvant Trastuzumab-Induced Cardiotoxicity in HER2-Positive Breast Cancer Patients
Hui Liu, Xiaoyan Hu, Lingyun Wang, Tao Du, Jing Feng, Ming Li, Lei Liu, Xiaofang Liu
Frontiers in Surgery.2022;[Epub] CrossRef - Use of Radionuclide-Based Imaging Methods in Breast Cancer
Betül Altunay, Agnieszka Morgenroth, Felix M. Mottaghy
Seminars in Nuclear Medicine.2022; 52(5): 561. CrossRef - Functional regulations between genetic alteration-driven genes and drug target genes acting as prognostic biomarkers in breast cancer
Li Wang, Lei Yu, Jian Shi, Feng Li, Caiyu Zhang, Haotian Xu, Xiangzhe Yin, Lixia Wang, Shihua Lin, Anastasiia Litvinova, Yanyan Ping, Shangwei Ning, Hongying Zhao
Scientific Reports.2022;[Epub] CrossRef - Human epidermal growth factor receptor-2 and endocrine resistance in hormone-dependent breast cancer
Anastasia Alataki, Mitch Dowsett
Endocrine-Related Cancer.2022; 29(8): R105. CrossRef - The Evolution of Targeted Radionuclide Diagnosis of HER2-Positive Breast Cancer
Olga D. Bragina, Sergei M. Deyev, Vladimir I. Chernov, Vladimir M. Tolmachev
Acta Naturae.2022; 14(2): 4. CrossRef - Deriving tumor purity from cancer next generation sequencing data: applications for quantitative ERBB2 (HER2) copy number analysis and germline inference of BRCA1 and BRCA2 mutations
Stephanie E. Siegmund, Danielle K. Manning, Phani K. Davineni, Fei Dong
Modern Pathology.2022; 35(10): 1458. CrossRef - Current challenges and unmet needs in treating patients with human epidermal growth factor receptor 2-positive advanced breast cancer
Matti Aapro, Fatima Cardoso, Giuseppe Curigliano, Alexandru Eniu, Joseph Gligorov, Nadia Harbeck, Andreas Mueller, Olivia Pagani, Shani Paluch-Shimon, Elzbieta Senkus, Beat Thürlimann, Khalil Zaman
The Breast.2022; 66: 145. CrossRef - [Retracted] Application of Fluorescence In Situ Hybridization Assisted by Fluorescence Microscope in Detection of Her2 Gene in Breast Cancer Patients
Fang Lu, Tingting Zhou, Yan Liu, Liying Song, Bin Zhang, Yuyan Li, Sorayouth Chumnanvej
Contrast Media & Molecular Imaging.2022;[Epub] CrossRef - High sensitivity label-free detection of HER2 using an Al–GaN/GaN high electron mobility transistor-based biosensor
Shivanshu Mishra, Pharyanshu Kachhawa, Amber Kumar Jain, Rajiv Ranjan Thakur, Nidhi Chaturvedi
Lab on a Chip.2022; 22(21): 4129. CrossRef - Indian Data on HER2 Fluorescence In Situ Hybridization in Invasive Breast Cancer with Immunohistochemically Equivocal Results As Per 2018 ASCO/CAP Guidelines
B. R. Nagarjun, Biren Parikh, Manaswi Nareshkumar Patel, Pina J. Trivedi, Dharmesh M. Patel
South Asian Journal of Cancer.2022; 11(04): 281. CrossRef - S‑phase fraction, lymph node status and disease staging as the main prognostic factors to differentiate between young and older patients with invasive breast carcinoma
António Pinto, João Matos, Teresa Pereira, Giovani Silva, Saudade André
Oncology Letters.2022;[Epub] CrossRef - Inferring tumor-specific cancer dependencies through integrating ex vivo drug response assays and drug-protein profiling
Alina Batzilla, Junyan Lu, Jarno Kivioja, Kerstin Putzker, Joe Lewis, Thorsten Zenz, Wolfgang Huber, James Gallo
PLOS Computational Biology.2022; 18(8): e1010438. CrossRef - Clinical possibilities of HER2-positive breast cancer diagnosis using alternative scaffold proteins
O. D. Bragina, V. I. Chernov, S. M. Deyev, V. M. Tolmachev
Bulletin of Siberian Medicine.2022; 21(3): 132. CrossRef - Molecular Pathology of Gastric Cancer
Moonsik Kim, An Na Seo
Journal of Gastric Cancer.2022; 22(4): 264. CrossRef - Loss of NECTIN1 triggers melanoma dissemination upon local IGF1 depletion
Julien Ablain, Amira Al Mahi, Harriet Rothschild, Meera Prasad, Sophie Aires, Song Yang, Maxim E. Dokukin, Shuyun Xu, Michelle Dang, Igor Sokolov, Christine G. Lian, Leonard I. Zon
Nature Genetics.2022; 54(12): 1839. CrossRef - Advanced diagnosis technologies for HER2 breast cancer markers
Mengxue Zhang
Highlights in Science, Engineering and Technology.2022; 14: 44. CrossRef - An Overview of Clinical Development of Agents for Metastatic or Advanced Breast Cancer Without ERBB2 Amplification (HER2-Low)
Aleix Prat, Aditya Bardia, Giuseppe Curigliano, M. Elizabeth H. Hammond, Sibylle Loibl, Sara M. Tolaney, Giuseppe Viale
JAMA Oncology.2022; 8(11): 1676. CrossRef - Development of T-cell engagers selective for cells co-expressing two antigens
Danielle M. Dicara, Sunil Bhakta, Mary Ann Go, James Ziai, Ron Firestein, Bill Forrest, Chen Gu, Steven R. Leong, Genee Lee, Shang-Fan Yu, Andrew G. Polson, Nicholas J. Agard
mAbs.2022;[Epub] CrossRef - The clinical significance of HER2 expression in DCIS
Ioanna Akrida, Francesk Mulita
Medical Oncology.2022;[Epub] CrossRef - Antibody-Drug Conjugates in Breast Cancer: Spotlight on HER2
Rachel Occhiogrosso Abelman, Arielle Medford, Laura Spring, Aditya Bardia
The Cancer Journal.2022; 28(6): 423. CrossRef - The Clinical Utility of Droplet Digital PCR for Profiling Circulating Tumor DNA in Breast Cancer Patients
Ugur Gezer, Abel J. Bronkhorst, Stefan Holdenrieder
Diagnostics.2022; 12(12): 3042. CrossRef - Lapatinib and lapatinib plus trastuzumab therapy versus trastuzumab therapy for HER2 positive breast cancer patients: an updated systematic review and meta-analysis
Ye Yuan, Xumei Liu, Yi Cai, Wenyuan Li
Systematic Reviews.2022;[Epub] CrossRef - Interactions dietary components with expression level of breast cancer-related genes
Fatemeh Bourbour, Azam Pourtaheri, Khadijeh Abbasi, Naeemeh Hasanpour Ardekanizadeh, Maryam Gholamalizadeh, Azadeh Hajipour, Sepideh Abdollahi, Seyedeh Elaheh Bagheri, Mina Ahmadzadeh, Saeid Doaei, Arezoo Haghighian
Egyptian Journal of Medical Human Genetics.2022;[Epub] CrossRef - HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging
Betül Altunay, Agnieszka Morgenroth, Mohsen Beheshti, Andreas Vogg, Nicholas C. L. Wong, Hong Hoi Ting, Hans-Jürgen Biersack, Elmar Stickeler, Felix M. Mottaghy
European Journal of Nuclear Medicine and Molecular Imaging.2021; 48(5): 1371. CrossRef - Risk-based decision-making in the treatment of HER2-positive early breast cancer: Recommendations based on the current state of knowledge
Christian Jackisch, Patricia Cortazar, Charles E. Geyer, Luca Gianni, Joseph Gligorov, Zuzana Machackova, Edith A. Perez, Andreas Schneeweiss, Sara M. Tolaney, Michael Untch, Andrew Wardley, Martine Piccart
Cancer Treatment Reviews.2021; 99: 102229. CrossRef - Histologic Patterns of Cutaneous Metastases of Breast Carcinoma: A Clinicopathologic Study of 232 Cases
Shira Ronen, David Suster, Wei-Shen Chen, Natali Ronen, Sri Krishna C. Arudra, Celestine Trinidad, Doina Ivan, Victor G. Prieto, Saul Suster
The American Journal of Dermatopathology.2021; 43(6): 401. CrossRef - Standardized pathology report for breast cancer
Soo Youn Cho, So Yeon Park, Young Kyung Bae, Jee Yeon Kim, Eun Kyung Kim, Woo Gyeong Kim, Youngmee Kwon, Ahwon Lee, Hee Jin Lee, Ji Shin Lee, Jee Young Park, Gyungyub Gong, Hye Kyoung Yoon
Journal of Pathology and Translational Medicine.2021; 55(1): 1. CrossRef - The impact of oral contraceptive use on breast cancer risk: State of the art and future perspectives in the era of 4P medicine
R. Bonfiglio, M.L. Di Pietro
Seminars in Cancer Biology.2021; 72: 11. CrossRef - The Co-Expression of Melanoma-Antigen Family a Proteins and New York Esophageal Squamous Cell Carcinoma-1 in Breast Cancer: A Pilot Study
Yu-Xin Wang, Feng-Lian Li, Li-Xin Du, Jun-Fang Liu, Li-Gang Huo, Shu-Qing Li, Bin Tian
Cancer Management and Research.2021; Volume 13: 6123. CrossRef - Targeting HER2 protein in individual cells using ICP-MS detection and its potential as prognostic and predictive breast cancer biomarker
A. Fernández Asensio, M. Corte-Rodríguez, J. Bettmer, L.M. Sierra, M. Montes-Bayón, E. Blanco- González
Talanta.2021; 235: 122773. CrossRef - Development of a 99mTc-Labeled Single-Domain Antibody for SPECT/CT Assessment of HER2 Expression in Breast Cancer
Lingzhou Zhao, Changcun Liu, Yan Xing, Jin He, Jim O’Doherty, Wenhua Huang, Jinhua Zhao
Molecular Pharmaceutics.2021; 18(9): 3616. CrossRef - WITHDRAWN: Nouvelles stratégies thérapeutiques dans les cancers du sein HER2-surexprimé
Benoîte Mery, Philippe Toussaint, Pierre-Etienne Heudel, Armelle Dufresne, Mélodie Carbonnaux, Hélène Vanacker, Thomas Bachelot, Olivier Trédan
Bulletin du Cancer.2021;[Epub] CrossRef - Retrospective observational study of HER2 immunohistochemistry in borderline breast cancer patients undergoing neoadjuvant therapy, with an emphasis on Group 2 (HER2/CEP17 ratio ≥2.0, HER2 copy number <4.0 signals/cell) cases
Emad A. Rakha, Islam M. Miligy, Cecily M. Quinn, Elena Provenzano, Abeer M. Shaaban, Caterina Marchiò, Michael S. Toss, Grace Gallagy, Ciara Murray, Janice Walshe, Ayaka Katayama, Karim Eldib, Nahla Badr, Bruce Tanchel, Rebecca Millican-Slater, Colin Purd
British Journal of Cancer.2021; 124(11): 1836. CrossRef - Loss of HER2‐positivity following neoadjuvant targeted therapy for breast cancer is not associated with inferior oncologic outcomes
Catherine L. Wetzel, Thomas L. Sutton, Stuart Gardiner, Maryam Farinola, Nathalie Johnson, Jennifer R. Garreau
Journal of Surgical Oncology.2021; 124(8): 1224. CrossRef - Clinical and Genetic Predictive Models for the Prediction of Pathological Complete Response to Optimize the Effectiveness for Trastuzumab Based Chemotherapy
Lun Li, Min Chen, Shuyue Zheng, Hanlu Li, Weiru Chi, Bingqiu Xiu, Qi Zhang, Jianjing Hou, Jia Wang, Jiong Wu
Frontiers in Oncology.2021;[Epub] CrossRef - tRNA‐derived fragments: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 as novel diagnostic biomarkers for breast cancer
Yue Zhang, Zhao Bi, Xiaohan Dong, Miao Yu, Kangyu Wang, Xingguo Song, Li Xie, Xianrang Song
Thoracic Cancer.2021; 12(17): 2314. CrossRef - Detection of secondary metastatic breast cancer by measurement of plasma CA 15.3
L. De Cock, J. Heylen, A. Wildiers, K. Punie, A. Smeets, C. Weltens, P. Neven, J. Billen, A. Laenen, H. Wildiers
ESMO Open.2021; 6(4): 100203. CrossRef - Standardized Pathology Report for Breast Cancer
Soo Youn Cho, So Yeon Park, Young Kyung Bae, Jee Yeon Kim, Eun Kyung Kim, Woo Gyeong Kim, Youngmee Kwon, Ahwon Lee, Hee Jin Lee, Ji Shin Lee, Jee Young Park, Gyungyub Gong, Hye Kyoung Yoon
Journal of Breast Cancer.2021; 24(1): 1. CrossRef - Circular RNA circ-ERBB2 promotes HER2-positive breast cancer progression and metastasis via sponging miR-136-5p and miR-198
Jin-xiu Zhong, Yun-yuan Kong, Rong-guang Luo, Guo-jin Xia, Wen-xing He, Xue-zhong Chen, Wei-wei Tan, Qing-jie Chen, Yu-yin Huang, Yan-xing Guan
Journal of Translational Medicine.2021;[Epub] CrossRef - Nouvelles stratégies thérapeutiques dans les cancers du sein HER2-surexprimé
Benoîte Mery, Philippe Toussaint, Pierre-Etienne Heudel, Armelle Dufresne, Mélodie Carbonnaux, Hélène Vanacker, Thomas Bachelot, Olivier Trédan
Bulletin du Cancer.2021; 108(11): 11S8. CrossRef - Association of Estrogen and Progesterone Receptors with Clinicopathological Prognostic Factors in Breast Cancer
Ali Abdul Hadi Abdul-Kareem, Qahtan A. Mahdi
Medical Journal of Babylon.2021; 18(2): 111. CrossRef - HER2 alterations in non-small-cell lung cancer – Druggable or undruggable?
Suresh Kumar Bondili, Ravindra Nandhana, Vanita Noronha, Swayamprabha Pawar, Nandini Menon, Omshree Shetty, Anuradha Chougule, Abhishek Mahajan, Rajiv Kumar, Vijay M. Patil, Amit Joshi, Kumar Prabhash
Cancer Research, Statistics, and Treatment.2021; 4(2): 374. CrossRef - UCNP-based Photoluminescent Nanomedicines for Targeted Imaging and Theranostics of Cancer
Evgenii L. Guryev, Anita S. Smyshlyaeva, Natalia Y. Shilyagina, Evgeniya A. Sokolova, Samah Shanwar, Alexey B. Kostyuk, Alexander V. Lyubeshkin, Alexey A. Schulga, Elena V. Konovalova, Quan Lin, Indrajit Roy, Irina V. Balalaeva, Sergey M. Deyev, Andrei V.
Molecules.2020; 25(18): 4302. CrossRef - Impact of the Updated Guidelines on Human Epidermal Growth Factor Receptor 2 (HER2) Testing in Breast Cancer
Min Chong Kim, Su Hwan Kang, Jung Eun Choi, Young Kyung Bae
Journal of Breast Cancer.2020; 23(5): 484. CrossRef
- A Comprehensive Model Outperformed the Single Radiomics Model in Noninvasively Predicting the HER2 Status in Patients with Breast Cancer
- Good Laboratory Standards for Clinical Next-Generation Sequencing Cancer Panel Tests
- Jihun Kim, Woong-Yang Park, Nayoung K. D. Kim, Se Jin Jang, Sung-Min Chun, Chang-Ohk Sung, Jene Choi, Young-Hyeh Ko, Yoon-La Choi, Hyo Sup Shim, Jae-Kyung Won
- J Pathol Transl Med. 2017;51(3):191-204. Published online May 10, 2017
- DOI: https://doi.org/10.4132/jptm.2017.03.14
- 29,115 View
- 1,118 Download
- 36 Web of Science
- 37 Crossref
-
 Abstract
Abstract
 PDF
PDF - Next-generation sequencing (NGS) has recently emerged as an essential component of personalized cancer medicine due to its high throughput and low per-base cost. However, no sufficient guidelines for implementing NGS as a clinical molecular pathology test are established in Korea. To ensure clinical grade quality without inhibiting adoption of NGS, a taskforce team assembled by the Korean Society of Pathologists developed laboratory guidelines for NGS cancer panel testing procedures and requirements for clinical implementation of NGS. This consensus standard proposal consists of two parts: laboratory guidelines and requirements for clinical NGS laboratories. The laboratory guidelines part addressed several important issues across multistep NGS cancer panel tests including choice of gene panel and platform, sample handling, nucleic acid management, sample identity tracking, library preparation, sequencing, analysis and reporting. Requirements for clinical NGS tests were summarized in terms of documentation, validation, quality management, and other required written policies. Together with appropriate pathologist training and international laboratory standards, these laboratory standards would help molecular pathology laboratories to successfully implement NGS cancer panel tests in clinic. In this way, the oncology community would be able to help patients to benefit more from personalized cancer medicine.
-
Citations
Citations to this article as recorded by- Tumour purity assessment with deep learning in colorectal cancer and impact on molecular analysis
Lydia A Schoenpflug, Aikaterini Chatzipli, Korsuk Sirinukunwattana, Susan Richman, Andrew Blake, James Robineau, Kirsten D Mertz, Clare Verrill, Simon J Leedham, Claire Hardy, Celina Whalley, Keara Redmond, Philip Dunne, Steven Walker, Andrew D Beggs, Ult
The Journal of Pathology.2025; 265(2): 184. CrossRef - Clinical Validation of Local Versus Commercial Genomic Testing in Cancer: A Comparison of Tissue and Plasma Concordance
Lucy G. Faulkner, Lynne Howells, Susann Lehman, Caroline Cowley, Zahirah Sidat, Jacqui Shaw, Anne L. Thomas
Cancer Investigation.2025; 43(2): 119. CrossRef - Diagnostic Implications of NGS-Based Molecular Profiling in Mature B-Cell Lymphomas with Potential Bone Marrow Involvement
Bernhard Strasser, Sebastian Mustafa, Josef Seier, Erich Wimmer, Josef Tomasits
Diagnostics.2025; 15(6): 727. CrossRef - Pragmatic nationwide master observational trial based on genomic alterations in advanced solid tumors: KOrean Precision Medicine Networking Group Study of MOlecular profiling guided therapy based on genomic alterations in advanced Solid tumors (KOSMOS)-II
Sun Young Kim, Jee Hyun Kim, Tae-Yong Kim, Sook Ryun Park, Shinkyo Yoon, Soohyeon Lee, Se-Hoon Lee, Tae Min Kim, Sae-Won Han, Hye Ryun Kim, Hongseok Yun, Sejoon Lee, Jihun Kim, Yoon-La Choi, Kui Son Choi, Heejung Chae, Hyewon Ryu, Gyeong-Won Lee, Dae Youn
BMC Cancer.2024;[Epub] CrossRef - Reporting of somatic variants in clinical cancer care: recommendations of the Swiss Society of Molecular Pathology
Yann Christinat, Baptiste Hamelin, Ilaria Alborelli, Paolo Angelino, Valérie Barbié, Bettina Bisig, Heather Dawson, Milo Frattini, Tobias Grob, Wolfram Jochum, Ronny Nienhold, Thomas McKee, Matthias Matter, Edoardo Missiaglia, Francesca Molinari, Sacha Ro
Virchows Archiv.2024; 485(6): 1033. CrossRef - Acute myeloid leukemia and myelodysplastic neoplasms: clinical implications of myelodysplasia-related genes mutations and TP53 aberrations
Hyunwoo Kim, Ja Young Lee, Shinae Yu, Eunkyoung Yoo, Hye Ran Kim, Sang Min Lee, Won Sik Lee
Blood Research.2024;[Epub] CrossRef - Validation and Clinical Application of ONCOaccuPanel for Targeted Next-Generation Sequencing of Solid Tumors
Moonsik Kim, Changseon Lee, Juyeon Hong, Juhee Kim, Ji Yun Jeong, Nora Jee-Young Park, Ji-Eun Kim, Ji Young Park
Cancer Research and Treatment.2023; 55(2): 429. CrossRef - Establishing molecular pathology curriculum for pathology trainees and continued medical education: a collaborative work from the Molecular Pathology Study Group of the Korean Society of Pathologists
Jiwon Koh, Ha Young Park, Jeong Mo Bae, Jun Kang, Uiju Cho, Seung Eun Lee, Haeyoun Kang, Min Eui Hong, Jae Kyung Won, Youn-La Choi, Wan-Seop Kim, Ahwon Lee
Journal of Pathology and Translational Medicine.2023; 57(5): 265. CrossRef - Clinical applications of next-generation sequencing in the diagnosis of genetic disorders in Korea: a narrative review
Jihoon G. Yoon, Man Jin Kim, Yong Jin Kwon, Jong-Hee Chae
Journal of the Korean Medical Association.2023; 66(10): 613. CrossRef - Obtaining spatially resolved tumor purity maps using deep multiple instance learning in a pan-cancer study
Mustafa Umit Oner, Jianbin Chen, Egor Revkov, Anne James, Seow Ye Heng, Arife Neslihan Kaya, Jacob Josiah Santiago Alvarez, Angela Takano, Xin Min Cheng, Tony Kiat Hon Lim, Daniel Shao Weng Tan, Weiwei Zhai, Anders Jacobsen Skanderup, Wing-Kin Sung, Hwee
Patterns.2022; 3(2): 100399. CrossRef - Update on Molecular Diagnosis in Extranodal NK/T-Cell Lymphoma and Its Role in the Era of Personalized Medicine
Ka-Hei (Murphy) Sun, Yin-Ting (Heylie) Wong, Ka-Man (Carmen) Cheung, Carmen (Michelle) Yuen, Yun-Tat (Ted) Chan, Wing-Yan (Jennifer) Lai, Chun (David) Chao, Wing-Sum (Katie) Fan, Yuen-Kiu (Karen) Chow, Man-Fai Law, Ho-Chi (Tommy) Tam
Diagnostics.2022; 12(2): 409. CrossRef - Defining Novel DNA Virus-Tumor Associations and Genomic Correlates Using Prospective Clinical Tumor/Normal Matched Sequencing Data
Chad M. Vanderbilt, Anita S. Bowman, Sumit Middha, Kseniya Petrova-Drus, Yi-Wei Tang, Xin Chen, Youxiang Wang, Jason Chang, Natasha Rekhtman, Klaus J. Busam, Sounak Gupta, Meera Hameed, Maria E. Arcila, Marc Ladanyi, Michael F. Berger, Snjezana Dogan, Ahm
The Journal of Molecular Diagnostics.2022; 24(5): 515. CrossRef - Performance Evaluation of Three DNA Sample Tracking Tools in a Whole Exome Sequencing Workflow
Gertjan Wils, Céline Helsmoortel, Pieter-Jan Volders, Inge Vereecke, Mauro Milazzo, Jo Vandesompele, Frauke Coppieters, Kim De Leeneer, Steve Lefever
Molecular Diagnosis & Therapy.2022; 26(4): 411. CrossRef - Clinical Quality Considerations when Using Next-Generation Sequencing (NGS) in Clinical Drug Development
Timothé Ménard, Alaina Barros, Christopher Ganter
Therapeutic Innovation & Regulatory Science.2021; 55(5): 1066. CrossRef - Fast Healthcare Interoperability Resources (FHIR)–Based Quality Information Exchange for Clinical Next-Generation Sequencing Genomic Testing: Implementation Study
Donghyeong Seong, Sungwon Jung, Sungchul Bae, Jongsuk Chung, Dae-Soon Son, Byoung-Kee Yi
Journal of Medical Internet Research.2021; 23(4): e26261. CrossRef - Status of Next-Generation Sequencing-Based Genetic Diagnosis in Hematologic Malignancies in Korea (2017-2018)
JinJu Kim, Ja Young Lee, Jungwon Huh, Myung-Hyun Nam, Myungshin Kim, Young-Uk Cho, Sun-Young Kong, Seung-Tae Lee, In-Suk Kim
Laboratory Medicine Online.2021; 11(1): 25. CrossRef - MSI-Testung
Josef Rüschoff, Gustavo Baretton, Hendrik Bläker, Wolfgang Dietmaier, Manfred Dietel, Arndt Hartmann, Lars-Christian Horn, Korinna Jöhrens, Thomas Kirchner, Ruth Knüchel, Doris Mayr, Sabine Merkelbach-Bruse, Hans-Ulrich Schildhaus, Peter Schirmacher, Mark
Der Pathologe.2021; 42(4): 414. CrossRef - Molecular biomarker testing for non–small cell lung cancer: consensus statement of the Korean Cardiopulmonary Pathology Study Group
Sunhee Chang, Hyo Sup Shim, Tae Jung Kim, Yoon-La Choi, Wan Seop Kim, Dong Hoon Shin, Lucia Kim, Heae Surng Park, Geon Kook Lee, Chang Hun Lee
Journal of Pathology and Translational Medicine.2021; 55(3): 181. CrossRef - MSI testing
Josef Rüschoff, Gustavo Baretton, Hendrik Bläker, Wolfgang Dietmaier, Manfred Dietel, Arndt Hartmann, Lars-Christian Horn, Korinna Jöhrens, Thomas Kirchner, Ruth Knüchel, Doris Mayr, Sabine Merkelbach-Bruse, Hans-Ulrich Schildhaus, Peter Schirmacher, Mark
Der Pathologe.2021; 42(S1): 110. CrossRef - 16S rDNA microbiome composition pattern analysis as a diagnostic biomarker for biliary tract cancer
Huisong Lee, Hyeon Kook Lee, Seog Ki Min, Won Hee Lee
World Journal of Surgical Oncology.2020;[Epub] CrossRef - Risk Stratification Using a Novel Genetic Classifier IncludingPLEKHS1Promoter Mutations for Differentiated Thyroid Cancer with Distant Metastasis
Chan Kwon Jung, Seung-Hyun Jung, Sora Jeon, Young Mun Jeong, Yourha Kim, Sohee Lee, Ja-Seong Bae, Yeun-Jun Chung
Thyroid.2020; 30(11): 1589. CrossRef - Biomarker testing for advanced lung cancer by next-generation sequencing; a valid method to achieve a comprehensive glimpse at mutational landscape
Anurag Mehta, Smreti Vasudevan, Sanjeev Kumar Sharma, Manoj Panigrahi, Moushumi Suryavanshi, Mumtaz Saifi, Ullas Batra
Applied Cancer Research.2020;[Epub] CrossRef - Application Areas of Traditional Molecular Genetic Methods and NGS in relation to Hereditary Urological Cancer Diagnosis
Dmitry S. Mikhaylenko, Alexander S. Tanas, Dmitry V. Zaletaev, Marina V. Nemtsova
Journal of Oncology.2020; 2020: 1. CrossRef - Assembling and Validating Bioinformatic Pipelines for Next-Generation Sequencing Clinical Assays
Jeffrey A SoRelle, Megan Wachsmann, Brandi L. Cantarel
Archives of Pathology & Laboratory Medicine.2020; 144(9): 1118. CrossRef - Standard operating procedure for somatic variant refinement of sequencing data with paired tumor and normal samples
Erica K. Barnell, Peter Ronning, Katie M. Campbell, Kilannin Krysiak, Benjamin J. Ainscough, Lana M. Sheta, Shahil P. Pema, Alina D. Schmidt, Megan Richters, Kelsy C. Cotto, Arpad M. Danos, Cody Ramirez, Zachary L. Skidmore, Nicholas C. Spies, Jasreet Hun
Genetics in Medicine.2019; 21(4): 972. CrossRef - A DNA pool of FLT3-ITD positive DNA samples can be used efficiently for analytical evaluation of NGS-based FLT3-ITD quantitation - Testing several different ITD sequences and rates, simultaneously
Zoltán A. Mezei, Dávid Tornai, Róza Földesi, László Madar, Andrea Sümegi, Mária Papp, Péter Antal-Szalmás
Journal of Biotechnology.2019; 303: 25. CrossRef - Pharmacogenomic Testing: Clinical Evidence and Implementation Challenges
Catriona Hippman, Corey Nislow
Journal of Personalized Medicine.2019; 9(3): 40. CrossRef - Cancer Panel Assay for Precision Oncology Clinic: Results from a 1-Year Study
Dohee Kwon, Binnari Kim, Hyeong Chan Shin, Eun Ji Kim, Sang Yun Ha, Kee-Taek Jang, Seung Tae Kim, Jeeyun Lee, Won Ki Kang, Joon Oh Park, Kyoung-Mee Kim
Translational Oncology.2019; 12(11): 1488. CrossRef - Analytical Evaluation of an NGS Testing Method for Routine Molecular Diagnostics on Melanoma Formalin-Fixed, Paraffin-Embedded Tumor-Derived DNA
Irene Mancini, Lisa Simi, Francesca Salvianti, Francesca Castiglione, Gemma Sonnati, Pamela Pinzani
Diagnostics.2019; 9(3): 117. CrossRef - Benchmark Database for Process Optimization and Quality Control of Clinical Cancer Panel Sequencing
Donghyeong Seong, Jongsuk Chung, Ki-Wook Lee, Sook-Young Kim, Byung-Suk Kim, Jung-Keun Song, Sungwon Jung, Taeseob Lee, Donghyun Park, Byoung-Kee Yi, Woong-Yang Park, Dae-Soon Son
Biotechnology and Bioprocess Engineering.2019; 24(5): 793. CrossRef - Use of the Ion PGM and the GeneReader NGS Systems in Daily Routine Practice for Advanced Lung Adenocarcinoma Patients: A Practical Point of View Reporting a Comparative Study and Assessment of 90 Patients
Simon Heeke, Véronique Hofman, Elodie Long-Mira, Virginie Lespinet, Salomé Lalvée, Olivier Bordone, Camille Ribeyre, Virginie Tanga, Jonathan Benzaquen, Sylvie Leroy, Charlotte Cohen, Jérôme Mouroux, Charles Marquette, Marius Ilié, Paul Hofman
Cancers.2018; 10(4): 88. CrossRef - Use of the Ion AmpliSeq Cancer Hotspot Panel in clinical molecular pathology laboratories for analysis of solid tumours: With emphasis on validation with relevant single molecular pathology tests and the Oncomine Focus Assay
Ahwon Lee, Sung-Hak Lee, Chan Kwon Jung, Gyungsin Park, Kyo Young Lee, Hyun Joo Choi, Ki Ouk Min, Tae Jung Kim, Eun Jung Lee, Youn Soo Lee
Pathology - Research and Practice.2018; 214(5): 713. CrossRef - Recent Advancement of the Molecular Diagnosis in Pediatric Brain Tumor
Jeong-Mo Bae, Jae-Kyung Won, Sung-Hye Park
Journal of Korean Neurosurgical Society.2018; 61(3): 376. CrossRef - The long tail of molecular alterations in non-small cell lung cancer: a single-institution experience of next-generation sequencing in clinical molecular diagnostics
Caterina Fumagalli, Davide Vacirca, Alessandra Rappa, Antonio Passaro, Juliana Guarize, Paola Rafaniello Raviele, Filippo de Marinis, Lorenzo Spaggiari, Chiara Casadio, Giuseppe Viale, Massimo Barberis, Elena Guerini-Rocco
Journal of Clinical Pathology.2018; 71(9): 767. CrossRef - Clinical laboratory utilization management and improved healthcare performance
Christopher Naugler, Deirdre L. Church
Critical Reviews in Clinical Laboratory Sciences.2018; 55(8): 535. CrossRef - Development of HLA-A, -B and -DR Typing Method Using Next-Generation Sequencing
Dong Hee Seo, Jeong Min Lee, Mi Ok Park, Hyun Ju Lee, Seo Yoon Moon, Mijin Oh, So Young Kim, Sang-Heon Lee, Ki-Eun Hyeong, Hae-Jin Hu, Dae-Yeon Cho
The Korean Journal of Blood Transfusion.2018; 29(3): 310. CrossRef - Value-based genomics
Jun Gong, Kathy Pan, Marwan Fakih, Sumanta Pal, Ravi Salgia
Oncotarget.2018; 9(21): 15792. CrossRef
- Tumour purity assessment with deep learning in colorectal cancer and impact on molecular analysis
Original Article
- Guidelines for Pathologic Study of Gastric Cancer.
- Korean J Pathol. 1992;26(2):154-163.
- 2,132 View
- 38 Download
-
 Abstract
Abstract
 PDF
PDF - Gastric cancer is the most common malignant neoplasm among Koreans, and the pathologists's daily diagnostic competency on gastric cancer at any hospital setting plays a critical implication not only in the quality of clinical service but also to the determination of patient's prognosis. Thus, adoption of a unified assessment system based on comprehensive understanding of pathologic features together with their active participation has been crucially demanded. Nevertheless, a considerable difference in handling procedures and diagnostic approach on gastric cancer among institutions apparently resulted in an extreme difficulty in exchange of clinicopathologic informations and in the nationwide survey. It is, therefore, essential and be the first step to develop a practical but scientific and reproducible classification of gastric cancer with its diverse gross and histologic findings. Based on the following basic principles, the Subcommittee on Gastric Cancer under the Gastrointestinal Study Group of the Korean Society of Pathologists has been requested to develop the guidelines of future pathologic study of gastric cancer to meet the above needs and be efficiently used with ease among the society members. 1) The prerequisite for pathologic classification of gastric cancer starts with consistency in handling of the resected stomach before its further examination. Thus, the guideline shall limits its scope only with the minimum agreement. 2) The classification should be simple and practical so that all pathologists can use with ease and with high reproducibility. 3) All the gross and microscopic findings which have been considered to be the prognosis-related factors should be included in every pathologic procedures to help future information exchange among pathologists and clinicians and to provide a meaningful role in determination of patient's prognosis. 4) The classification should be interchangeable and stand with compatibility among WHO and other internationally accepted classifications. 5) The guideline accepts in part the staging system of American Joint Commitee on Cancer, classification of early gastric cancer proposed by the Japanese Society of Gastrointestinal Endoscopy, and the General Rules of Stomach Cancer Study by Japanese Research Society of Gastric Cancer, until otherwise developed and accepted by the Korean Society of Pathologists. 6) The guideline should not interfere with each institution-based special study. The details of the handling procedures of the resected stomach cancer, its gross and histologic classifications and descriptive methods of prognostic factors are supplemented with illustrations.

 E-submission
E-submission

 First
First Prev
Prev



