Search
- Page Path
- HOME > Search
- The evolving role of TRPS1 in dermatopathology: insights from the past 4 years
- Mokhtar H. Abdelhammed, Woo Cheal Cho
- Received September 11, 2025 Accepted November 25, 2025 Published online January 29, 2026
- DOI: https://doi.org/10.4132/jptm.2025.11.25 [Epub ahead of print]
- 597 View
- 34 Download
-
 Abstract
Abstract
 PDF
PDF - Over the past 4 years, trichorhinophalangeal syndrome type 1 (TRPS1) has rapidly gained attention among practicing pathologists, with numerous studies emerging that both support and question its diagnostic utility. Initially regarded as a highly specific marker for tumors of mammary origin, TRPS1 is now recognized to have broader expression patterns, including in a variety of cutaneous neoplasms. This is likely due to embryologic parallels between breast tissue and skin adnexal structures, an overlap that was underappreciated in early investigations. Although TRPS1 lacks absolute specificity—even among cutaneous neoplasms—it can still offer meaningful diagnostic value when interpreted alongside conventional immunohistochemical markers and within the appropriate morphologic context. Noteworthy diagnostic applications include mammary Paget disease, primary extramammary Paget disease, rare adnexal neoplasms such as endocrine mucin-producing sweat gland carcinoma and primary cutaneous NUT adnexal carcinoma, and cutaneous metastases from breast carcinoma. In this review, we present the most comprehensive and up-to-date evaluation of the utility and limitations of TRPS1 immunohistochemistry in dermatopathology. Our aim is to deepen understanding of this emerging marker and provide practical guidance on its optimal integration with established immunohistochemical panels to enhance diagnostic accuracy in routine practice.
- Mucocele of the rectal stump: mucinous cystic neoplasm with low-grade dysplasia simulating low-grade appendiceal mucinous neoplasm
- Hasan Basri Aydin, Maria Faraz, A. David Chismark, Haiyan Qiu, Hwajeong Lee
- J Pathol Transl Med. 2025;59(2):139-146. Published online February 26, 2025
- DOI: https://doi.org/10.4132/jptm.2024.12.27
- 2,811 View
- 171 Download
-
 Abstract
Abstract
 PDF
PDF - Mucoceles, commonly observed in the appendix, are mucin-filled, dilated structures arising from a range of etiologies. Cases associated with dysplastic or neoplastic epithelium can rupture and disseminate within the abdominopelvic cavity. Similar lesions in other parts of the colon are exceedingly rare, with only 16 colonic mucoceles having been reported. The first case of a colonic mucinous neoplasm with dysplasia resembling a low-grade appendiceal mucinous neoplasm involving rectal stump was described in 2016. Here, we present the second such case arising in the rectal stump, identified in a 44-year-old male with extensive surgical history. Microscopic examination revealed low-grade dysplastic epithelium lining the cyst and mucin dissecting into the stroma, without evidence of rupture or extramural mucin. The patient was followed for 16 months without recurrence or peritoneal disease. The exact etiology and outcome of these rare lesions remain unknown, requiring close follow-up.
- TRPS1 expression in non-melanocytic cutaneous neoplasms: an immunohistochemical analysis of 200 cases
- Yi A. Liu, Phyu P. Aung, Yunyi Wang, Jing Ning, Priyadharsini Nagarajan, Jonathan L. Curry, Carlos A. Torres-Cabala, Doina Ivan, Victor G. Prieto, Qingqing Ding, Woo Cheal Cho
- J Pathol Transl Med. 2024;58(2):72-80. Published online February 26, 2024
- DOI: https://doi.org/10.4132/jptm.2024.01.23
- 7,030 View
- 392 Download
- 13 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Although trichorhinophalangeal syndrome type 1 (TRPS1) was initially thought to be highly sensitive and specific for carcinomas and mesenchymal tumors of mammary origin, more recent data suggest its expression is not limited to breast neoplasms but also can be seen in other cutaneous neoplasms, such as extramammary Paget disease and squamous cell carcinoma (SCC) in situ.
Methods
Two-hundred cases of non-melanocytic cutaneous neoplasm, including basal cell carcinomas (BCCs) (n = 41), SCCs (n = 35), Merkel cell carcinomas (MCCs) (n = 25), and adnexal neoplasms (n = 99), were tested for TRPS1 expression using a monoclonal anti- TRPS1 rabbit anti-human antibody.
Results
TRPS1 expression was present in almost all cases of SCC (94%), with a median H-score of 200, while it was either absent or only focally present in most BCCs (90%), with a median H-score of 5. The difference between BCCs and SCCs in H-score was significant (p < .001). All MCCs (100%) lacked TRPS1 expression. TRPS1 expression was frequently seen in most adnexal neoplasms, benign and malignant, in variable intensity and proportion but was consistently absent in apocrine carcinomas. All endocrine mucin-producing sweat gland carcinomas (EMPSGCs) (100%, 6/6) showed diffuse and strong TRPS1 immunoreactivity, with a median H-score of 300, which was significantly different (p < .001) than that of BCCs.
Conclusions
Our study shows that TRPS1 may be an effective discriminatory marker for BCCs and SCCs. It also has a role in distinguishing BCCs from EMPSGCs. -
Citations
Citations to this article as recorded by- Metastatic Vulvar Paget's Disease Presenting in a Supraclavicular Lymph Node: A Diagnostic Challenge on Fine Needle Aspiration Cytology
Thiri Htoo Aung, Neha Seth, Anam Khan, Kasturi Das
Diagnostic Cytopathology.2026;[Epub] CrossRef - Trichorhinophalangeal syndrome type 1 (TRPS1) in breast pathology: diagnostic utility and pitfalls
Atif Ali Hashmi, Edi Brogi, Hannah Y. Wen
Diagnostic Pathology.2025;[Epub] CrossRef - Refining NTRK Fusion Detection in Papillary Thyroid Carcinoma Through Pan-TRK Immunohistochemistry and Histopathologic Features
Hyun Lee, Sue Youn Kim, Ji Min Park, Seung-Hyun Jung, Ozgur Mete, Chan Kwon Jung
Endocrine Pathology.2025;[Epub] CrossRef - Endocrine mucin-producing sweat gland carcinoma: Case report and literature review
Nan Guo, Zhenlin Fan, Yitong Chen, Qian Li, Limin Guo
European Journal of Ophthalmology.2025;[Epub] CrossRef - Updates on utility of immunohistochemistry in diagnosis of metastatic breast cancer
Hongxia Sun, Aysegul A. Sahin, Qingqing Ding
Human Pathology.2025; 162: 105821. CrossRef - Primary Cutaneous NUT Adnexal Carcinoma With BRD4::NUTM1 Fusion: A 19-Year Follow-Up
Elsayed Ibrahim, Richard K. Yang, Maria A. Gubbiotti, Victor G. Prieto, Woo Cheal Cho
The American Journal of Dermatopathology.2025; 47(9): 731. CrossRef - Primary mucinous carcinoma of the skin with co-expression of TRPS1 and GATA3: a case report
Liling Song, Ning Zhu, Lei Jiang, Dong Gao, Guohua Yu
Frontiers in Oncology.2025;[Epub] CrossRef - Diagnostic Algorithm for Secondary Extramammary Paget Disease from Institutional Cases and Literature Review
Salin Kiratikanon, Ayaka Fukui, Masahiro Hirata, Jakob M. T. Moran, Masakazu Fujimoto, Mai P. Hoang
Cancers.2025; 17(24): 4014. CrossRef - TRPS1 Expression Is Frequently Seen in a Subset of Cutaneous Mesenchymal Neoplasms and Tumors of Uncertain Differentiation: A Potential Diagnostic Pitfall
Moon Joo Kim, Yi A. Liu, Yunyi Wang, Jing Ning, Woo Cheal Cho
Dermatopathology.2024; 11(3): 200. CrossRef - TRPS1 expression in MPNST is correlated with PRC2 inactivation and loss of H3K27me3
Rossana Lazcano, Davis R. Ingram, Gauri Panse, Alexander J. Lazar, Wei-Lien Wang, Jeffrey M. Cloutier
Human Pathology.2024; 151: 105632. CrossRef - Syringocystadenoma Papilliferum-Like Features in Poroma: An Unusual Morphologic Pattern of Poroma or True Synchronous Occurrence of 2 Distinct Neoplasms?
Mouaz Alsawas, Fiorinda F. Muhaj, Phyu P. Aung, Priyadharsini Nagarajan, Woo Cheal Cho
The American Journal of Dermatopathology.2024; 46(12): 871. CrossRef - A Comprehensive Review of TRPS1 as a Diagnostic Immunohistochemical Marker for Primary Breast Carcinoma: Latest Insights and Diagnostic Pitfalls
Antonia-Carmen Georgescu, Tiberiu-Augustin Georgescu, Simona-Alina Duca-Barbu, Lucian Gheorghe Pop, Daniela Oana Toader, Nicolae Suciu, Dragos Cretoiu
Cancers.2024; 16(21): 3568. CrossRef - Expression of TRPS1 in Metastatic Tumors of the Skin: An Immunohistochemical Study of 72 Cases
Kassiani Boulogeorgou, Christos Topalidis, Triantafyllia Koletsa, Georgia Karayannopoulou, Jean Kanitakis
Dermatopathology.2024; 11(4): 293. CrossRef
- Metastatic Vulvar Paget's Disease Presenting in a Supraclavicular Lymph Node: A Diagnostic Challenge on Fine Needle Aspiration Cytology
- Standardization of the pathologic diagnosis of appendiceal mucinous neoplasms
- Dong-Wook Kang, Baek-hui Kim, Joon Mee Kim, Jihun Kim, Hee Jin Chang, Mee Soo Chang, Jin-Hee Sohn, Mee-Yon Cho, So-Young Jin, Hee Kyung Chang, Hye Seung Han, Jung Yeon Kim, Hee Sung Kim, Do Youn Park, Ha Young Park, So Jeong Lee, Wonae Lee, Hye Seung Lee, Yoo Na Kang, Younghee Choi
- J Pathol Transl Med. 2021;55(4):247-264. Published online July 8, 2021
- DOI: https://doi.org/10.4132/jptm.2021.05.28
- 20,938 View
- 1,114 Download
- 18 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Although the understanding of appendiceal mucinous neoplasms (AMNs) and their relationship with disseminated peritoneal mucinous disease have advanced, the diagnosis, classification, and treatment of AMNs are still confusing for pathologists and clinicians. The Gastrointestinal Pathology Study Group of the Korean Society of Pathologists (GPSG-KSP) proposed a multicenter study and held a workshop for the “Standardization of the Pathologic Diagnosis of the Appendiceal Mucinous Neoplasm” to overcome the controversy and potential conflicts. The present article is focused on the diagnostic criteria, terminologies, tumor grading, pathologic staging, biologic behavior, treatment, and prognosis of AMNs and disseminated peritoneal mucinous disease. In addition, GPSG-KSP proposes a checklist of standard data elements of appendiceal epithelial neoplasms to standardize pathologic diagnosis. We hope the present article will provide pathologists with updated knowledge on how to handle and diagnose AMNs and disseminated peritoneal mucinous disease.
-
Citations
Citations to this article as recorded by- Intrasplenic metastasis of appendiceal low-grade mucinous neoplasm – A case report and review of the literature
P. Meister, J. Rawitzer, M. Reschke, H.A. Baba, U. Neumann, M. Kaths
Current Problems in Cancer: Case Reports.2025; 18: 100364. CrossRef - Complete laparoscopic resection of giant appendiceal mucinous neoplasm, case report, and literature review
Shatha Awad Althobaiti, Rayan Z. Makeen, Abrar J. Filfilan, Ahmed Abdulaziz Hawsawi
Saudi Surgical Journal.2025; 13(1): 35. CrossRef - Survival Outcomes and Prognostic Factors in Metastatic Unresectable Appendiceal Adenocarcinoma Treated with Palliative Systemic Chemotherapy: A 10-Year Retrospective Analysis from Australia
Jirapat Wonglhow, Hui-Li Wong, Michael Michael, Alexander Heriot, Glen Guerra, Catherine Mitchell, Jeanne Tie
Cancers.2025; 17(20): 3297. CrossRef - Lower Gastrointestinal Bleeding Secondary to Appendiceal Mucinous Neoplasm: A Report of Two Cases and a Review of the Literature
Jesús Omar Soto Llanes, Samanta Kin Dosal Limón, Ana Jimena Iberri Jaime, Mario Zambrano Lara, Billy Jiménez Bobadilla
Cureus.2024;[Epub] CrossRef - Predicting Survival in Mucinous Adenocarcinoma of the Appendix: Demographics, Disease Presentation, and Treatment Methodology
Paul H. McClelland, Stephanie N. Gregory, Shirley K. Nah, Jonathan M. Hernandez, Jeremy L. Davis, Andrew M. Blakely
Annals of Surgical Oncology.2024; 31(9): 6237. CrossRef - Histoséminaire biopsies péritonéales tumorales. Néoplasies mucineuses appendiculaires
Peggy Dartigues
Annales de Pathologie.2024; 44(4): 274. CrossRef - Histoséminaire biopsies péritonéales tumorales. Cas no 2
Peggy Dartigues
Annales de Pathologie.2024; 44(4): 245. CrossRef - A Case of Low-Grade Appendiceal Mucinous Neoplasm: The Role of Preoperative Imaging and Surgical Technique in Achieving Favorable Outcomes
Daniel A Meza-Martinez, Yeudiel Suro Santos, Samantha J Andrade-Ordoñez, Julio A Palomino-Payan, Brando J Fematt-Rodriguez
Cureus.2024;[Epub] CrossRef - Incidental Appendiceal Mucinous Neoplasm Found During Appendectomy in a 15-Year-Old Patient: A Case Report
Fernando Aguilar-Ruiz, Kevin Joseph Fuentes-Calvo, Sara Fernanda Arechavala-Lopez, Irving Fuentes-Calvo, Luis F Arias-Ruiz
Cureus.2024;[Epub] CrossRef - Uncovering the Hidden Threat: Ileocolic Intussusception in an Adult With Appendicular Tumor
Mrunal Panchal, Shishir Kumar, Khushboo Jha, Kaushik Saha, Abhijit Kundu
Cureus.2024;[Epub] CrossRef - Low-Grade Appendiceal Mucinous Neoplasm vs. Appendiceal Diverticulum: Distinction with Histomorphologic Features
Cevriye Cansiz Ersöz, Siyar Ersöz, Berna Savas, Arzu Ensari
Gastrointestinal Disorders.2024; 6(4): 905. CrossRef - Appendiceal perforation secondary to endometriosis with intestinal metaplasia: A case report
Minghua Wang, Jing Liu, Boxin Hu, Simin Wang, Ping Xie, Ping Li
Experimental and Therapeutic Medicine.2023;[Epub] CrossRef - Primary and secondary tumors of the peritoneum: key imaging features and differential diagnosis with surgical and pathological correlation
Javier Miguez González, Francesc Calaf Forn, Laura Pelegrí Martínez, Pilar Lozano Arranz, Rafael Oliveira Caiafa, Jordi Català Forteza, Lina Maria Palacio Arteaga, Ferrán Losa Gaspà, Isabel Ramos Bernadó, Pedro Barrios Sánchez, Juan Ramón Ayuso Colella
Insights into Imaging.2023;[Epub] CrossRef - Muzinöse Tumoren des Peritoneums
Anne Kristin Fischer, Andrea Tannapfel, Alexander Quaas
Die Chirurgie.2023; 94(10): 823. CrossRef - Landscape of Genetic Mutations in Appendiceal Cancers
Marian Constantin, Cristina Mătanie, Livia Petrescu, Alexandra Bolocan, Octavian Andronic, Coralia Bleotu, Mihaela Magdalena Mitache, Sorin Tudorache, Corneliu Ovidiu Vrancianu
Cancers.2023; 15(14): 3591. CrossRef - Delivery of an Incidental Appendiceal Mucinous Neoplasm
Madison Bowles, Jessica Y Ng, Hajir Nabi
Cureus.2022;[Epub] CrossRef - Unearthing novel fusions as therapeutic targets in solid tumors using targeted RNA sequencing
Sungbin An, Hyun Hee Koh, Eun Sol Chang, Juyoung Choi, Ji-Young Song, Mi-Sook Lee, Yoon-La Choi
Frontiers in Oncology.2022;[Epub] CrossRef
- Intrasplenic metastasis of appendiceal low-grade mucinous neoplasm – A case report and review of the literature
- Clinicopathologic characteristics of HER2-positive pure mucinous carcinoma of the breast
- Yunjeong Jang, Hera Jung, Han-Na Kim, Youjeong Seo, Emad Alsharif, Seok Jin Nam, Seok Won Kim, Jeong Eon Lee, Yeon Hee Park, Eun Yoon Cho, Soo Youn Cho
- J Pathol Transl Med. 2020;54(1):95-102. Published online November 13, 2019
- DOI: https://doi.org/10.4132/jptm.2019.10.24
- 10,730 View
- 293 Download
- 22 Web of Science
- 19 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Pure mucinous carcinoma (PMC) is a rare type of breast cancer, estimated to represent 2% of invasive breast cancer. PMC is typically positive for estrogen receptors (ER) and progesterone receptors (PR) and negative for human epidermal growth factor receptor 2 (HER2). The clinicopathologic characteristics of HER2-positive PMC have not been investigated.
Methods
Pathology archives were searched for PMC diagnosed from January 1999 to April 2018. Clinicopathologic data and microscopic findings were reviewed and compared between HER2-positive PMC and HER2-negative PMC. We also analyzed the differences in disease-free survival (DFS) and overall survival according to clinicopathologic parameters including HER2 status in overall PMC cases.
Results
There were 21 HER2-positive cases (4.8%) in 438 PMCs. The average tumor size of HER2-positive PMC was 32.21 mm (± 26.55). Lymph node metastasis was present in seven cases. Compared to HER2-negative PMC, HER2-positive PMC presented with a more advanced T category (p < .001), more frequent lymph node metastasis (p = .009), and a higher nuclear and histologic grade (p < .001). Microscopically, signet ring cells were frequently observed in HER2-positive PMC (p < .001), whereas a micropapillary pattern was more frequent in HER2-negative PMC (p = .012). HER2-positive PMC was more frequently negative for ER (33.3% vs. 1.2%) and PR (28.6% vs. 7.2%) than HER2-negative PMC and showed a high Ki-67 labeling index. During follow-up, distant metastasis and recurrence developed in three HER2-positive PMC patients. Multivariate analysis revealed that only HER2-positivity and lymph node status were significantly associated with DFS.
Conclusions
Our results suggest that HER2-positive PMC is a more aggressive subgroup of PMC. HER2 positivity should be considered for adequate management of PMC. -
Citations
Citations to this article as recorded by- Mucin‐producing breast lesions: a practical approach to diagnosis
Sunayana Misra, Mihir Gudi, Kimberly H Allison, Edi Brogi, Cecily Quinn, Hannah Y Wen, Puay Hoon Tan
Histopathology.2026;[Epub] CrossRef - Clinicopathological characteristics of mucinous breast cancer: a retrospective analysis of a 6-years study from national cancer center in Vietnam
Thi Huyen Phung, Thanh Tung Pham, Huu Thang Nguyen, Dinh Thach Nguyen, Thanh Long Nguyen, Thi Hoai Hoang
Breast Cancer Research and Treatment.2025; 209(3): 667. CrossRef - Poor response of HER2-positive mucinous carcinomas of breast to neoadjuvant HER2-targeted therapy: A study of four cases
Min Han, Daniel Schmolze, Javier A. Arias-Stella, Christina H. Wei, Joanne Mortimer, Fang Fan
Annals of Diagnostic Pathology.2025; 74: 152396. CrossRef - Comprehensive Immunohistochemical Analysis of Mesonephric Marker Expression in Low-grade Endometrial Endometrioid Carcinoma
Yurimi Lee, Sangjoon Choi, Hyun-Soo Kim
International Journal of Gynecological Pathology.2024; 43(3): 221. CrossRef - Clinicopathological features and prognosis of mucinous breast carcinoma with a micropapillary structure
Beibei Yang, Menglu Shen, Bo Sun, Jing Zhao, Meng Wang
Thoracic Cancer.2024; 15(36): 2530. CrossRef - Pure Mucinous Carcinoma of the Breast: Radiologic-Pathologic Correlation
Cherie M Kuzmiak, Benjamin C Calhoun
Journal of Breast Imaging.2023;[Epub] CrossRef - Role of circ-FOXO3 and miR-23a in radiosensitivity of breast cancer
Elahe Abdollahi, Hossein Mozdarani, Behrooz Z. Alizadeh
Breast Cancer.2023; 30(5): 714. CrossRef - On Ultrasonographic Features of Mucinous Carcinoma with Micropapillary Pattern
Wei-Sen Yang, Yang Li, Ya Gao
Breast Cancer: Targets and Therapy.2023; Volume 15: 473. CrossRef - Spectrum of Mucin-containing Lesions of the Breast: Multimodality Imaging Review with Pathologic Correlation
Janice N. Thai, Melinda F. Lerwill, Shinn-Huey S. Chou
RadioGraphics.2023;[Epub] CrossRef - Mesonephric-like Adenocarcinoma of the Ovary: Clinicopathological and Molecular Characteristics
Hyun Hee Koh, Eunhyang Park, Hyun-Soo Kim
Diagnostics.2022; 12(2): 326. CrossRef - Alveolar Soft Part Sarcoma of the Uterus: Clinicopathological and Molecular Characteristics
Yurimi Lee, Kiyong Na, Ha Young Woo, Hyun-Soo Kim
Diagnostics.2022; 12(5): 1102. CrossRef - Metastasis of the Mucionous adenocarcinoma of breast to the mandibular gingiva: Rare case report
Ivana Mijatov, Aleksandra Fejsa Levakov, Aleksandar Spasić, Jelena Nikolić, Saša Mijatov
Medicine.2022; 101(38): e30732. CrossRef - Endometrioid Carcinomas of the Ovaries and Endometrium Involving Endocervical Polyps: Comprehensive Clinicopathological Analyses
Jihee Sohn, Yurimi Lee, Hyun-Soo Kim
Diagnostics.2022; 12(10): 2339. CrossRef - Serous Carcinoma of the Endometrium with Mesonephric-Like Differentiation Initially Misdiagnosed as Uterine Mesonephric-Like Adenocarcinoma: A Case Report with Emphasis on the Immunostaining and the Identification of Splice Site TP53 Mutation
Sangjoon Choi, Yoon Yang Jung, Hyun-Soo Kim
Diagnostics.2021; 11(4): 717. CrossRef - HER2 positive mucinous carcinoma of breast with micropapillary features: Report of a case and review of literature
Dinesh Chandra Doval, Rupal Tripathi, Sunil Pasricha, Pankaj Goyal, Chaturbhuj Agrawal, Anurag Mehta
Human Pathology: Case Reports.2021; 25: 200531. CrossRef - Carcinoma mucosecretor de mama HER2-positivo, un caso clínico
A.M. González Aranda, E. Martínez Gómez, A. Santana Costa, F. Arnanz Velasco, M.H. González de Diego, A. Zapico Goñi
Clínica e Investigación en Ginecología y Obstetricia.2021; 48(4): 100685. CrossRef - Clinicopathologic features of unexpectedly HER2 positive breast carcinomas: An institutional experience
Carissa LaBoy, Kalliopi P. Siziopikou, Lauren Rosen, Luis Z. Blanco, Jennifer L. Pincus
Pathology - Research and Practice.2021; 222: 153441. CrossRef - Mesonephric-like Differentiation of Endometrial Endometrioid Carcinoma: Clinicopathological and Molecular Characteristics Distinct from Those of Uterine Mesonephric-like Adenocarcinoma
Sujin Park, Go Eun Bae, Jiyoung Kim, Hyun-Soo Kim
Diagnostics.2021; 11(8): 1450. CrossRef - Mesonephric-like Adenocarcinoma of the Uterine Corpus: Comprehensive Immunohistochemical Analyses Using Markers for Mesonephric, Endometrioid and Serous Tumors
Hyunjin Kim, Kiyong Na, Go Eun Bae, Hyun-Soo Kim
Diagnostics.2021; 11(11): 2042. CrossRef
- Mucin‐producing breast lesions: a practical approach to diagnosis
- Coexisting Mucinous Cystic Neoplasm of the Pancreas and Type 1 Autoimmune Pancreatitis
- Mee-Jeong Kim, Tae Jun Song, Hyoung Jung Kim, Song-Cheol Kim, Myung-Hwan Kim, Seung-Mo Hong
- J Pathol Transl Med. 2019;53(2):125-128. Published online November 14, 2018
- DOI: https://doi.org/10.4132/jptm.2018.10.25
- 10,257 View
- 122 Download
- 3 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - Type 1 autoimmune pancreatitis (AIP1) is an IgG4-related systemic disease that mimics tumors. We report a rare case of AIP1 accompanied by mucinous cystic neoplasm (MCN). A pancreatic lesion was incidentally detected in a woman in her 60s. After 6 years of follow-up, the lesion abruptly increased in size. Computed tomography showed a 3.5 cm unilocular cyst in the tail of the pancreas and distal pancreatectomy was performed. On microscopic examination, the cyst was lined by mucinous and non-mucinous epithelial cells with mild cytologic atypia. The surrounding stroma comprised ovarian-type spindle cells with progesterone receptor positivity. The pericystic pancreas exhibited multifocal lymphoid follicles, lymphoplasmacytic infiltrations, obliterative phlebitis, and storiform fibrosis. IgG4-positive plasma cell infiltration (215 cells high-power field) and the IgG4/IgG ratio (57%) were increased. Cases of MCN coexisting with AIP1 are extremely rare; only two such cases have been reported in the English-language literature. This third case featured low-grade MCN with AIP1.
-
Citations
Citations to this article as recorded by- Utilizing Immunoglobulin G4 Immunohistochemistry for Risk Stratification in Patients with Papillary Thyroid Carcinoma Associated with Hashimoto Thyroiditis
Faridul Haq, Gyeongsin Park, Sora Jeon, Mitsuyoshi Hirokawa, Chan Kwon Jung
Endocrinology and Metabolism.2024; 39(3): 468. CrossRef - Histological features of autoimmune pancreatitis and IgG4-related sclerosing cholangitis with a correlation with imaging findings
Kenji NOTOHARA
Choonpa Igaku.2023; 50(1): 55. CrossRef - Imaging Features and Risk Factors of Pancreatic Cystic Lesions Complicating
Autoimmune Pancreatitis: A Retrospective Study
Bin-Bin Zhang, Xin-Meng Hou, Yu-Qi Chen, Jian-Wei Huo, Er-Hu Jin
Current Medical Imaging Reviews.2023;[Epub] CrossRef - Histological features of autoimmune pancreatitis and IgG4-related sclerosing cholangitis with a correlation with imaging findings
Kenji Notohara
Journal of Medical Ultrasonics.2021; 48(4): 581. CrossRef - 自己免疫性膵炎診療ガイドライン2020
Suizo.2020; 35(6): 465. CrossRef - Mucinous cystic neoplasm of the pancreas with type-1 autoimmune pancreatitis-like lesion
Kevin Gowing, David F. Schaeffer, Hui-Min Yang
Human Pathology: Case Reports.2019; 18: 200339. CrossRef
- Utilizing Immunoglobulin G4 Immunohistochemistry for Risk Stratification in Patients with Papillary Thyroid Carcinoma Associated with Hashimoto Thyroiditis
- Primary Cutaneous Mucinous Carcinoma with Extramammary Paget’s Disease: Eccrine or Apocrine?
- Sun-Ju Oh, Young-Ok Kim
- J Pathol Transl Med. 2018;52(4):238-242. Published online January 25, 2018
- DOI: https://doi.org/10.4132/jptm.2017.11.21
- 9,790 View
- 146 Download
- 3 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - Primary cutaneous mucinous carcinoma (PCMC) is an uncommon tumor of the sweat gland origin. The occurrence of PCMC is mostly in middle-aged and older patients, with a slight male predominance. Most cases of PCMC arise on the head, with a preference for eyelids. The histogenesis of PCMC, whether eccrine or apocrine, remains controversial. We report a rare case of PCMC with secondary extramammary Paget’s disease in the groin of a 75-year-old man, which favored an apocrine origin. Furthermore, based on a review of the literature, we provide several histologic clues that can be used to differentiate PCMC from metastatic mucinous carcinoma.
-
Citations
Citations to this article as recorded by- Primary cutaneous mucinous carcinoma of the scalp masquerading as a benign dermatological mass – A case report
Fadi Alnehlaoui, Nafad Mohamed Lotfy Elhadidi, Shafik Fwakhrji, Shekhar V. Shikare, Majid Hassan Alhammadi, Salman Yousuf Guraya
International Journal of Surgery Case Reports.2024; 114: 109175. CrossRef - Primary cutaneous mucinous carcinoma in a periorbital lesion: two case reports and literature review
Jun Woo Kim, Sung Eun Kim
Archives of Craniofacial Surgery.2024; 25(2): 90. CrossRef - Primary Cutaneous Mucinous Carcinoma: A Review of the Literature
Timothy Freeman, Aaron J. Russell, M. Laurin Council
Dermatologic Surgery.2023; 49(12): 1091. CrossRef - A Case of Eccrine Mucinous Carcinoma Involving Scalp
Ramsha Saleem, Sachin Vaidya
Cureus.2021;[Epub] CrossRef
- Primary cutaneous mucinous carcinoma of the scalp masquerading as a benign dermatological mass – A case report
- Prognostic Significance of a Micropapillary Pattern in Pure Mucinous Carcinoma of the Breast: Comparative Analysis with Micropapillary Carcinoma
- Hyun-Jung Kim, Kyeongmee Park, Jung Yeon Kim, Guhyun Kang, Geumhee Gwak, Inseok Park
- J Pathol Transl Med. 2017;51(4):403-409. Published online June 9, 2017
- DOI: https://doi.org/10.4132/jptm.2017.03.18
- 9,289 View
- 201 Download
- 17 Web of Science
- 18 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Mucinous carcinoma of the breast is an indolent tumors with a favorable prognosis; however, micropapillary features tend to lead to aggressive behavior. Thus, mucinous carcinoma and micropapillary carcinoma exhibit contrasting biologic behaviors. Here, we review invasive mucinous carcinoma with a focus on micropapillary features and correlations with clinicopathological factors.
Methods
A total of 64 patients with invasive breast cancer with mucinous or micropapillary features were enrolled in the study. Of 36 pure mucinous carcinomas, 17 (47.2%) had micropapillary features and were termed mucinous carcinoma with micropapillary features (MUMPC), and 19 (52.8%) had no micropapillary features and were termed mucinous carcinoma without micropapillary features. MUMPC were compared with 15 invasive micropapillary carcinomas (IMPC) and 13 invasive ductal and micropapillary carcinomas (IDMPC).
Results
The clinicopathological factors of pure mucinous carcinoma and MUMPC were not significantly different. In contrast to IMPC and IDMPC, MUMPC had a low nuclear grade, lower mitotic rate, higher expression of hormone receptors, negative human epidermal growth factor receptor 2 (HER2) status, lower Ki-67 proliferating index, and less frequent lymph node metastasis (p < .05). According to univariate analyses, progesterone receptor, HER2, T-stage, and lymph node metastasis were significant risk factors for overall survival; however, only T-stage remained significant in a multivariate analysis (p < .05).
Conclusions
In contrast to IMPC and IDMPC, the micropapillary pattern in mucinous carcinoma does not contribute to aggressive behavior. However, further analysis of a larger series of patients is required to clarify the prognostic significance of micropapillary patterns in mucinous carcinoma of the breast. -
Citations
Citations to this article as recorded by- Pure mucinous adenocarcinoma of the breast with the rare lymphoplasmacytic infiltration: A case report with review of literature
Yash Hasmukhbhai Prajapati, Vishal Bhabhor, Kahan Samirkumar Mehta, Mithoon Barot, Husen Boriwala, Mohamed Omar
Clinical Case Reports.2024;[Epub] CrossRef - Clinicopathological features and prognosis of mucinous breast carcinoma with a micropapillary structure
Beibei Yang, Menglu Shen, Bo Sun, Jing Zhao, Meng Wang
Thoracic Cancer.2024; 15(36): 2530. CrossRef - Expression of autocrine motility factor receptor (AMFR) in human breast and lung invasive micropapillary carcinomas
Jing Xu, Hongfei Ma, Qi Wang, Hui Zhang
International Journal of Experimental Pathology.2023; 104(1): 43. CrossRef - The Spectrum of Mucinous Lesions of the Breast
Upasana Joneja, Juan Palazzo
Archives of Pathology & Laboratory Medicine.2023; 147(1): 19. CrossRef - Pure Mucinous Carcinoma of the Breast: Radiologic-Pathologic Correlation
Cherie M Kuzmiak, Benjamin C Calhoun
Journal of Breast Imaging.2023; 5(2): 180. CrossRef - On Ultrasonographic Features of Mucinous Carcinoma with Micropapillary Pattern
Wei-Sen Yang, Yang Li, Ya Gao
Breast Cancer: Targets and Therapy.2023; Volume 15: 473. CrossRef - Micropapillary Breast Carcinoma: From Molecular Pathogenesis to Prognosis
Georgios-Ioannis Verras, Levan Tchabashvili, Francesk Mulita, Ioanna Maria Grypari, Sofia Sourouni, Evangelia Panagodimou, Maria-Ioanna Argentou
Breast Cancer: Targets and Therapy.2022; Volume 14: 41. CrossRef - Mucinous carcinoma of the breast: distinctive histopathologic and genetic characteristics
Minjung Jung
Kosin Medical Journal.2022; 37(3): 176. CrossRef - Triple-Positive Breast Carcinoma: Histopathologic Features and Response to Neoadjuvant Chemotherapy
Jennifer Zeng, Marcia Edelweiss, Dara S. Ross, Bin Xu, Tracy-Ann Moo, Edi Brogi, Timothy M. D'Alfonso
Archives of Pathology & Laboratory Medicine.2021; 145(6): 728. CrossRef - HER2 positive mucinous carcinoma of breast with micropapillary features: Report of a case and review of literature
Dinesh Chandra Doval, Rupal Tripathi, Sunil Pasricha, Pankaj Goyal, Chaturbhuj Agrawal, Anurag Mehta
Human Pathology: Case Reports.2021; 25: 200531. CrossRef - Sonographic Features of Pure Mucinous Breast Carcinoma With Micropapillary Pattern
Wu Zhou, Yong-Zhong Li, Li-Min Gao, Di-Ming Cai
Frontiers in Oncology.2021;[Epub] CrossRef - Clinicopathologic characteristics of HER2-positive pure mucinous carcinoma of the breast
Yunjeong Jang, Hera Jung, Han-Na Kim, Youjeong Seo, Emad Alsharif, Seok Jin Nam, Seok Won Kim, Jeong Eon Lee, Yeon Hee Park, Eun Yoon Cho, Soo Youn Cho
Journal of Pathology and Translational Medicine.2020; 54(1): 95. CrossRef - Mucinous carcinoma with micropapillary features is morphologically, clinically and genetically distinct from pure mucinous carcinoma of breast
Peng Sun, Zaixuan Zhong, Qianyi Lu, Mei Li, Xue Chao, Dan Chen, Wenyan Hu, Rongzhen Luo, Jiehua He
Modern Pathology.2020; 33(10): 1945. CrossRef - Micropapillary pattern in pure mucinous carcinoma of the breast – does it matter or not?
Xiaoli Xu, Rui Bi, Ruohong Shui, Baohua Yu, Yufan Cheng, Xiaoyu Tu, Wentao Yang
Histopathology.2019; 74(2): 248. CrossRef - An Update of Mucinous Lesions of the Breast
Beth T. Harrison, Deborah A. Dillon
Surgical Pathology Clinics.2018; 11(1): 61. CrossRef - The clinicopathological significance of micropapillary pattern in colorectal cancers
Jung-Soo Pyo, Mee Ja Park, Dong-Wook Kang
Human Pathology.2018; 77: 159. CrossRef - The sonographic findings of micropapillary pattern in pure mucinous carcinoma of the breast
Heqing Zhang, Li Qiu, Yulan Peng
World Journal of Surgical Oncology.2018;[Epub] CrossRef - Diagnostic dilemma of micropapillary variant of mucinous breast cancer
Geok Hoon Lim, Zhiyan Yan, Mihir Gudi
BMJ Case Reports.2018; 2018: bcr-2018-225775. CrossRef
- Pure mucinous adenocarcinoma of the breast with the rare lymphoplasmacytic infiltration: A case report with review of literature
- Mucinous Cystadenoma of the Testis: A Case Report with Immunohistochemical Findings
- Gilhyang Kim, Dohee Kwon, Hee Young Na, Sehui Kim, Kyung Chul Moon
- J Pathol Transl Med. 2017;51(2):180-184. Published online February 13, 2017
- DOI: https://doi.org/10.4132/jptm.2016.08.30
- 10,631 View
- 126 Download
- 5 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF - Mucinous cystadenoma of the testis is a very rare tumor. Herein, we report a case of mucinous cystadenoma arising in the testis of a 61-year-old man, along with a literature review. Computed tomography showed a 2.5-cm-sized poorly enhancing cystic mass. Grossly, the tumor was a unilocular cystic mass filled with mucinous material and confined to the testicular parenchyma. Histologically, the cyst had a fibrotic wall lined by mucinous columnar epithelium without atypia. Immunohistochemical staining was positive for cytokeratin 20 and CDX2, as well as focally positive for cytokeratin 7. The pathologic diagnosis was mucinous cystadenoma.
-
Citations
Citations to this article as recorded by- Review of Paratesticular Appendageal Tumors, Morphology, Immunohistochemistry, and Recent Molecular Advances
Mathew Vega, Muhammad T. Idrees
Surgical Pathology Clinics.2025; 18(1): 119. CrossRef - Cistoadenoma Mucinoso Paratesticular: Caso Interesante en el Instituto Guatemalteco de Seguridad Social
Edgar Estuardo González López, Carlos Gonzalo Estrada Pazos
Revista Guatemalteca de Urología.2023; 10(2): 16. CrossRef - Primary borderline mucinous tumor of the testis with postoperative metastasis: A rare case report
Yingyu Shi, Ling Song, Yan Luo
Radiology Case Reports.2023; 18(9): 3203. CrossRef - Case report: Misdiagnosis of primary mucinous cystadenoma of the testicle by ultrasound
Linlin Zhang, Jianyuan Xuan, Manxi Li, Mei Zhang, Yu Song, Ziang Pan, Bo Fan, Lin Lu, Hongyan Zhou, Yang Li
Frontiers in Oncology.2023;[Epub] CrossRef - Primary Borderline Mucinous Testicular Tumor: A Case Report and Literature Review
Changjuan Hao, Chunsong Kang, Xiaoyan Kang, Zhuanzhuan Yu, Tingting Li, Jiping Xue
Frontiers in Oncology.2021;[Epub] CrossRef - Ovarian-type Tumors (Mullerian Tumors) of the Testis: Clinicopathologic Findings with Recent Advances
Michelle S Lin, Alberto G Ayala, Jae Y Ro
annals of urologic oncology.2019; : 1. CrossRef - Borderline Mucinous Testicular Tumour: Diagnostic and Management difficulties
Krishan Pratap, Marlon Perera, Frances Malczewski, Rachel Esler
BMJ Case Reports.2018; 2018: bcr-2017-223787. CrossRef - Mucinous tumor arising in a giant sacrococcygeal teratoma
Fengtian Zhang, Xiaolong Yu, Jin Zeng, Min Dai
Medicine.2017; 96(47): e8759. CrossRef
- Review of Paratesticular Appendageal Tumors, Morphology, Immunohistochemistry, and Recent Molecular Advances
- Mucinous Carcinoma with Extensive Signet Ring Cell Differentiation: A Case Report
- Hye Min Kim, Eun Kyung Kim, Ja Seung Koo
- J Pathol Transl Med. 2017;51(2):176-179. Published online December 5, 2016
- DOI: https://doi.org/10.4132/jptm.2016.08.17
- 11,637 View
- 167 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - Breast cancers that present with mucin include mucinous carcinoma and carcinoma with signet ring cell differentiation. The former shows extracellular mucin and the latter shows abundant intracellular mucin. Here, we report a case of breast cancer showing both extracellular mucin and extensive signet ring cell differentiation due to abundant intracellular mucin. Unlike mucinous carcinoma, this case had the features of high-grade nuclear pleomorphism, high mitotic index, estrogen receptor negativity, progesterone receptor negativity, human epidermal growth factor receptor-2 positivity, and ductal type with positivity for E-cadherin. In a case with signet ring cell differentiation, differential diagnosis with metastatic signet ring cell carcinoma of the stomach and colon is essential. In this case, the presence of accompanied ductal carcinoma in situ component and mammaglobin and gross cystic disease fluid protein-15 positivity were findings that suggested the breast as the origin.
-
Citations
Citations to this article as recorded by- Signet ring cell carcinoma of the urachus: survival and pathologic outcomes from the national cancer database
Deerush Kannan Sakthivel, Pushan Prabhakar, Mohamed Javid Raja Iyub, Rohan Garje, Murugesan Manoharan
International Urology and Nephrology.2025;[Epub] CrossRef - Research on the Histological Features and Pathological Types of Gastric Adenocarcinoma With Mucinous Differentiation
Nian-Long Meng, Yang-kun Wang, Hai-Li Wang, Jun-Ling Zhou, Su-nan Wang
Frontiers in Medicine.2022;[Epub] CrossRef - Clinicopathologic characteristics of HER2-positive pure mucinous carcinoma of the breast
Yunjeong Jang, Hera Jung, Han-Na Kim, Youjeong Seo, Emad Alsharif, Seok Jin Nam, Seok Won Kim, Jeong Eon Lee, Yeon Hee Park, Eun Yoon Cho, Soo Youn Cho
Journal of Pathology and Translational Medicine.2020; 54(1): 95. CrossRef - Human Epidermal Growth Factor Receptor 2-positive Mucinous Carcinoma with Signet Ring Cell Differentiation, Which Showed Complete Response after Neoadjuvant Chemotherapy
Yunjeong Jang, Eun Yoon Cho, Soo Youn Cho
Journal of Breast Cancer.2019; 22(2): 336. CrossRef
- Signet ring cell carcinoma of the urachus: survival and pathologic outcomes from the national cancer database
- Size of Non-lepidic Invasive Pattern Predicts Recurrence in Pulmonary Mucinous Adenocarcinoma: Morphologic Analysis of 188 Resected Cases with Reappraisal of Invasion Criteria
- Soohyun Hwang, Joungho Han, Misun Choi, Myung-Ju Ahn, Yong Soo Choi
- J Pathol Transl Med. 2017;51(1):56-68. Published online October 16, 2016
- DOI: https://doi.org/10.4132/jptm.2016.09.17
- 12,319 View
- 237 Download
- 8 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
We reviewed a series of 188 resected pulmonary mucinous adenocarcinomas (MAs) to clarify the prognostic significance of lepidic and non-lepidic patterns.
Methods
Non-lepidic patterns were divided into bland, non-distorted acini with uncertain invasiveness (pattern 1), unequivocal invasion into stroma (pattern 2), or invasion into alveolar spaces (pattern 3).
Results
The mean proportion of invasive patterns (patterns 2 and 3) was lowest in small (≤ 3 cm) tumors, and gradually increased in intermediate (> 3 cm and ≤ 7 cm) and large (> 7 cm) tumors (8.4%, 34.3%, and 50.1%, respectively). Adjusted T (aT) stage, as determined by the size of invasive patterns, was positively correlated with adverse histologic and clinical features including older age, male sex, and ever smokers. aTis tumors, which were exclusively composed of lepidic pattern (n = 9), or a mixture of lepidic and pattern 1 (n = 40) without any invasive patterns, showed 100% disease- free survival (DFS). The aT1mi tumors, with minimal (≤ 5 mm) invasive patterns (n = 63), showed a 95.2% 5-year DFS, with recurrences (n = 2) limited to tumors greater than 3 cm in total size (n = 23). Both T and aT stage were significantly associated with DFS; however, survival within the separate T-stage subgroups was stratified according to the aT stage, most notably in the intermediatestage subgroups. In multivariate analysis, the size of invasive patterns (p = .020), pleural invasion (p < .001), and vascular invasion (p = .048) were independent predictors of recurrence, whereas total size failed to achieve statistical significance (p = .121).
Conclusions
This study provides a rationale for histologic risk stratification in pulmonary MA based on the extent of invasive growth patterns with refined criteria for invasion. -
Citations
Citations to this article as recorded by- Distinct Recurrence Pattern and Survival Outcomes of Invasive Mucinous Adenocarcinoma of the Lung: The Potential Role of Local Therapy in Intrapulmonary Spread
Dong Woog Yoon, Soohyun Hwang, Tae Hee Hong, Yoon-La Choi, Hong Kwan Kim, Yong Soo Choi, Jhingook Kim, Young Mog Shim, Jong Ho Cho
Annals of Surgical Oncology.2024; 31(1): 201. CrossRef - Pulmonary invasive mucinous adenocarcinoma
Wei‐Chin Chang, Yu Zhi Zhang, Andrew G Nicholson
Histopathology.2024; 84(1): 18. CrossRef - Micropapillary Pattern in Invasive Mucinous Adenocarcinoma of the Lung: Comparison With Invasive Non-Mucinous Adenocarcinoma
Hui He, Lue Li, Yuan-yuan Wen, Li-yong Qian, Zhi-qiang Yang
International Journal of Surgical Pathology.2024; 32(5): 926. CrossRef - Radiological and clinical features of screening-detected pulmonary invasive mucinous adenocarcinoma
Dae Hyeon Kim, So Young Bae, Kwon Joong Na, Samina Park, In Kyu Park, Chang Hyun Kang, Young Tae Kim
Interactive CardioVascular and Thoracic Surgery.2022; 34(2): 229. CrossRef - Micropapillary Pattern in Invasive Mucinous Adenocarcinoma of the Lung: Comparison with Invasive Non-Mucinous Adenocarcinoma
Hui He, Yuanyuan Wen, Liyong Qian, Zhiqiang Yang
SSRN Electronic Journal .2022;[Epub] CrossRef - Optimal method for measuring invasive size that predicts survival in invasive mucinous adenocarcinoma of the lung
Tomonari Oki, Keiju Aokage, Shogo Nomura, Kenta Tane, Tomohiro Miyoshi, Norihiko Shiiya, Kazuhito Funai, Masahiro Tsuboi, Genichiro Ishii
Journal of Cancer Research and Clinical Oncology.2020; 146(5): 1291. CrossRef - Prognostic Impact of Histopathologic Features in Pulmonary Invasive Mucinous Adenocarcinomas
Wei-Chin Chang, Yu Zhi Zhang, Eric Lim, Andrew G Nicholson
American Journal of Clinical Pathology.2020; 154(1): 88. CrossRef
- Distinct Recurrence Pattern and Survival Outcomes of Invasive Mucinous Adenocarcinoma of the Lung: The Potential Role of Local Therapy in Intrapulmonary Spread
- A Pyloric Gland-Phenotype Ovarian Mucinous Tumor Resembling Lobular Endocervical Glandular Hyperplasia in a Patient with Peutz-Jeghers Syndrome
- Eun Na Kim, Gu-Hwan Kim, Jiyoon Kim, In Ah Park, Jin Ho Shin, Yun Chai, Kyu-Rae Kim
- J Pathol Transl Med. 2017;51(2):159-164. Published online August 22, 2016
- DOI: https://doi.org/10.4132/jptm.2016.07.01
- 10,114 View
- 210 Download
- 10 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF - We describe an ovarian mucinous neoplasm that histologically resembles lobular endocervical glandular hyperplasia (LEGH) containing pyloric gland type mucin in a patient with Peutz-Jeghers syndrome (PJS). Although ovarian mucinous tumors rarely occur in PJS patients, their pyloric gland phenotype has not been clearly determined. The histopathologic features of the ovarian mucinous tumor were reminiscent of LEGH. The cytoplasmic mucin was stained with periodic acid-Schiff reaction after diastase treatment but was negative for Alcian blue pH 2.5, suggesting the presence of neutral mucin. Immunohistochemically, the epithelium expressed various gastric markers, including MUC6, HIK1083, and carbonic anhydrase-IX. Multiple ligation-dependent probe amplification detected a germline heterozygous deletion mutation at exons 1–7 of the STK11 gene (c.1-?_920+?del) in peripheral blood leukocytes and mosaic loss of heterozygosity in ovarian tumor tissue. Considering that LEGH and/or gastric-type cervical adenocarcinoma can be found in patients with PJS carrying germline and/or somatic STK11 mutations, our case indicates that STK11 mutations have an important role in the proliferation of pyloric-phenotype mucinous epithelium at various anatomical locations.
-
Citations
Citations to this article as recorded by- Molecular evidence of a clonal relationship of synchronous/multifocal gastric‐type lesions of the female genital tract
Min Shi, Hong Yang, Fang Zhang, Ting Hou, Huageng Huang, Yi Lu, Yehan Zhou, Ting Lan, Juan Ji, Jun Hou, Chengmin Zhou, Zhou Zhang, Sheng Qin, Zongyao Huang, Yang Liu
The Journal of Pathology.2026; 268(1): 27. CrossRef - Serine/threonine kinase 11 (STK11) associated adnexal tumors: from biology to therapeutic impact
Guanxiang Huang, Wenyu Lin, Tingting Jiang, Yuanjun Cai, Chengbin Lin, Pengming Sun
Human Genomics.2025;[Epub] CrossRef - Novel ultrasound features and diagnostic clues of gastric-type endocervical adenocarcinoma: a case series
Liwen Yang, Yangyang Wang, Jian Cai, Ying Xiong, Juan Li, Qi Zhou, Nan Ye, Hua Lai, Tianjiao Liu, Liuying Zhou
Frontiers in Oncology.2025;[Epub] CrossRef - Ovarian Mucinous Tumor Presenting Atypical Lobular Endocervical Glandular Hyperplasia-Like Appearance in a Patient With Germline STK11 p.F354L Variant: A Case Report
Hiroshi Yoshida, Kengo Hiranuma, Mariko Nakahara, Mayumi Kobayashi-Kato, Yasuhito Tanase, Masaya Uno, Kouya Shiraishi, Mitsuya Ishikawa, Tomoyasu Kato
International Journal of Surgical Pathology.2024; 32(2): 394. CrossRef - Preoperative multimodal ultrasonic imaging in a case of Peutz-Jeghers syndrome complicated by atypical lobular endocervical glandular hyperplasia: a case report and literature review
Liwen Yang, Duan Duan, Ying Xiong, Tianjiao Liu, Lijun Zhao, Fan Lai, Dingxian Gu, Liuying Zhou
Hereditary Cancer in Clinical Practice.2024;[Epub] CrossRef - Gastric‐type glandular lesions of the female genital tract excluding the cervix: emerging pathological entities
Richard W‐C Wong, Karen L Talia, W Glenn McCluggage
Histopathology.2024; 85(1): 20. CrossRef - Gastric-phenotype Mucinous Carcinoma of the Fallopian Tube with Secondary Ovarian Involvement in a Woman with Peutz-Jeghers Syndrome: A Case Report
Mónica Bronte Anaut, Javier Arredondo Montero, Maria Pilar Fernández Seara, Rosa Guarch Troyas
International Journal of Surgical Pathology.2023; 31(1): 92. CrossRef - Molecular characterization of gastric-type endocervical adenocarcinoma using next-generation sequencing
Swati Garg, Teddy S. Nagaria, Blaise Clarke, Orit Freedman, Zanobia Khan, Joerg Schwock, Marcus Q. Bernardini, Amit M. Oza, Kathy Han, Adam C. Smith, Tracy L. Stockley, Marjan Rouzbahman
Modern Pathology.2019; 32(12): 1823. CrossRef - The developing spectrum of gastric-type cervical glandular lesions
Karen L. Talia, W. Glenn McCluggage
Pathology.2018; 50(2): 122. CrossRef
- Molecular evidence of a clonal relationship of synchronous/multifocal gastric‐type lesions of the female genital tract
- ThinPrep Cytological Findings of Desmoplastic Small Round Cell Tumor with Extensive Glandular Differentiation: A Case Study
- Hyun-Jung Kim, Byeong Seok Sohn, Ji-Eun Kwon, Jeong Yeon Kim, Kyeongmee Park
- Korean J Pathol. 2013;47(2):182-187. Published online April 24, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.2.182
- 8,370 View
- 70 Download
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Desmoplastic small round cell tumor (DSRCT) is a rare and highly aggressive neoplasm. The cytological diagnosis of this tumor has only been reported in a few cases. In most of these cases, the diagnosis was made using fine-needle aspiration cytology. Most DSRCTs resemble disseminated carcinomatoses in their clinical manifestation as well as cytomorphologically, even in young-adult patients. These authors report a case of using peritoneal-washing and pleural-effusion ThinPrep cytology to diagnose DSRCT, with extensive glandular differentiation and mucin vacuoles. We found that fibrillary stromal fragment, clinical setting, and adjunctive immunocytochemical staining were most helpful for avoiding misdiagnosis.
-
Citations
Citations to this article as recorded by- Desmoplastic Small Round Cell Tumor: Study of Cytomorphologic and Immunophenotypical Features in Seven Cases, One With Unusual Rhabdoid Morphology
Sameer Chhetri Aryal, Khalid Shittu, Mohamed Mustafa, Fatimah I. Alruwaii, Kyle D. Perry, Lisi Yuan
Diagnostic Cytopathology.2026;[Epub] CrossRef - A Rare and Aggressive Abdominopelvic Tumor: A Case of Desmoplastic Small Round Cell Tumor in a Young Male
Yağmur Sena Tosun, Aytan Babazade, Emine Sena Sözen, Betül Erişmiş, Enes Seyda Şahiner
Caucasian Medical Journal.2025; 3(1): 4. CrossRef - Desmoplastic Small Round Cell Tumor Involving Serous Fluid: Cytologic Features and Diagnostic Pitfalls: A Series of 8 Cases
Nibras L Fakhri, Qiong Gan
American Journal of Clinical Pathology.2023; 160(4): 417. CrossRef - A Review of Effusion Cytomorphology of Small Round Cell Tumors
Lucy M. Han, Christopher J. VandenBussche, Mads Abildtrup, Ashish Chandra, Poonam Vohra
Acta Cytologica.2022; 66(4): 336. CrossRef - Intra-abdominal desmoplastic small blue round cell tumor: A case report
Tareq Hamed Al Taei, Hasan Al Fardan, Sarah Ali Al Mail
Radiology Case Reports.2022; 17(12): 4502. CrossRef - Desmoplastic Small Round Cell Tumor of the Kidney: Report of a Case, Literature Review, and Comprehensive Discussion of the Distinctive Morphologic, Immunohistochemical, and Molecular Features in the Differential Diagnosis of Small Round Cell Tumors Affec
Carlos A. Galliani, Michele Bisceglia, Antonio Del Giudice, Giuseppe Cretì
Advances in Anatomic Pathology.2020; 27(6): 408. CrossRef - Intra-abdominal desmoplastic small round cell tumors: CT and FDG-PET/CT findings with histopathological association
JINGJING CHEN, ZENGJIE WU, BINBIN SUN, DACHENG LI, ZHENGUANG WANG, FANGJUN LIU, HUI HUA
Oncology Letters.2016; 11(5): 3298. CrossRef - Desmoplastic small round cell tumor with sphere‐like clusters mimicking adenocarcinoma
Yukinori Hattori, Akihiko Yoshida, Naoshi Sasaki, Yasuo Shibuki, Kenji Tamura, Koji Tsuta
Diagnostic Cytopathology.2015; 43(3): 214. CrossRef - Tumor intraabdominal desmoplásico de células pequeñas y redondas
Andrés Alejandro Briseño-Hernández, Deissy Roxana Quezada-López, Lilia Edith Corona-Cobián, Agar Castañeda-Chávez, Alfonso Tonatiuh Duarte-Ojeda, Michel Dassaejv Macías-Amezcua
Cirugía y Cirujanos.2015; 83(3): 243. CrossRef - Intra-abdominal desmoplastic small round cell tumour
Andrés Alejandro Briseño-Hernández, Deissy Roxana Quezada-López, Lilia Edith Corona-Cobián, Agar Castañeda-Chávez, Alfonso Tonatiuh Duarte-Ojeda, Michel Dassaejv Macías-Amezcua
Cirugía y Cirujanos (English Edition).2015; 83(3): 243. CrossRef - Diagnostic Pitfalls of Differentiating Desmoplastic Small Round Cell Tumor (DSRCT) From Wilms Tumor (WT)
Michael A. Arnold, Lynn Schoenfield, Berkeley N. Limketkai, Christina A. Arnold
American Journal of Surgical Pathology.2014; 38(9): 1220. CrossRef
- Desmoplastic Small Round Cell Tumor: Study of Cytomorphologic and Immunophenotypical Features in Seven Cases, One With Unusual Rhabdoid Morphology
- Microsatellite Instability Status in Gastric Cancer: A Reappraisal of Its Clinical Significance and Relationship with Mucin Phenotypes
- Joo-Yeun Kim, Na Ri Shin, Ahrong Kim, Hyun-Jeong Lee, Won-young Park, Jee-Yeon Kim, Chang-Hun Lee, Gi-Young Huh, Do Youn Park
- Korean J Pathol. 2013;47(1):28-35. Published online February 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.1.28
- 12,591 View
- 126 Download
- 76 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Gastric cancers with microsatellite instabilities (MSI) have been reported to be associated with favorable prognosis. However, the significance of the effect of MSI on the clinicopathological features, as well as its association with mucin phenotype, remains unclear.
Methods MSI status was assessed in 414 cases of gastric cancer using polymerase chain reaction analysis of five microsatellite loci, as recommended by National Cancer Institution criteria. The expression of mucins (MUC5AC, MUC6, MUC2, and CD10) was assessed.
Results Out of 414 total cases of gastric cancer, 380 (91.7%), 11 (2.7%), and 23 (5.6%) were microsatellite stable (MSS), low-level MSI (MSI-L), and high-level MSI (MSI-H), respectively. Compared to MSS/MSI-L, MSI-H gastric cancers were associated with older age (p=0.010), tumor size (p=0.014), excavated gross (p=0.042), intestinal type (p=0.028), aggressive behaviors (increase of T stage [p=0.009]), perineural invasion [p=0.022], and lymphovascular emboli [p=0.027]). MSI-H gastric cancers were associated with tumor necrosis (p=0.041), tumor-infiltrating lymphocytes (≥2/high power field, p<0.001), expanding growth patterns (p=0.038), gastric predominant mucin phenotypes (p=0.028), and MUC6 expression (p=0.016). Tumor necrosis (≥10% of mass, p=0.031), tumor-infiltrating lymphocytes (p<0.001), intestinal type (p=0.014), and gastric mucin phenotypes (p=0.020) could represent independent features associated with MSI-H gastric cancers. MSI-H intestinal type gastric cancers had a tendency for poor prognosis in univariate analysis (p=0.054) but no association in Cox multivariate analysis (p=0.197).
Conclusions Our data suggest that MSI-H gastric cancers exhibit distinct aggressive biologic behaviors and a gastric mucin phenotype. This contradicts previous reports that describe MSI-H gastric cancer as being associated with favorable prognosis.
-
Citations
Citations to this article as recorded by- Intestinal Subtype as a Biomarker of Response to Neoadjuvant Immunochemotherapy in Locally Advanced Gastric Adenocarcinoma: Insights from a Prospective Phase II Trial
Lei Wang, Mengting Sun, Jinyang Li, Linghong Wan, Yuting Tan, Shuoran Tian, Yongying Hou, Linyu Wu, Ziyi Peng, Xiao Hu, Qihua Zhang, Zening Huang, Mengyi Han, Shiyin Peng, Yuwei Pan, Yuanfeng Ren, Mengsi Zhang, Dongfeng Chen, Qin Liu, Xianfeng Li, Zhong-y
Clinical Cancer Research.2025; 31(1): 74. CrossRef - How do I treat dMMR/MSI gastro-oesophageal adenocarcinoma in 2025? A position paper from the EORTC-GITCG gastro-esophageal task force
Christelle de la Fouchardière, Antonella Cammarota, Magali Svrcek, Maria Alsina, Tania Fleitas-Kanonnikoff, Radka Lordick Obermannová, Anna Dorothea Wagner, Dominic Yap Wei Ting, Diana Enea, Angelica Petrillo, Elizabeth C. Smyth
Cancer Treatment Reviews.2025; 134: 102890. CrossRef - T-bet+CD8+ T cells govern anti-PD-1 responses in microsatellite-stable gastric cancers
Shiying Tang, Xiaofang Che, Jinyan Wang, Ce Li, Xin He, Kezuo Hou, Xiaojie Zhang, Jia Guo, Bowen Yang, Danni Li, Lili Cao, Xiujuan Qu, Zhenning Wang, Yunpeng Liu
Nature Communications.2025;[Epub] CrossRef - Prediction of a Panel of Programmed Cell Death Protein-1 (PD-1) Inhibitor–Sensitive Biomarkers Using Multiphase Computed Tomography Imaging Textural Features: Retrospective Cohort Analysis
Shiqi Wang, Na Chai, Jingji Xu, Pengfei Yu, Luguang Huang, Quan Wang, Zhifeng Zhao, Bin Yang, Jiangpeng Wei, Xiangjie Wang, Gang Ji, Minwen Zheng
JMIR Cancer.2025; 11: e67379. CrossRef - Isolated tumor cell clusters (ITC) in lymph nodes and PD-L1 expression on tumor-associated immune cells are prognostic factors for microsatellite instable-high gastric cancers
Menghan Cui, Yangli Zhou, Yin Han, Nannan Chen, Min Zhao, Yan Wang, Fengxia He
Translational Oncology.2025; 59: 102465. CrossRef - Microsatellite Instability and BAT-26 Marker Expression in a Mexican Prostate Cancer Population with Different Gleason Scores
Ana K. Flores-Islas, Manuel A. Rico-Méndez, Marisol Godínez-Rubí, Martha Arisbeth Villanueva-Pérez, Erick Sierra-Díaz, Ana Laura Pereira-Suárez, Saul A. Beltrán-Ontiveros, Perla Y. Gutiérrez-Arzapalo, José M. Moreno-Ortiz, Adrián Ramírez-de-Arellano
Diseases.2025; 13(7): 202. CrossRef - Prognostic implications of ERBB2 amplification and mismatch repair in gastric adenocarcinoma: a single-center study
Han Xia, Zekang Li, Minyi Wang, Dachuan Zhang, Xiao Zheng, Jun Wu, Chen Wu
Gastrointestinal Tumors.2025;[Epub] CrossRef - Microsatellite Instability in Chronic Gastritis Associated with Gastric Cancer
A. V. Kononov, V. A. Rubtsov, E. V. Demidova, M. N. Parygina, A. G. Shimanskaya, S. I. Mozgovoi, E. G. Pomorgailo, М. V. Markelova
Russian Journal of Gastroenterology, Hepatology, Coloproctology.2025; 35(4): 48. CrossRef - Non–Pure Intestinal Phenotype as an Indicator of Progression in Sporadic Nonampullary Duodenal Adenomas: A Multicenter Retrospective Cohort Study
Ryotaro Uema, Yoshito Hayashi, Masato Komori, Narihiro Shibukawa, Noriko Hayashi, Masayoshi Horimoto, Takuya Yamada, Masashi Yamamoto, Satoshi Hiyama, Kazuo Kinoshita, Hideharu Ogiyama, Shinjiro Yamaguchi, Satoshi Egawa, Takashi Kanesaka, Minoru Kato, Shu
Clinical and Translational Gastroenterology.2024; 15(1): e00649. CrossRef - Intratumoral and peritumoral CT-based radiomics for predicting the microsatellite instability in gastric cancer
Xingchi Chen, Zijian Zhuang, Lin Pen, Jing Xue, Haitao Zhu, Lirong Zhang, Dongqing Wang
Abdominal Radiology.2024; 49(5): 1363. CrossRef - The tumor immune composition of mismatch repair deficient and Epstein-Barr virus-positive gastric cancer: A systematic review
J. Bos, T.S. Groen-van Schooten, C.P. Brugman, F.S. Jamaludin, H.W.M. van Laarhoven, S. Derks
Cancer Treatment Reviews.2024; 127: 102737. CrossRef - Potent therapeutic strategy in gastric cancer with microsatellite instability-high and/or deficient mismatch repair
Akira Ooki, Hiroki Osumi, Koichiro Yoshino, Kensei Yamaguchi
Gastric Cancer.2024; 27(5): 907. CrossRef - The mechanism of RGS5 regulating gastric cancer mismatch repair protein
Zhenwei Yang, Ranran Zhang, Jialong Liu, Sufang Tian, Hailin Zhang, Lingxiu Zeng, Yangyang Zhang, Liping Gao, Meng Wang, Wenqing Shan, Jing Liu
Molecular Carcinogenesis.2024; 63(9): 1750. CrossRef - Prognostic significance of microsatellite instability in patients with resectable gastric cancer
Marina Alessandra Pereira, Marcus Fernando Kodama Pertille Ramos, Leonardo Cardili, André Roncon Dias, Venancio Avancini Ferreira Alves, Evandro Sobroza de Mello, Ulysses Ribeiro
Journal of Gastrointestinal Surgery.2024; 28(10): 1687. CrossRef - Access to radiotherapy in improving gastric cancer care quality and equality
Minmin Wang, Kepei Huang, Xiaohan Fan, Jia Wang, Yinzi Jin, Zhi-Jie Zheng
Communications Medicine.2024;[Epub] CrossRef - Deep learning captures selective features for discrimination of microsatellite instability from pathologic tissue slides of gastric cancer
Sung Hak Lee, Yujin Lee, Hyun‐Jong Jang
International Journal of Cancer.2023; 152(2): 298. CrossRef - Novel Biomarkers of Gastric Cancer: Current Research and Future Perspectives
Yasushi Sato, Koichi Okamoto, Yutaka Kawano, Akinari Kasai, Tomoyuki Kawaguchi, Tamotsu Sagawa, Masahiro Sogabe, Hiroshi Miyamoto, Tetsuji Takayama
Journal of Clinical Medicine.2023; 12(14): 4646. CrossRef - The results of treatment for resectable gastric cancer with microsatellite instability
H. Sun, S. N. Nered, A. A. Tryakin, E. V. Artamonova, A. E. Kalinin, V. E. Bugaev, A. M. Stroganova, N. S. Besova, P. P. Arkhiri, V. I. Marshall, R. Sh. Abdulaeva, I. S. Stilidi
Pelvic Surgery and Oncology.2023; 13(2): 17. CrossRef - Heterogeneity and Adjuvant Therapeutic Approaches in MSI-H/dMMR Resectable Gastric Cancer: Emerging Trends in Immunotherapy
Hui Wu, Wenyuan Ma, Congfa Jiang, Ning Li, Xin Xu, Yongfeng Ding, Haiping Jiang
Annals of Surgical Oncology.2023; 30(13): 8572. CrossRef - Dual-layer spectral-detector CT for predicting microsatellite instability status and prognosis in locally advanced gastric cancer
Yongjian Zhu, Peng Wang, Bingzhi Wang, Zhichao Jiang, Ying Li, Jun Jiang, Yuxin Zhong, Liyan Xue, Liming Jiang
Insights into Imaging.2023;[Epub] CrossRef - Concordance between microsatellite instability testing and immunohistochemistry for mismatch repair proteins and efficient screening of mismatch repair deficient gastric cancer
Gou Yamamoto, Tetsuya Ito, Okihide Suzuki, Nao Kamae, Miho Kakuta, Akemi Takahashi, Katsuya Iuchi, Tomio Arai, Hideyuki Ishida, Kiwamu Akagi
Oncology Letters.2023;[Epub] CrossRef - Low incidence of microsatellite instability in gastric cancers and its association with the clinicopathological characteristics: a comparative study
Fateme Fooladi Talari, Ali Bozorg, Sirous Zeinali, Mohammadreza Zali, Zhale Mohsenifar, Hamid Asadzadeh Aghdaei, Kaveh Baghaei
Scientific Reports.2023;[Epub] CrossRef - Mutational separation and clinical outcomes of TP53 and CDH1 in gastric cancer
He-Li Liu, Huan Peng, Chang-Hao Huang, Hai-Yan Zhou, Jie Ge
World Journal of Gastrointestinal Surgery.2023; 15(12): 2855. CrossRef - Genomic and Immunologic Markers of Intrinsic Resistance to Pembrolizumab Monotherapy in Microsatellite Instability-High Gastric Cancer: Observations from a Prospective Phase II Study
Haibo Qiu
Global Medical Genetics.2022; 09(02): 060. CrossRef - Clinicopathological features of PD-L1 protein expression, EBV positivity, and MSI status in patients with advanced gastric and esophagogastric junction adenocarcinoma in Japan
Tsutomu Yoshida, Go Ogura, Mikiko Tanabe, Takuo Hayashi, Chiho Ohbayashi, Mizutomo Azuma, Chikara Kunisaki, Yoichi Akazawa, Soji Ozawa, Sohei Matsumoto, Takayoshi Suzuki, Akira Mitoro, Tetsu Fukunaga, Akiko Shimizu, Go Fujimoto, Takashi Yao
Cancer Biology & Therapy.2022; 23(1): 191. CrossRef - Development of Tissue-Agnostic Treatments for Patients with Cancer
Steven Lemery, Lola Fashoyin-Aje, Leigh Marcus, Sandra Casak, Julie Schneider, Marc Theoret, Paul Kluetz, Richard Pazdur, Julia A. Beaver
Annual Review of Cancer Biology.2022; 6(1): 147. CrossRef - A multicenter study on the preoperative prediction of gastric cancer microsatellite instability status based on computed tomography radiomics
Xiuqun Liang, Yinbo Wu, Ying Liu, Danping Yu, Chencui Huang, Zhi Li
Abdominal Radiology.2022; 47(6): 2036. CrossRef - Combination of AKT1 and CDH1 mutations predicts primary resistance to immunotherapy in dMMR/MSI-H gastrointestinal cancer
Zhenghang Wang, Qi Zhang, Changsong Qi, Yuezong Bai, Feilong Zhao, Hui Chen, Zhongwu Li, Xicheng Wang, Mifen Chen, Jifang Gong, Zhi Peng, Xiaotian Zhang, Jinping Cai, Shiqing Chen, Xiaochen Zhao, Lin Shen, Jian Li
Journal for ImmunoTherapy of Cancer.2022; 10(6): e004703. CrossRef - Eldest gastric cancer patient with high microsatellite instability responding to pembrolizumab
Akinobu Wakasugi, Akinori Sasaki, Risa Okamoto, Yasuaki Motomura
International Cancer Conference Journal.2022; 12(1): 59. CrossRef - Baseline lesion number as an efficacy predictive and independent prognostic factor and its joint utility with TMB for PD-1 inhibitor treatment in advanced gastric cancer
Xiao-Li Wei, Jian-Ying Xu, De-Shen Wang, Dong-Liang Chen, Chao Ren, Jia-Ning Li, Feng Wang, Feng-Hua Wang, Rui-Hua Xu
Therapeutic Advances in Medical Oncology.2021;[Epub] CrossRef - Clinical and morphological portrait of tumors with microsatellite instability
A. A. Musaelyan, V. D. Nazarov, A. S. Budnikova, S. V. Lapin, S. L. Vorobyev, V. L. Emanuel, A. A. Zakharenko, S. V. Orlov
Advances in Molecular Oncology.2021; 8(2): 52. CrossRef - How to Best Exploit Immunotherapeutics in Advanced Gastric Cancer: Between Biomarkers and Novel Cell-Based Approaches
Michele Ghidini, Angelica Petrillo, Andrea Botticelli, Dario Trapani, Alessandro Parisi, Anna La Salvia, Elham Sajjadi, Roberto Piciotti, Nicola Fusco, Shelize Khakoo
Journal of Clinical Medicine.2021; 10(7): 1412. CrossRef - Microsatellite instability in Gastric Cancer: Between lights and shadows
Elisabetta Puliga, Simona Corso, Filippo Pietrantonio, Silvia Giordano
Cancer Treatment Reviews.2021; 95: 102175. CrossRef - Impact of microsatellite status on negative lymph node count and prognostic relevance after curative gastrectomy
Zhenghao Cai, Junjun Ma, Shuchun Li, Abe Fingerhut, Jing Sun, Lu Zang, Chao Yan, Wentao Liu, Zhenggang Zhu, Minhua Zheng
Journal of Surgical Oncology.2021;[Epub] CrossRef - A greater lymph node yield is required during pathological examination in microsatellite instability-high gastric cancer
Zhenghao Cai, Haiqin Song, Abe Fingerhut, Jing Sun, Junjun Ma, Luyang Zhang, Shuchun Li, Chaoran Yu, Minhua Zheng, Lu Zang
BMC Cancer.2021;[Epub] CrossRef - Determinants of Response and Intrinsic Resistance to PD-1 Blockade in Microsatellite Instability–High Gastric Cancer
Minsuk Kwon, Minae An, Samuel J. Klempner, Hyuk Lee, Kyoung-Mee Kim, Jason K. Sa, Hee Jin Cho, Jung Yong Hong, Taehyang Lee, Yang Won Min, Tae Jun Kim, Byung-Hoon Min, Woong-Yang Park, Won Ki Kang, Kyu-Tae Kim, Seung Tae Kim, Jeeyun Lee
Cancer Discovery.2021; 11(9): 2168. CrossRef - Advanced Gastric Cancer: Current Treatment Landscape and a Future Outlook for Sequential and Personalized Guide: Swiss Expert Statement Article
Alexander R. Siebenhüner, Sara De Dosso, Daniel Helbling, Christoforos Astaras, Petr Szturz, Peter Moosmann, Stefanie Pederiva, Thomas Winder, Philippe Von Burg, Markus Borner
Oncology Research and Treatment.2021; 44(9): 485. CrossRef - High homogeneity of mismatch repair deficiency in advanced prostate cancer
Christoph Fraune, Ronald Simon, Doris Höflmayer, Katharina Möller, David Dum, Franziska Büscheck, Claudia Hube-Magg, Georgia Makrypidi-Fraune, Martina Kluth, Andrea Hinsch, Eike Burandt, Till Sebastian Clauditz, Waldemar Wilczak, Guido Sauter, Stefan Steu
Virchows Archiv.2020; 476(5): 745. CrossRef - High homogeneity of MMR deficiency in ovarian cancer
Christoph Fraune, Janina Rosebrock, Ronald Simon, Claudia Hube-Magg, Georgia Makrypidi-Fraune, Martina Kluth, Franziska Büscheck, Doris Höflmayer, Barbara Schmalfeldt, Volkmar Müller, Linn Wölber, Isabell Witzel, Peter Paluchowski, Christian Wilke, Uwe He
Gynecologic Oncology.2020; 156(3): 669. CrossRef - Molecular Classification of Gastric Cancer among Alaska Native People
Holly Martinson, Dominic Mallari, Christine Richter, Tsung-Teh Wu, James Tiesinga, Steven Alberts, Matthew Olnes
Cancers.2020; 12(1): 198. CrossRef - Tumor immune response and immunotherapy in gastric cancer
Yoonjin Kwak, An Na Seo, Hee Eun Lee, Hye Seung Lee
Journal of Pathology and Translational Medicine.2020; 54(1): 20. CrossRef - MMR deficiency in urothelial carcinoma of the bladder presents with temporal and spatial homogeneity throughout the tumor mass
Christoph Fraune, Ronald Simon, Claudia Hube-Magg, Georgia Makrypidi-Fraune, Christian Kähler, Martina Kluth, Doris Höflmayer, Franziska Büscheck, David Dum, Andreas M. Luebke, Eike Burandt, Till Sebastian Clauditz, Waldemar Wilczak, Guido Sauter, Stefan
Urologic Oncology: Seminars and Original Investigations.2020; 38(5): 488. CrossRef - MMR Deficiency is Homogeneous in Pancreatic Carcinoma and Associated with High Density of Cd8-Positive Lymphocytes
Christoph Fraune, Eike Burandt, Ronald Simon, Claudia Hube-Magg, Georgia Makrypidi-Fraune, Martina Kluth, Franziska Büscheck, Doris Höflmayer, Niclas Ch. Blessin, Tim Mandelkow, Wenchao Li, Daniel Perez, Jakob R. Izbicki, Waldemar Wilczak, Guido Sauter, J
Annals of Surgical Oncology.2020; 27(10): 3997. CrossRef - CD73's Potential as an Immunotherapy Target in Gastrointestinal Cancers
Jerry B. Harvey, Luan H. Phan, Oscar E. Villarreal, Jessica L. Bowser
Frontiers in Immunology.2020;[Epub] CrossRef - Tumor copy-number alterations predict response to immune-checkpoint-blockade in gastrointestinal cancer
Zhihao Lu, Huan Chen, Shuang Li, Jifang Gong, Jian Li, Jianling Zou, Lihong Wu, Jianing Yu, Wenbo Han, Huaibo Sun, Xi Jiao, Xiaotian Zhang, Zhi Peng, Ming Lu, Zhenghang Wang, Henghui Zhang, Lin Shen
Journal for ImmunoTherapy of Cancer.2020; 8(2): e000374. CrossRef - Protein expression-based classification of gastric cancer by immunohistochemistry of tissue microarray
Chong Zhao, Zhiqiang Feng, Hongzhen He, Dan Zang, Hong Du, Hongli Huang, Yanlei Du, Jie He, Yongjian Zhou, Yuqiang Nie, Girijesh Kumar Patel
PLOS ONE.2020; 15(10): e0238836. CrossRef - Clinicopathologic Characteristics and Long-Term Outcome of Gastric Cancer Patients with Family History: Seven-Year Follow-Up Study for Korean Health Check-Up Subjects
Jooyoung Lee, Su Jin Chung, Ji Min Choi, Yoo Min Han, Joo Sung Kim, Greger Lindberg
Gastroenterology Research and Practice.2020; 2020: 1. CrossRef - Implication of expression of MMR proteins and clinicopathological characteristics in gastric cancer
Renu Verma, Puja Sakhuja, Ritu Srivastava, Prakash Chand Sharma
Asia-Pacific Journal of Oncology.2020; : 1. CrossRef - Prognostic significance of microsatellite‐instability in gastric and gastroesophageal junction cancer patients undergoing neoadjuvant chemotherapy
Georg Martin Haag, Elena Czink, Aysel Ahadova, Thomas Schmidt, Leila Sisic, Susanne Blank, Ulrike Heger, Leonidas Apostolidis, Anne Katrin Berger, Christoph Springfeld, Felix Lasitschka, Dirk Jäger, Magnus von Knebel Doeberitz, Matthias Kloor
International Journal of Cancer.2019; 144(7): 1697. CrossRef - Serological Markers Associated With Response to Immune Checkpoint Blockade in Metastatic Gastrointestinal Tract Cancer
Zhihao Lu, Jianling Zou, Ying Hu, Shuang Li, Tao Zhou, Jifang Gong, Jian Li, Xiaotian Zhang, Jun Zhou, Ming Lu, Xicheng Wang, Zhi Peng, Changsong Qi, Yanyan Li, Jie Li, Yan Li, Jianyin Zou, Xiao Du, Henghui Zhang, Lin Shen
JAMA Network Open.2019; 2(7): e197621. CrossRef - Assessing molecular subtypes of gastric cancer: microsatellite unstable and Epstein-Barr virus subtypes. Methods for detection and clinical and pathological implications
Carolina Martinez-Ciarpaglini, Tania Fleitas-Kanonnikoff, Valentina Gambardella, Marta Llorca, Cristina Mongort, Regina Mengual, Gema Nieto, Lara Navarro, Marisol Huerta, Susana Rosello, Desamparados Roda, Noelia Tarazona, Samuel Navarro, Gloria Ribas, An
ESMO Open.2019; 4(3): e000470. CrossRef - The role of pembrolizumab in the treatment of PD-L1 expressing gastric and gastroesophageal junction adenocarcinoma
Gagandeep Brar, Manish A. Shah
Therapeutic Advances in Gastroenterology.2019;[Epub] CrossRef - Novel Biomarkers for Prediction of Response to Preoperative Systemic Therapies in Gastric Cancer
Alessandro Cavaliere, Valeria Merz, Simona Casalino, Camilla Zecchetto, Francesca Simionato, Hayley Louise Salt, Serena Contarelli, Raffaela Santoro, Davide Melisi
Journal of Gastric Cancer.2019; 19(4): 375. CrossRef - MICROSATELLITE INSTABILITY AND GASTRIC CARCINOMA. REVIEW OF THELITERATURE
D. L. Rotin, O. V. Paklina, I. O. Tin’kova, D. N. Grekov
Russian Journal of Biotherapy.2019; 18(4): 17. CrossRef - Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer
K Polom, L Marano, D Marrelli, R De Luca, G Roviello, V Savelli, P Tan, F Roviello
Journal of British Surgery.2018; 105(3): 159. CrossRef - Gastric poorly cohesive carcinoma: a correlative study of mutational signatures and prognostic significance based on histopathological subtypes
Chae H Kwon, Young K Kim, Sojeong Lee, Ahrong Kim, Hye J Park, Yuri Choi, Yeo J Won, Do Y Park, Gregory Y Lauwers
Histopathology.2018; 72(4): 556. CrossRef - Microsatellite instability in gastric cancer: molecular bases, clinical perspectives, and new treatment approaches
Margherita Ratti, Andrea Lampis, Jens C. Hahne, Rodolfo Passalacqua, Nicola Valeri
Cellular and Molecular Life Sciences.2018; 75(22): 4151. CrossRef - High-throughput Protein and mRNA Expression–based Classification of Gastric Cancers Can Identify Clinically Distinct Subtypes, Concordant With Recent Molecular Classifications
Sangjeong Ahn, So-Jeong Lee, Yonugkeum Kim, Ahrong Kim, Nari Shin, Kyung Un Choi, Chang-Hun Lee, Gi Yeong Huh, Kyong-Mee Kim, Namrata Setia, Gregory Y. Lauwers, Do Youn Park
American Journal of Surgical Pathology.2017; 41(1): 106. CrossRef - Molecular Testing for Gastrointestinal Cancer
Hye Seung Lee, Woo Ho Kim, Yoonjin Kwak, Jiwon Koh, Jeong Mo Bae, Kyoung-Mee Kim, Mee Soo Chang, Hye Seung Han, Joon Mee Kim, Hwal Woong Kim, Hee Kyung Chang, Young Hee Choi, Ji Y. Park, Mi Jin Gu, Min Jin Lhee, Jung Yeon Kim, Hee Sung Kim, Mee-Yon Cho
Journal of Pathology and Translational Medicine.2017; 51(2): 103. CrossRef - Molecular testing of gastrointestinal tumours
Matthew Evans, Matthew Smith, Brendan O'Sullivan, Philippe Taniere
Diagnostic Histopathology.2017; 23(10): 442. CrossRef - Gastric Carcinomas With Lymphoid Stroma
Raul S Gonzalez, Justin M M Cates, Frank Revetta, Loralee A McMahon, Kay Washington
American Journal of Clinical Pathology.2017; 148(6): 477. CrossRef - Meta-Analysis of Prognostic Role of Ki-67 Labeling Index in Gastric Carcinoma
Jung-Soo Pyo, Nae Yu Kim
The International Journal of Biological Markers.2017; 32(4): 447. CrossRef - Tissue-Agnostic Drug Development
Keith T. Flaherty, Dung T. Le, Steven Lemery
American Society of Clinical Oncology Educational Book.2017; (37): 222. CrossRef - Programmed death ligand-1 and MET co-expression is a poor prognostic factor in gastric cancers after resection
Mi Jung Kwon, Kab-Choong Kim, Eun Sook Nam, Seong Jin Cho, Hye-Rim Park, Soo Kee Min, Jinwon Seo, Ji-Young Choe, Hye Kyung Lee, Ho Suk Kang, Kyueng-Whan Min
Oncotarget.2017; 8(47): 82399. CrossRef - Hypermutation and microsatellite instability in gastrointestinal cancers
Kizuki Yuza, Masayuki Nagahashi, Satoshi Watanabe, Kazuaki Takabe, Toshifumi Wakai
Oncotarget.2017; 8(67): 112103. CrossRef - The Emerging Role of Immunotherapy in Gastric and Esophageal Adenocarcinoma
Bruno Bockorny, Eirini Pectasides
Future Oncology.2016; 12(15): 1833. CrossRef - Expression of Mismatch Repair Proteins in Early and Advanced Gastric Cancer in Poland
Katarzyna Karpińska-Kaczmarczyk, Magdalena Lewandowska, Małgorzata Ławniczak, Andrzej Białek, Elżbieta Urasińska
Medical Science Monitor.2016; 22: 2886. CrossRef - Immunotherapy for Gastroesophageal Cancer
Emily Goode, Elizabeth Smyth
Journal of Clinical Medicine.2016; 5(10): 84. CrossRef - Lauren classification and individualized chemotherapy in gastric cancer
JUNLI MA, HONG SHEN, LINDA KAPESA, SHAN ZENG
Oncology Letters.2016; 11(5): 2959. CrossRef - High-risk and low-risk gastric cancer areas in Italy and its association with microsatellite instability
Karol Polom, Daniele Marrelli, Valeria Pascale, Giandomenico Roviello, Costantino Voglino, Henry Rho, Carla Vindigni, Mario Marini, Raffaele Macchiarelli, Franco Roviello
Journal of Cancer Research and Clinical Oncology.2016; 142(8): 1817. CrossRef - MUC2 Expression Is Correlated with Tumor Differentiation and Inhibits Tumor Invasion in Gastric Carcinomas: A Systematic Review and Meta-analysis
Jung-Soo Pyo, Jin Hee Sohn, Guhyun Kang, Dong-Hoon Kim, Kyungeun Kim, In-Gu Do, Dong Hyun Kim
Journal of Pathology and Translational Medicine.2015; 49(3): 249. CrossRef - Correlation between microsatellite instability-high phenotype and occult lymph node metastasis in gastric carcinoma
Jiwoon Choi, Soo Kyung Nam, Do Joong Park, Hwal Woong Kim, Hyung-Ho Kim, Woo Ho Kim, Hye Seung Lee
APMIS.2015; 123(3): 215. CrossRef - Clinicopathologic and molecular features associated with patient age in gastric cancer
Ji Yeon Seo, Eun Hyo Jin, Hyun Jin Jo, Hyuk Yoon, Cheol Min Shin, Young Soo Park, Nayoung Kim, Hyun Chae Jung, Dong Ho Lee
World Journal of Gastroenterology.2015; 21(22): 6905. CrossRef - Molecular classification of gastric cancer: Towards a pathway-driven targeted therapy
Ismael Riquelme, Kathleen Saavedra, Jaime A. Espinoza, Helga Weber, Patricia García, Bruno Nervi, Marcelo Garrido, Alejandro H. Corvalán, Juan Carlos Roa, Carolina Bizama
Oncotarget.2015; 6(28): 24750. CrossRef - A phylogenetic model for understanding the effect of gene duplication on cancer progression
Qin Ma, Jaxk H. Reeves, David A. Liberles, Lili Yu, Zheng Chang, Jing Zhao, Juan Cui, Ying Xu, Liang Liu
Nucleic Acids Research.2014; 42(5): 2870. CrossRef - The analysis of microsatellite instability in extracolonic gastrointestinal malignancy
Andrew S. Williams, Weei-Yuarn Huang
Pathology.2013; 45(6): 540. CrossRef
- Intestinal Subtype as a Biomarker of Response to Neoadjuvant Immunochemotherapy in Locally Advanced Gastric Adenocarcinoma: Insights from a Prospective Phase II Trial
- Primary Mucinous Cystadenocarcinoma of the Breast: Cytologic Finding and Expression of MUC5 Are Different from Mucinous Carcinoma
- Sung Eun Kim, Ji Hye Park, SoonWon Hong, Ja Seung Koo, Joon Jeong, Woo-Hee Jung
- Korean J Pathol. 2012;46(6):611-616. Published online December 26, 2012
- DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.6.611
- 9,661 View
- 66 Download
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF Mucinous cystadenocarcinoma (MCA) in the breast is a rare neoplasm. There have been 13 cases of primary breast MCA reported. The MCA presents as a large, partially cystic mass in postmenopausal woman with a good prognosis. The microscopic findings resemble those of ovarian, pancreatic, or appendiceal MCA. The aspiration findings showed mucin-containing cell clusters in the background of mucin and necrotic material. The cell clusters had intracytoplasmic mucin displacing atypical nuclei to the periphery. Histologically, the tumor revealed an abundant mucin pool with small floating clusters of mucin-containing tumor cells. There were also small cysts lined by a single layer of tall columnar mucinous cells, resembling those of the uterine endocervix. The cancer cells were positive for mucin (MUC) 5 and negative for MUC2 and MUC6. This mucin profile is different from ordinary mucinous carcinoma and may be a unique characteristic of breast MCA.
-
Citations
Citations to this article as recorded by- BMP2 alterations in mucinous cystadenocarcinoma of the breast: insights from whole-exome sequencing
Zhenyu Li, Yi Gong, Guangxin Li, Qingming Jiang, Juanhui Dong, Rui Chen
PeerJ.2025; 13: e19948. CrossRef - Primary mucinous cystadenocarcinoma of the breast commonly harbours TP53 mutations and PI3K/AKT pathway alterations
Cheng Xu, Jing Wu, Ying Ding, Zhihong Zhang, Cong Wang
Virchows Archiv.2025; 487(6): 1235. CrossRef - Mucinous cystadenocarcinoma of the breast harbours TRPS1 expressions and PIK3CA alterations
Wei‐Yu Chen, Yu‐Hsuan Hu, Yu‐Hsin Tsai, Jen‐Fan Hang, Puay Hoon Tan, Chih‐Jung Chen
Histopathology.2024; 84(3): 550. CrossRef - Pure mucinous adenocarcinoma of the breast with the rare lymphoplasmacytic infiltration: A case report with review of literature
Yash Hasmukhbhai Prajapati, Vishal Bhabhor, Kahan Samirkumar Mehta, Mithoon Barot, Husen Boriwala, Mohamed Omar
Clinical Case Reports.2024;[Epub] CrossRef - HER2‐positive mucinous cystadenocarcinoma of the breast coexisting with invasive lobular carcinoma: A case report and review of the literature
Ismail Guzelis, Betul Bolat Kucukzeybek, Mehmet Ali Uyaroglu, Melek Bekler Gokova, Gulten Sezgin, Yuksel Kucukzeybek
Diagnostic Cytopathology.2024;[Epub] CrossRef - Primary mucinous cystadenocarcinoma of the breast: A case report and literature review
Xi Cao, Yongchao Luo, Songjie Shen, Xinyu Ren
Oncology Letters.2024;[Epub] CrossRef - Mammary mucinous cystadenocarcinoma with long-term follow-up: molecular information and literature review
Ting Lei, Yong Qiang Shi, Tong Bing Chen
Diagnostic Pathology.2023;[Epub] CrossRef - Primary Mucinous Cystadenocarcinoma of the Breast Intermixed with Pleomorphic Invasive Lobular Carcinoma: The First Report of This Rare Association
Federica Vegni, Nicoletta D’Alessandris, Angela Santoro, Giuseppe Angelico, Giulia Scaglione, Angela Carlino, Damiano Arciuolo, Michele Valente, Stefania Sfregola, Maria Natale, Alejandro Martin Sanchez, Valeria Masciullo, Gian Franco Zannoni, Antonino Mu
Journal of Personalized Medicine.2023; 13(6): 948. CrossRef - Special Histologic Type and Rare Breast Tumors – Diagnostic Review and Clinico-Pathological Implications
Benjamin Yongcheng Tan, Elaine Hsuen Lim, Puay Hoon Tan
Surgical Pathology Clinics.2022; 15(1): 29. CrossRef - Mucinous cystadenocarcinoma of the breast: a new entity with broad differentials—a case report
Kanwalpreet Kaur, Ashini Shah, Jahnvi Gandhi, Priti Trivedi
Journal of the Egyptian National Cancer Institute.2022;[Epub] CrossRef - Mucinous carcinoma of the breast: distinctive histopathologic and genetic characteristics
Minjung Jung
Kosin Medical Journal.2022; 37(3): 176. CrossRef - Primary Mucinous Cystadenocarcinoma of the Breast: A Rare Case Report With Review of Literature
Ekta Jain, Abhishek Kumar, Raajul Jain, Shivani Sharma
International Journal of Surgical Pathology.2021; 29(7): 740. CrossRef - Mucinous Cystadenocarcinoma of the Breast: Report of 2 Cases Including One With Long-Term Local Recurrence
Anupma Nayak, Ira J. Bleiweiss, Kimberly Dumoff, Tawfiqul A. Bhuiya
International Journal of Surgical Pathology.2018; 26(8): 749. CrossRef - Mucinous breast carcinoma with tall columnar cells
N Tsoukalas, M Kiakou, M Tolia, ID Kostakis, M Galanopoulos, G Nakos, D Tryfonopoulos, G Kyrgias, G Koumakis
The Annals of The Royal College of Surgeons of England.2018; 100(5): e132. CrossRef - Radiologic Findings of Primary Mucinous Cystadenocarcinoma of the Breast: A Report of Two Cases and a Literature Review
Minjung Seong, Eun Young Ko, Boo-Kyung Han, Soo Youn Cho, Eun Yoon Cho, Se Kyung Lee, Jeong Eon Lee
Journal of Breast Cancer.2016; 19(3): 330. CrossRef - Primary Mucinous Cystadenocarcinoma of the Breast with Endocervical-Like Mucinous Epithelium
Dong-Liang Lin, Ji-Lin Hu, Shi-Hong Shao, Dong-Mei Sun, Ji-Gang Wang
Breast Care.2013; 8(6): 445. CrossRef
- BMP2 alterations in mucinous cystadenocarcinoma of the breast: insights from whole-exome sequencing
- Primary Thymic Mucinous Adenocarcinoma: A Case Report
- Jamshid Abdul-Ghafar, Suk-Joong Yong, Woocheol Kwon, Il Hwan Park, Soon-Hee Jung
- Korean J Pathol. 2012;46(4):377-381. Published online August 23, 2012
- DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.4.377
- 9,869 View
- 83 Download
- 21 Crossref
-
 Abstract
Abstract
 PDF
PDF Primary thymic mucinous adenocarcinoma is an extremely rare aggressive subtype of thymic carcinoma. With a review of literatures, only nine cases have been reported up to present. A 36-year-old woman was admitted for further evaluation and treatment of a mediastinal mass. The patient had no medical history of cancer. The clinicoradiological examination disclosed no tumor elsewhere. After the surgical excision of mediastinal mass, it was grossly a round semi-solid mass with mucin-filled cystic areas. Microscopically solid areas showed cords, small nests and dilated glands infiltrating the fibrotic parenchyma, while the cystic areas were lined by mucinous epithelium with tumor cells floating in extracellular-mucin pools. Some cystic walls underwent malignant transformation of the benign thymic epithelium. Immunohistochemically, the tumor cells were positive for cytokeratin (CK) 7, CK20, CD5, and CDX-2, and negative for thyroid transcription factor-1. In conclusion, the mucinous thymic adenocarcinoma should be recognized as a separate histopathological entity and considered in the differential diagnosis of mediastinal carcinomas.
-
Citations
Citations to this article as recorded by- Unresectable Primary Enteric‐Type Thymic Adenocarcinoma Treated With FOLFOX Chemotherapy: A Case Report
Carl He, Georgia Bentick, Patrick Hosking, Andrew Mant
Cancer Reports.2025;[Epub] CrossRef - Enteric thymic adenocarcinoma: Understanding a unique pathology in the mediastinum
Raja Chhabra, Kartik Mittal, Anmol Tufchi, Sajjan Rajpurohit, Deepak Kumar Mittal, Aditya Vidushi, Swati Saxena, Md Ali Osama
Indian Journal of Pathology and Microbiology.2025; 68(4): 820. CrossRef - Primary mucinous adenocarcinoma of the thymus
Kohei Soejima, Hidehito Matsuoka
The Journal of the Japanese Association for Chest Surgery.2024; 38(6): 545. CrossRef - Stage IV thymic mucinous adenocarcinoma under long-term disease control after primary tumor resection: A case report
Chihaya Maeda, Tomoyuki Hishida, Kyohei Masai, Keisuke Asakura, Katsura Emoto, Hisao Asamura
The Journal of the Japanese Association for Chest Surgery.2022; 36(2): 156. CrossRef - Lenvatinib-refractory thymic mucinous carcinoma with PIK3CA mutation
Akihiro Tsukaguchi, Shoichi Ihara, Hironao Yasuoka, Seigo Minami
International Cancer Conference Journal.2022; 12(1): 36. CrossRef - Thymic mucinous adenocarcinoma: A case report
Hideki Tsubouchi, Naoki Ozeki, Yuka Suzuki, Koji Kawaguchi, Takayuki Fukui, Toyofumi F. Chen-Yoshikawa
The Journal of the Japanese Association for Chest Surgery.2021; 35(5): 547. CrossRef - Metastatic thymic-enteric adenocarcinoma responding to chemoradiation plus anti-angiogenic therapy: A case report
Man Li, Xiao-Yu Pu, Li-Hua Dong, Peng-Yu Chang
World Journal of Clinical Cases.2021; 9(7): 1676. CrossRef - Primary Mucinous Adenocarcinoma of the Thymus: a Rare Type of Thymic Carcinoma—Case Report
Koichi Tomoshige, Tomoshi Tsuchiya, Keitaro Matsumoto, Takuro Miyazaki, Ryoichiro Doi, Ryusuke Machino, Satoshi Mizoguchi, Takamune Matumoto, Yutaka Maeda, Takeshi Nagayasu
SN Comprehensive Clinical Medicine.2021; 3(5): 1233. CrossRef - Primary mucinous adenocarcinoma of the thymus: A case report
Tomoka Hamahiro, Ryo Maeda, Takanori Ayabe, Yuichiro Sato, Masaki Tomita
Respiratory Medicine Case Reports.2021; 34: 101497. CrossRef - Primary Thymic Mucinous Adenocarcinoma: A Case Report Focusing on Radiological Findings and Review of the Literature
Young Hoon Koo, Jae Wook Lee, Jai Soung Park, Kyung Eun Shin, Heon Lee, Susie Chin
Iranian Journal of Radiology.2020;[Epub] CrossRef - Mucinous adenocarcinoma of the thymus: report of a case
Fumihiko Kinoshita, Fumihiro Shoji, Kazuki Takada, Gouji Toyokawa, Tatsuro Okamoto, Tokujiro Yano, Yoshinao Oda, Yoshihiko Maehara
General Thoracic and Cardiovascular Surgery.2018; 66(2): 111. CrossRef - Thymic enteric type adenocarcinoma: A case report with cytological features
Marie Tamai, Mitsuaki Ishida, Yusuke Ebisu, Hisashi Okamoto, Chika Miyasaka, Chisato Ohe, Yoshiko Uemura, Tomohito Saito, Tomohiro Murakawa, Koji Tsuta
Diagnostic Cytopathology.2018; 46(1): 92. CrossRef - Primary thymic adenocarcinomas: a clinicopathological and immunohistochemical study of 16 cases with emphasis on the morphological spectrum of differentiation
Neda Kalhor, Cesar A. Moran
Human Pathology.2018; 74: 73. CrossRef - Histologic characteristics of thymic adenocarcinomas: Clinicopathologic study of a nine-case series and a review of the literature
Ah-Young Kwon, Joungho Han, Jinah Chu, Yong Soo Choi, Byeong-Ho Jeong, Myung-Ju Ahn, Yong Chan Ahn
Pathology - Research and Practice.2017; 213(2): 106. CrossRef - Cytologic Characteristics of Thymic Adenocarcinoma with Enteric Differentiation: A Study of Four Fine-Needle Aspiration Specimens
Ah-Young Kwon, Joungho Han, Hae-yon Cho, Seokhwi Kim, Heejin Bang, Jiyeon Hyeon
Journal of Pathology and Translational Medicine.2017; 51(5): 509. CrossRef - Mucinous cystic tumor with CK20 and CDX2 expression of the thymus: Is this a benign counterpart of adenocarcinoma of the thymus, enteric type?
Jun Akiba, Hiroshi Harada, Shintaro Yokoyama, Toshihiro Hashiguchi, Akihiko Kawahara, Masahiro Mitsuoka, Shinzo Takamori, Hirohisa Yano
Pathology International.2016; 66(1): 29. CrossRef - Colon cancer chemotherapy for a patient with CDX2-expressing metastatic thymic adenocarcinoma: a case report and literature review
Akihiko Sawaki, Mikiya Ishihara, Yuji Kozuka, Hiroyasu Oda, Satoshi Tamaru, Yumiko Sugawara, Yoshiki Yamashita, Toshiro Mizuno, Taizo Shiraishi, Naoyuki Katayama
International Cancer Conference Journal.2016; 5(2): 113. CrossRef - Adenocarcinoma of the Thymus, Enteric Type
Bernhard Moser, Ana Iris Schiefer, Stefan Janik, Alexander Marx, Helmut Prosch, Wolfgang Pohl, Barbara Neudert, Anke Scharrer, Walter Klepetko, Leonhard Müllauer
American Journal of Surgical Pathology.2015; 39(4): 541. CrossRef - A Rare Case of Primary Thymic Adenocarcinoma Mimicking Small Cell Lung Cancer
Eun Na Cho, Hye Sung Park, Tae Hoon Kim, Min Kwang Byun, Hyung Jung Kim, Chul Min Ahn, Yoon Soo Chang
Tuberculosis and Respiratory Diseases.2015; 78(2): 112. CrossRef - A Rare Case of Primary Tubular Adenocarcinoma of the Thymus, Enteric Immunophenotype: A Case Study and Review of the Literature
Hae Yoen Jung, Hyundeuk Cho, Jin-Haeng Chung, Sang Byoung Bae, Ji-Hye Lee, Hyun Ju Lee, Si-Hyong Jang, Mee-Hye Oh
Journal of Pathology and Translational Medicine.2015; 49(4): 331. CrossRef - Primary mucinous adenocarcinoma of the thymus: a case report
Tadashi Sakane, Kotaro Mizuno, Risa Oda, Takuya Matsui, Makoto Ito, Takeshi Yamada
The Journal of the Japanese Association for Chest Surgery.2014; 28(7): 904. CrossRef
- Unresectable Primary Enteric‐Type Thymic Adenocarcinoma Treated With FOLFOX Chemotherapy: A Case Report
- Urachal Mucinous Tumor of Uncertain Malignant Potential: A Case Report
- Jung-Woo Choi, Ju-Han Lee, Young-Sik Kim
- Korean J Pathol. 2012;46(1):83-86. Published online February 23, 2012
- DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.1.83
- 11,044 View
- 63 Download
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF Urachal mucinous tumor of uncertain malignant potential is very rare and is characterized by a multilocular cyst showing the proliferation of atypical mucin-secreting cells without stromal invasion. As in ovarian and appendiceal borderline tumors, it represents a transitional stage of mucinous carcinogenesis in the urachus. In addition, this tumor may recur locally and develop into pseudomyxoma peritonei. Due to its scarcity and diagnostic challenges, we report a mucinous tumor of uncertain malignant potential arising in the urachus.
-
Citations
Citations to this article as recorded by- Exploring the Enigma of a Urachal Mucinous Cystic Tumor of Low Malignant Potential (MCTLMP): A Case Report and Literature Review
Klaas De Corte, Ali Ramadhan
Cureus.2025;[Epub] CrossRef - Mucinous Cystadenoma of the Urachus and Review of Current Classification of Urachal Mucinous Cystic Neoplasms
Diping Wang, Norbert Sule
Archives of Pathology & Laboratory Medicine.2019; 143(2): 258. CrossRef - Urachal Mucinous Cystic Tumor of Low Malignant Potential with Concurrent Sigmoid Colon Adenocarcinoma
Kelly Brennan, Paul Johnson, Heather Curtis, Thomas Arnason
Case Reports in Gastrointestinal Medicine.2019; 2019: 1. CrossRef - Pseudomyxoma Peritonei Arising from Mucinous Cystadenoma of the Urachus with Postoperative Disease-Free Survival over 15 Years
Tomoki Kobayashi, Shinichi Mizuno, Hideki Matsuba, Min Kanamori, Toshio Tamauchi, Makoto Urano
The Japanese Journal of Gastroenterological Surgery.2019; 52(6): 307. CrossRef - Urachal borderline mucinous cystadenoma
Jingjun Wu, Ailian Liu, Anliang Chen, Pengxin Zhang
Medicine.2017; 96(47): e8740. CrossRef - Incidental Finding of a Rare Urachal Pathology: Urachal Mucinous Cystic Tumour of Low Malignant Potential
Luke L. Wang, Heath Liddell, Sharman Tan Tanny, Briony Norris, Sree Appu, David Pan
Case Reports in Urology.2016; 2016: 1. CrossRef - A Case of Borderline Mucinous Cystadenoma Thought to be of Urachal Origin
Kiichiro YAGUCHI, Yoshihito GOMYO, Hiroyasu SAITO, Tatsuo IKENO, Hiromi SAKAGUCHI, Hideo MIYAMOTO
Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association).2014; 75(5): 1418. CrossRef - An unexpected mass of the urachus: a case report
Monica C. Pasternak, Jonathan D. Black, Natalia Buza, Masoud Azodi, Aileen Gariepy
American Journal of Obstetrics and Gynecology.2014; 211(4): e1. CrossRef
- Exploring the Enigma of a Urachal Mucinous Cystic Tumor of Low Malignant Potential (MCTLMP): A Case Report and Literature Review
- Simultaneous Pancreatic Serous Microcystic Adenoma and Intraductal Papillary Mucinous Tumor of the Pancreas: A Case Report.
- Hyoung Jong Kwak, Young Kon Kim, Baik Hwan Cho, Woo Sung Moon
- Korean J Pathol. 2011;45:S29-S31.
- DOI: https://doi.org/10.4132/KoreanJPathol.2011.45.S1.S29
- 3,531 View
- 24 Download
-
 Abstract
Abstract
 PDF
PDF - Serous cystadenomas of the pancreas account for approximately a third of pancreatic cystic neoplasms. Their coexistence with a second tumor is extremely rare. We now report a case of a serous microcystic adenoma combined with an intraductal papillary mucinous tumor of the pancreas in a 69-year-old man. Abdominal computed tomography scans demonstrated an incidental cystic mass in the body with cystic dilatation of the duct in the head of the pancreas. Central pancreatectomy with pancreatico-jejunostomy, and cyst excision of the pancreatic head were performed. Histologic examination demonstrated a serous microcystic cystadenoma in the body coexisting with an intraductal papillary mucinous adenoma in the head of the pancreas. This case study highlights the importance of careful intra-operative and pathologic examination for synchronous pancreatic tumors.
- Ovarian Large Cell Neuroendocrine Carcinoma Associated with Endocervical-like Mucinous Borderline Tumor: A Case Report and Literature Review.
- Jun Mo Kim, Hyeong Chan Shin, Mi Jin Kim
- Korean J Pathol. 2011;45(5):523-528.
- DOI: https://doi.org/10.4132/KoreanJPathol.2011.45.5.523
- 5,077 View
- 34 Download
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - Ovarian large cell neuroendocrine carcinoma is a rare tumor that is usually associated with surface epithelial tumors. Mucinous tumors are most common surface epithelial component identified in reported cases. Ovarian mucinous tumor associated with large cell neuroendocrine carcinoma is almost always an intestinal type. However, large cell neuroendocrine carcinoma associated with pure mucinous borderline tumor of endocervical-like type has not been described previously. The present case report describes a large cell neuroendocrine carcinoma associated with endocervical-like mucinous borderline tumor of the ovary in a 35-year-old woman. The tumor was confirmed by histopathology and immunohistochemistry. A review of the pertinent literature is included.
-
Citations
Citations to this article as recorded by- The puzzle of gynecologic neuroendocrine carcinomas: State of the art and future directions
Giuseppe Caruso, Carolina Maria Sassu, Federica Tomao, Violante Di Donato, Giorgia Perniola, Margherita Fischetti, Pierluigi Benedetti Panici, Innocenza Palaia
Critical Reviews in Oncology/Hematology.2021; 162: 103344. CrossRef - Pathological features, clinical presentations and prognostic factors of ovarian large cell neuroendocrine carcinoma: a case report and review of published literature
Xiaohang Yang, Junyu Chen, Ruiying Dong
Journal of Ovarian Research.2019;[Epub] CrossRef - Primary pure large cell neuroendocrine carcinoma of the ovary
Chen-Hsien Lin, Yu-Chieh Lin, Mu-Hsien Yu, Her-Young Su
Taiwanese Journal of Obstetrics and Gynecology.2014; 53(3): 413. CrossRef - Pure Large Cell Neuroendocrine Carcinoma of Ovary: A Rare Clinical Entity and Review of Literature
P. N. Shakuntala, K. Uma Devi, K. Shobha, U. D. Bafna, M. Geetashree
Case Reports in Oncological Medicine.2012; 2012: 1. CrossRef
- The puzzle of gynecologic neuroendocrine carcinomas: State of the art and future directions
- Cytologic Distinctive Features of Brenner Tumor.
- Jung Sik Jang, An Na Seo, Seon Jae Lee, Ji Young Park
- Korean J Pathol. 2011;45(2):223-226.
- DOI: https://doi.org/10.4132/KoreanJPathol.2011.45.2.223
- 4,703 View
- 33 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - Herein, we present two cases of Brenner tumor, a rarely occurring neoplasm in the ovaries, obtained via intraoperative fine needle aspiration. The borderline Brenner tumor exhibited marked squamous metaplasia, characterized by individually distributed atypical squamous cells. A benign Brenner tumor associated with mucinous cystadenoma evidenced typical mucinous metaplastic features and transitional foci. These distinctive features may prove helpful in differential diagnosis of varied ovarian tumors, and particularly for intraoperative consultation.
-
Citations
Citations to this article as recorded by- Pre‐operative cytodiagnosis of an adult granulosa cell tumour: report of a case with its differential diagnosis
S. R. Jinkala, S. E. Jacob, S. Neelaiah, B. A. Badhe
Cytopathology.2014; 25(1): 63. CrossRef
- Pre‐operative cytodiagnosis of an adult granulosa cell tumour: report of a case with its differential diagnosis
- A Case of Ovarian Microinvasive Mucinous Carcinoma and Co-existent Angiosarcoma.
- Jin Hyung Heo, Yoon Hee Lee, Gwang Il Kim, Tae Heon Kim, Haeyoun Kang, Hee Jung An, Bo Sung Yoon, Seok Ju Seong, Hyun Park, Ji Young Kim
- Korean J Pathol. 2011;45(1):96-100.
- DOI: https://doi.org/10.4132/KoreanJPathol.2011.45.1.96
- 4,043 View
- 24 Download
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF - Primary ovarian angiosarcoma is very rare with only 27 cases reported so far in the medical literature. We report here on a rare case of ovarian microinvasive mucinous carcinoma that was coexistent with angiosarcoma in a 54-year-old woman. The tumor was a 26x19x10 cm-sized multilocular cystic mass with a 4x3 cm-sized solid hematoma-like nodule in the center. Microscopically, it was composed mostly of mucinous tumor of various grades from borderline to microinvasive carcinoma. The hematoma-like area turned out to be an angiosarcoma, composed of pleomorphic cells that formed slit-like spaces, spindle cells that formed short fascicles and anastomosing vascular channels with atypical endothelial cells. All these cells were positive for CD31, CD34 and factor VIII-related antigen. The patient developed peritoneal and pleural metastases, which were angiosarcoma and mucinous carcinoma, respectively. We believe this case is only the fourth example of an ovarian collision tumor of angiosarcoma and surface epithelial tumor.
-
Citations
Citations to this article as recorded by- Ovarian angiosarcoma: A systematic review of literature and survival analysis
Shafi Rehman, Arya Harikrishna, Amisha Silwal, B.R. Sumie, Safdar Mohamed, Nisha Kolhe, Meghana Maddi, Linh Huynh, Jesus Gutierrez, Yoshita Rao Annepu, Ameer Mustafa Farrukh
Annals of Diagnostic Pathology.2024; 73: 152331. CrossRef - Tumor to Tumor Metastasis: A Case Report of Metastatic Angiosarcoma to an Ovarian Brenner Tumor and Review of the Literature
Bilge Dundar, Audai Alrwashdeh, Laila Dahmoush
International Journal of Gynecological Pathology.2023; 42(2): 176. CrossRef - Collision Tumors in Ovary: Case Series and Literature Review
Borges A, Loddo A, Martins A, Peiretti M, Fanni D, Djokovic D
Journal of Surgical Oncology.2019; : 1. CrossRef - Angiosarcoma Arising in Ovarian Mucinous Tumor: A Challenge in Intraoperative Frozen Section Diagnosis
Surapan Khunamornpong, Jongkolnee Settakorn, Kornkanok Sukpan, Tip Pongsuvareeyakul, Sumalee Siriaunkgul
Case Reports in Pathology.2016; 2016: 1. CrossRef - Impact of body burden of pesticide residues on the reproductive tract of buffalo
KARANPREET KAUR, SARVPREET SINGH GHUMAN, OPINDER SINGH, JASBIR SINGH BEDI, JATINDER PAUL SINGH GILL
The Indian Journal of Animal Sciences.2016;[Epub] CrossRef
- Ovarian angiosarcoma: A systematic review of literature and survival analysis
- Molecular Biological Characteristics of Differentiated Early Gastric Cancer on the Basis of Mucin Expression.
- Nari Shin, Hye Yeon Kim, Woo Kyung Kim, Min Gyung Park, Kyung Bin Kim, Dong Hoon Shin, Kyung Un Choi, Jee Yeon Kim, Chang Hun Lee, Gi Young Huh, Mee Young Sol, Do Youn Park
- Korean J Pathol. 2011;45(1):69-78.
- DOI: https://doi.org/10.4132/KoreanJPathol.2011.45.1.69
- 4,721 View
- 23 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
It is clear that the biologic characteristics of gastric cancer are different on the basis of mucin phenotypes. However, there are unabated controversies on the exact biologic differences of mucin expression in gastric cancer.
METHODS
We analyzed various protein expressions and microsatellite instability (MSI) status based on mucin expression in 130 differentiated early gastric adenocarcinoma cases. Furthermore, we evaluated the genomic alternation in 10 selected differentiated early gastric adenocarcinoma cases using array based comparative genomic hybridization (aCGH).
RESULTS
Intestinal mucin predominant subtype showed significantly elevated p53 protein and caudal-related homeobox 2 expression, and delocalization of beta catenin expressions compared to the gastric mucin predominant subtype. On MSI status, the gastric mucin predominant subtype more frequently showed unstable status than the intestinal mucin predominant subtype. CGH study showed more frequent chromosomal gain and loss in the intestinal mucin predominant subtype than the gastric mucin predominant subtype, albeit without statistical significance. Interestingly, there were significant differences in chromosomal alternation between four mucin phenotypes.
CONCLUSIONS
Study results suggest possible different points of biologic behaviors in early differentiated gastric adenocarcinomas by mucin expression type. -
Citations
Citations to this article as recorded by- Mucin Expression in Gastric Cancer: Reappraisal of Its Clinicopathologic and Prognostic Significance
Dae Hwan Kim, Nari Shin, Gwang Ha Kim, Geum Am Song, Tae-Yong Jeon, Dong-Heon Kim, Gregory Y. Lauwers, Do Youn Park
Archives of Pathology & Laboratory Medicine.2013; 137(8): 1047. CrossRef - Microsatellite Instability Status in Gastric Cancer: A Reappraisal of Its Clinical Significance and Relationship with Mucin Phenotypes
Joo-Yeun Kim, Na Ri Shin, Ahrong Kim, Hyun-Jeong Lee, Won-young Park, Jee-Yeon Kim, Chang-Hun Lee, Gi-Young Huh, Do Youn Park
Korean Journal of Pathology.2013; 47(1): 28. CrossRef
- Mucin Expression in Gastric Cancer: Reappraisal of Its Clinicopathologic and Prognostic Significance
- Clinical Outcome of Surgically Resected Pancreatic Intraductal Papillary Mucinous Neoplasm According to the Marginal Status: A Single Center Experience.
- Sun A Kim, Eunsil Yu, Song Cheol Kim, Jihun Kim
- Korean J Pathol. 2010;44(4):410-419.
- DOI: https://doi.org/10.4132/KoreanJPathol.2010.44.4.410
- 4,392 View
- 26 Download
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Surgical resection is the treatment of choice of intraductal papillary mucinous neoplasm (IPMN) of the pancreas. However, the benefit of clearing resection margin is still controversial.
METHODS
We reviewed 281 surgically resected cases of IPMN. The recurrences were compared according to the histologic grade (benign or borderline IPMN, malignant noninvasive IPMN, invasive carcinoma) and size (pancreatic intraepithelial neoplasia, PanIN, less than 0.5 cm in the long axis; and IPMN, greater than or equal to 0.5 cm) of the residual lesions at the resection margin.
RESULTS
Sixty cases (21.4%) were invasive carcinoma, and 221 (78.6%) noninvasive cases included 87 (31.0%) benign, 107 (38.1%) borderline and 11 (3.9%) malignant noninvasive IPMN cases. In noninvasive IPMN, increased recurrence in patients with five or more years of follow-up was only related to the involvement of resection margin by severe dysplasia. The recurrence of invasive carcinoma was high (27.3%) even when the resection margin was clear, and was not related to the grade or size of residual tumors at the resection margin.
CONCLUSIONS
Invasiveness is a strong risk factor for recurrence in IPMN regardless of the status of the resection margin. However, in noninvasive IPMN, histologic grading of residual lesions at the resection margin predicts local recurrence. -
Citations
Citations to this article as recorded by- Systematic review of challenging issues in pathology of intraductal papillary mucinous neoplasms
Laura D. Wood, N. Volkan Adsay, Olca Basturk, Lodewijk A.A. Brosens, Noriyoshi Fukushima, Seung-Mo Hong, Sung-Joo Kim, Jae W. Lee, Claudio Luchini, Michaël Noë, Martha B. Pitman, Aldo Scarpa, Aatur D. Singhi, Mariko Tanaka, Toru Furukawa
Pancreatology.2023; 23(7): 878. CrossRef - The Use of Intraoperative Frozen Sections in Guiding the Extent of Pancreatic Resections for Intraductal Papillary Mucinous Neoplasms
Zhikai Chi, Deepti Dhall, Richard Mertens
Pancreas.2022; 51(1): 63. CrossRef - Recurrence of non-invasive intraductal papillary municious neoplasm seven years following total pancreatectomy
Nayima M. Clermont Dejean, Sinziana Dumitra, Jeffrey S. Barkun
International Journal of Surgery Case Reports.2013; 4(9): 789. CrossRef
- Systematic review of challenging issues in pathology of intraductal papillary mucinous neoplasms
- Expression of MUC1 and MUC4 and Its Prognostic Significance in Non-Small Cell Lung Carcinoma.
- Ji Min Jeon, Hye Won Lee, Ji Young Park, Hye Ra Jung, Ilseon Hwang, Sun Young Kwon, Mi Sun Choe, Yu Na Kang, Sang Pyo Kim, Sang Sook Lee, Won Il Choi, Kun Young Kwon
- Korean J Pathol. 2010;44(4):397-403.
- DOI: https://doi.org/10.4132/KoreanJPathol.2010.44.4.397
- 5,496 View
- 60 Download
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Mucin (MUC)1 and MUC4 (MUC1, 4) are high molecular weight glycoproteins expressed in normal and malignant epithelial cells, and these expressions are related to the prognosis of some carcinomas. In non-small cell lung carcinoma (NSCLC), the relationship between MUC1, 4 expressions and their prognostic significance is not well known. We evaluated these relationships in a series of NSCLC: 1) between MUC1, 4 expression levels and histologic subtypes, and 2) between high expression of MUC1, 4 and their prognostic significance.
METHODS
We performed immunohistochemical staining for MUC1, 4 in paraffin-embedded tissues from 165 NSCLC cases arranged in a tissue microarray.
RESULTS
We found a significant correlation between MUC1, 4 expressions and NSCLC histologic subtypes (p < 0.05). High MUC1 expression was characteristic of adenocarcinoma. Low MUC1, 4 expressions were characteristic of squamous cell carcinoma. In adenocarcinoma, we found significant association between diffuse MUC1 expression and short patient survival (p = 0.005). In squamous cell carcinoma, diffuse MUC4 expression showed long patient survival trend (p = 0.128).
CONCLUSIONS
MUC1, 4 expression levels were significantly correlated with NSCLC histologic subtypes. Diffuse MUC1 expression was significantly associated with shortened survival in NSCLC patients, especially in adenocarcinoma. -
Citations
Citations to this article as recorded by- Inhibition of MUC1-C Increases ROS and Cell Death in Mouse Embryonic Stem Cells
Jeong-A Park, Sangkyu Park, Jun-Kyu Choi, Myung-Kwan Han, Younghee Lee
International Journal of Stem Cells.2020;[Epub] CrossRef - Assessing the prognostic significance of MUC4β in mucoepidermoid carcinoma of the salivary glands: An immunohistochemical study
Poonam R. Sawant, Anita Spadigam, Anita Dhupar, Shaheen Syed, Karla Carvalho
Heliyon.2019; 5(11): e02753. CrossRef - Ultrasensitive cytosensing based on an aptamer modified nanobiosensor with a bioconjugate: Detection of human non-small-cell lung cancer cells
Tanveer A. Mir, Jang-Hee Yoon, N.G. Gurudatt, Mi-Sook Won, Yoon-Bo Shim
Biosensors and Bioelectronics.2015; 74: 594. CrossRef - Expression of MUC1 and MUC4 in Gallbladder Adenocarcinoma
Su-Mi Kim, Sun-Ju Oh, Bang Hur
Korean Journal of Pathology.2012; 46(5): 429. CrossRef - MUC4 and MUC1 Expression in Adenocarcinoma of the Stomach Correlates with Vessel Invasion and Lymph Node Metastasis: An Immunohistochemical Study of Early Gastric Cancer
Yukihiro Tamura, Michiyo Higashi, Sho Kitamoto, Seiya Yokoyama, Masahiko Osako, Michiko Horinouchi, Takeshi Shimizu, Mineo Tabata, Surinder K. Batra, Masamichi Goto, Suguru Yonezawa, Fazlul H. Sarkar
PLoS ONE.2012; 7(11): e49251. CrossRef - Prognostic significance of membrane-associated mucins 1 and 4 in gastric adenocarcinoma
ILSEON HWANG, YU NA KANG, JIN YOUNG KIM, YOUNG ROK DO, HONG SUK SONG, KEON UK PARK
Experimental and Therapeutic Medicine.2012; 4(2): 311. CrossRef
- Inhibition of MUC1-C Increases ROS and Cell Death in Mouse Embryonic Stem Cells
- A Case of Endocrine Mucin-Producing Sweat Gland Carcinoma Co-existing with Mucinous Carcinoma: A Case Report.
- Sunhee Chang, Sang Hwa Shim, Mee Joo, Hanseong Kim, Yong Kyu Kim
- Korean J Pathol. 2010;44(1):97-100.
- DOI: https://doi.org/10.4132/KoreanJPathol.2010.44.1.97
- 5,534 View
- 51 Download
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF - An endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare skin tumor that most commonly occurs on the eyelids of elderly women. This tumor is morphologically analogous to endocrine ductal carcinoma in situ and solid papillary carcinoma of the breast. We describe one case of a 51-year-old male with an EMPSGC co-existing with mucinous carcinoma of the eyelid. The tumor was composed of dilated ducts with a smooth border and was partially filled with a papillary proliferation. Tumor cells were uniform, small-to-medium in size, and oval-to-polygonal with light eosinophilic cytoplasm. Nuclei were bland with diffusely stippled chromatin and inconspicuous nucleoli. Tumor cells expressed chromogranin, synaptophysin, estrogen and progesterone receptors, cytokeratin 7, and epithelial membrane antigen.
-
Citations
Citations to this article as recorded by- A Case of Endocrine Mucin-Producing Sweat Gland Carcinoma of the Eyelid
Ji Eon Kang, Sung Eun Kim, Suk-Woo Yang
Journal of the Korean Ophthalmological Society.2023; 64(2): 149. CrossRef - Endocrine mucin-producing sweat gland carcinoma: a systematic review and meta-analysis
Michael H. Froehlich, Keith R. Conti, Ivy I. Norris, Jordan J. Allensworth, Nicole A. Ufkes, Shaun A. Nguyen, Evelyn T. Bruner, Joel Cook, Terry A. Day
Journal of Dermatological Treatment.2022; 33(4): 2182. CrossRef - Next-generation sequencing analysis suggests varied multistep mutational pathogenesis for endocrine mucin-producing sweat gland carcinoma with comments on INSM1 and MUC2 suggesting a conjunctival origin
Joseph G. Mathew, Anita S. Bowman, Jad Saab, Klaus J. Busam, Kishwer Nehal, Melissa Pulitzer
Journal of the American Academy of Dermatology.2022; 86(5): 1072. CrossRef - Endocrine mucin‐producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: Review of the literature
Rebecca Tian Mei Au, Manish M. Bundele
Journal of Cutaneous Pathology.2021; 48(9): 1156. CrossRef - An Update on Endocrine Mucin-producing Sweat Gland Carcinoma
Meghana Agni, Meisha L. Raven, Randy C. Bowen, Nora V. Laver, Patricia Chevez-Barrios, Tatyana Milman, Charles G. Eberhart, Steven Couch, Daniel D. Bennett, Daniel M. Albert, R. Nick Hogan, Paul O. Phelps, Hillary Stiefel, Norberto Mancera, Martin Hyrcza,
American Journal of Surgical Pathology.2020; 44(8): 1005. CrossRef - A Case of Endocrine Mucin-Producing Sweat Gland Carcinoma: Is it Still an Under-Recognized Entity?
Khaled A. Murshed, Mohamed Ben-Gashir
Case Reports in Dermatology.2020; 12(3): 255. CrossRef - Endocrine Mucin-Producing Sweat Gland Carcinoma, a Histological Challenge
Mary Anne Brett, Samih Salama, Gabriella Gohla, Salem Alowami
Case Reports in Pathology.2017; 2017: 1. CrossRef - Endocrine mucin‐producing sweat gland carcinoma occurring on extra‐facial site: a case report
Jia‐Huei Tsai, Tzu‐Lin Hsiao, Yi‐Ying Chen, Cheng‐Hsiang Hsiao, Jau‐Yu Liau
Journal of Cutaneous Pathology.2014; 41(6): 544. CrossRef - Endocrine Mucin-Producing Sweat Gland Carcinoma
Catharine A. Dhaliwal, Antonia Torgersen, Jonathan J. Ross, James W. Ironside, Asok Biswas
The American Journal of Dermatopathology.2013; 35(1): 117. CrossRef
- A Case of Endocrine Mucin-Producing Sweat Gland Carcinoma of the Eyelid
- Intraductal Papillary Mucinous Tumor Simultaneously Involving the Liver and Pancreas: A Case Report.
- Bong Hee Park, Jae Hee Suh, Hee Jeong Cha, Young Min Kim, Hye Jeong Choi
- Korean J Pathol. 2010;44(1):83-86.
- DOI: https://doi.org/10.4132/KoreanJPathol.2010.44.1.83
- 4,183 View
- 31 Download
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - We describe here a 67-year-old man who was diagnosed with a rare case of intraductal papillary mucinous tumors that occurred simultaneously in the liver and pancreas. Abdominal computed tomography showed a tubular and cystic dilatation of the pancreatic duct in the pancreas tail, which suggested an intraductal papillary mucinous tumor (IPMT), and multiple intrahepatic duct stones. The patient underwent a distal pancreatectomy with splenectomy and a lateral segmentectomy of the liver. Microscopic examination showed an intraductal papillary mucinous neoplasms of borderline malignancy in the pancreas and a non-invasive intraductal papillary mucinous tumor with moderate dysplasia of the bile duct. Although several cases of intraductal papillary mucinous neoplasm of the liver (IPNL) without any pancreatic association have been described, the simultaneous presentation of both IPMT of the pancreas and IPNL is very rare. The patient has been doing well for 10 months postoperatively.
-
Citations
Citations to this article as recorded by- Surgical resection for simultaneous intraductal papillary mucinous neoplasm of the bile duct and pancreatic duct: A case report
Xiao-Rui Huang, Deng-Sheng Zhu, Ya-Hong Yu
World Journal of Gastrointestinal Surgery.2025;[Epub] CrossRef - Reoperation for heterochronic intraductal papillary mucinous neoplasm of the pancreas after bile duct neoplasm resection: A case report
Gang Xiao, Tao Xia, Yi-Ping Mou, Yu-Cheng Zhou
World Journal of Gastrointestinal Surgery.2023; 15(7): 1542. CrossRef - Intraductal papillary neoplasm of the bile duct: The new frontier of biliary pathology
Federico Mocchegiani, Paolo Vincenzi, Grazia Conte, Daniele Nicolini, Roberta Rossi, Andrea Benedetti Cacciaguerra, Marco Vivarelli
World Journal of Gastroenterology.2023; 29(38): 5361. CrossRef - Multicentric recurrence of intraductal papillary neoplasm of bile duct after spontaneous detachment of primary tumor: A case report
Hiroki Fukuya, Akifumi Kuwano, Shigehiro Nagasawa, Yusuke Morita, Kosuke Tanaka, Masayoshi Yada, Akihide Masumoto, Kenta Motomura
World Journal of Clinical Cases.2022; 10(3): 1000. CrossRef - Co-occurrence of IPMN and malignant IPNB complicated by a pancreatobiliary fistula: A case report and review of the literature
Xu Ren, Chun-Lan Zhu, Xu-Fu Qin, Hong Jiang, Tian Xia, Yong-Ping Qu
World Journal of Clinical Cases.2019; 7(1): 102. CrossRef - Synchronous pancreatic adenocarcinoma and intrahepatic cholangiocarcinoma arising in the context of intraductal papillary neoplasms
Anmol Bansal, Swan N. Thung, Hongfa Zhu, Myron Schwartz, Sara Lewis
Clinical Imaging.2016; 40(5): 897. CrossRef
- Surgical resection for simultaneous intraductal papillary mucinous neoplasm of the bile duct and pancreatic duct: A case report
- Retroperitoneal Mucinous Tumor: Report of two Cases.
- Eun Kyung Kim, Seong Ran Hong, Hy Sook Kim, Young Hyeh Ko, Jung Dal Lee
- Korean J Pathol. 1992;26(6):632-634.
- 1,997 View
- 15 Download
-
 Abstract
Abstract
 PDF
PDF - Primary retroperitoneal mucinous tumor is very rare. In the worldwide literature, only 14 cases have been reported. Herein, we report two cases of retroperitoneal mucinous tumor found at laparotomy. The patients were females, aged 24 and 21 years, respectively. They had bilateral normal ovaries. Histologically, one was mucinous cystadenoma and the other showed borderline histology. The connective tissue wall resembling ovarian stroma was noted in one case. The pathogenesis was discussed.
- Minimal Deviation Adenocarcinoma, Mucinous Type, of the Uterine Cervix: Report of a Case with Extensive Metastasis to the Uterine Corpus and Bilateral Adnexae.
- Eundeok Chang, Eunjung Lee, Kyoungmee Kim, Okran Shin, Youngmi Ku, Heejung An, Changsuk Kang
- Korean J Pathol. 2004;38(2):121-125.
- 2,040 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - Minimal deviation adenocarcinoma is an extremely well differentiated variant of cervical adenocarcinoma, and is frequently misdiagnosed due to its benign-looking histopathological features. A 38-year-old woman was diagnosed as having had a minimal deviation adenocarcinoma in the cervix, metastasizing to the uterine body and bilateral adnexae. She had a history of right salpingo-oophorectomy 3 years ago, and was diagnosed as having a mucinous cystadenoma. Histologically, the tumor cells were so well-differentiated that they appeared to be almost the same as those of the non-neoplastic cervical glands. Similar glands were found in both ovaries and in the left fallopian tube. PAS staining showed a negative or apical positive pattern in the endocervical-like glands. Immunohistochemical studies for CEA, ER/PR, cytokeratin 20, and p53 were negative, but positive for cytokeratin 7. The HPV DNA microarray test was negative. Clinically, this proved to be an advanced, biologically aggressive disease.
- Genetic Expression Pattern of Gastric Carcinomas According to Cellular Mucin Phenotypes.
- Won Ae Lee, In Soo Suh, Ying Hua Li, Ji Hyun Eum, Wan Sik Yu, Han Ik Bae
- Korean J Pathol. 2007;41(5):307-315.
- 2,151 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Gastric carcinomas (GCs) have recently been reclassified according to the mucin phenotypes. We aimed to characterize the relationship between the mucin phenotypes and the genetic alterations or the clinicopathologic parameters of GCs.
METHODS
Immunohistochemistry was performed for MUC1, MUC5AC, MUC6, MUC2, CD10, p53, hMLH1, CerbB2 and E-cadherin in 150 GCs. The mucin phenotypes of the GCs were classified as 4 phenotypes: gastric, intestinal, mixed and unclassified.
RESULTS
MUC1, MUC5AC, MUC6, MUC2 and CD10 were expressed in 63.3%, 42.7%, 14.0%, 24.7% and 14.0% of the GCs, respectively. The mucin phenotypes of the GCs corresponded to the gastric type in 31.3%, the intestinal type in 20.0%, the mixed type in 15.3% and the unclassified type in 33.3%. The incidence of a p53 overexpression was higher in the gastric or mixed phenotype than in the intestinal or unclassified phenotype. MUC5AC expression, p53 overexpression and the gastric or mixed phenotype were associated with poor patient survival by multivariate analysis.
CONCLUSION
This study suggests the gastric or mixed mucin phenotype may more likely go through the p53 pathway in carcinogenesis and the mucin phenotype may be considered as a prognostic indicator.
- Differential Diagnosis of Ovarian Mucinous, Serous, and Endometrioid Adenocarcinoma in Peritoneal Washing Cytology .
- Shi Nae Lee, In Ae Park
- J Pathol Transl Med. 2000;11(2):83-88.
- 2,989 View
- 77 Download
-
 Abstract
Abstract
 PDF
PDF - This study presents the cytologic features of peritoneal washings, with particular emphasis on the cytologic discrimination among serous, mucinous, and endometrioid adenocarcinoma of the ovary. We selected histologically confirmed 27 cases of peritoneal washing : 8 cases of serous cystadenocarcinomas, 5 cases of mucinous cystadenocarcinomas, and 14 cases of endometrioid adenocarcinomas. The most frequent cytologic pattern of three tumors was clusters. Ball pattern was found in serous cystadenocarcinoma(36%) and acinar pattern in endometrioid adenocarcinoma (36%). Mucinous adenocarcinoma showed mucoid background(100%) and endometrioid adenocarcinoma revealed inflammatory background(43%). The cytoplasmic vacuoles were noted in 80%, 13%, and 43% of mucinous, serous, and endometrioid adenocarcinoma, respectively. The endometrioid adenocarcinoma showed prominent nucleoli(64%). In conclusion, the cytologic findings of mucinous cystadenocarcinoma were different from that of serous and endometrioid carcinomas, such as mucoid background, abundant cytoplasm with vacuolated cytoplasm, and peripherally located cytoplasm. Although endometrioid carcinoma showed acinar pattern and prominent nucleoli, the differential diagnosis between serous cystadenocarcinoma and endometrioid adenocarcinoma in peritoneal washing cytology was not always possible.
- Mucinous Adenocarcinoma in a Horseshoe Kidney.
- Man Hoon Han, Sang Chul Nam, Bup Wan Kim, Ghil Suk Yoon
- Korean J Pathol. 2008;42(1):60-62.
- 1,939 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - We report here on a case of mucinous adenocarcinoma that probably originated in the renal pelvis of a horseshoe kidney. A 61-year-old woman presented with a palpable mass in the left upper quadrant of the abdomen, and this mass had been present for several months. Computed tomography (CT) revealed a left renal pelvic tumor in the horseshoe kidney. Grossly, a 10x9x8 cm unilocular cystic mass filled with chocolate colored mucinous fluid was seen. A connection between the cystic mass and the renal pelvis was demonstrated on retrograde pyelography. Microscopically, the cyst contained anaplastic columnar mucosecretory epithelial cells. Some atypical cell clusters were freely floating in the mucinous lakes. The histopathological findings were consistent with mucinous adenocarcinoma. In addition, glandular metaplasia was noted in the cystic wall. Immunohistochemical assessment of the pelvic adenocarcinoma revealed the positive expressions of carcinoembryonic antigen (CEA) and cytokeratin 20 (CK20) and a weak positive expression of cytokeratin 7 (CK7).
- Ultrastructural Changes in Human Gallbladder Epithelium in Acalculous and Calculous Cholecystitis.
- Sung Chul Lim, Chae Hong Suh
- Korean J Pathol. 1995;29(6):714-726.
- 2,025 View
- 15 Download
-
 Abstract
Abstract
 PDF
PDF - Cholelithiasis is defined as the presence of stones within the lumen of the gallbladder or in the extrahepatic biliary tree. Cholecystitis, secondary to gallstones, is a common surgical disorder in Korea. Detailed microscopic descriptions, particularly the ultrastructural changes, of these diseases were not available. The goal of this study was to identify the ultrastructural alterations of gallbladder epithelium in cholecystitis with and without a stone, according to the degree of severity of inflammation, and the nature of the stone. The gallbladders of the control group, and cholecystitis cases without stone and with stone were fixed and examined with routine stain, special stain, immunohistochemical stain and trans-mission electron microscopy. The number and the volume density of the mucin containing secretary granules were not significantly increased in the calculous cholecystitis cases compared with those of the acalculous cholecystitis cases. Major findings were that the calculous cholecystitis cases showed a markedly reduced total lysosome area and volume density of the lysosome compared with those of the acalculous chole-cystitis cases. The differences between the mucin secretary granules and lysosomes, according to the degree of severity of cholecystitis and the natures of gallstones, were statistically not significant.
- Metastatic Adenocarcinoma with Mucin Emboli in the Lung: A case report.
- Chung Yeul Kim, Kwang Il Kim, Sung Hwan Park, Eung Suk Lee, Han Kygum Kim
- Korean J Pathol. 1999;33(6):450-452.
- 2,018 View
- 12 Download
-
 Abstract
Abstract
 PDF
PDF - Lung is known as a vulnerable organ to metastatic tumors. Metastasis occurs mainly through lymphatics but seldom via blood vessels. Adenocarcinoma with mucin emboli is very rare. Primary foci reported in the literature were breast, lung, ovary and pancreas. A lung biopsy from a 60-year-old male patient showed floating mucin associated with metastatic adenocarcinoma to the lung in the blood vessels. The tumor cells spreaded along the vascular endothelium as if they were vascular endothelial cells. The tumor cells and mucin in the vessel were strong positive for PAS staining. Elastic and immunohistochemical staining for smooth muscle actin highlighted the vascular wall in the mucin containing structure with infarction of the lung. The primary focus was identified in the pancreatic head through the abdominal ultra-sonographic and computer-tomographic examination.
- Oncocytic Type Intraductal Papillary Mucinous Neoplasm Mimicking Mucinous Cystic Neoplasm of the Pancreas: A Case Report.
- Tae Jung Jang, Jong Im Lee
- Korean J Pathol. 2008;42(3):172-174.
- 2,300 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - Oncocytic type intraductal papillary mucinous neoplasm is a newly defined subgroup of intraductal papillary mucinous neoplasms. A 35-year-old woman presented complaining of epigastric pain for one month. Enhanced computed tomography revealed a multilocular cystic mass in the distal body and tail of the pancreas. Endoscopic retrograde cholangiopancreatography showed no communication between the mass and the main pancreatic duct. The patient underwent a distal pancreatectomy and splenectomy. Microscopical examination showed a fibrous cyst wall; polypoid tumors exhibiting thin stalks, with extensive arborizing papillary growths from these stalks; and a focally cribriform pattern, lined by plump cells with abundant eosinophilic and granular cytoplasm. Red granules were detected in the cytoplasm of tumor cells on modified Gomori trichrome stain. Ultrastructurally, the tumor cells contained abundant cytoplasm packed with numerous mitochondria and intracellular and intercellular lumina. We describe an oncocytic type intraductal papillary mucinous neoplasm having the clinical characteristics of a mucinous cystic neoplasm.
- Fine Needle Aspiration Cytology of the Intraductal Papillary Mucinous Tumor of the Pancreas: A Case Report.
- In Gu Do, Jae Hoon Park, Youn Wha Kim, Ju Hie Lee, Moon Ho Yang, Sung Wha Hong, Yong Koo Park
- J Pathol Transl Med. 2003;14(2):91-95.
- 1,922 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - Intraductal papillary mucinous tumor of the pancreas is characterized by intraductal papillary proliferation of mucin-producing epithelial cells with or without excessive mucin secretion. According to the degree of epithelial dysplasia, intraductal papillary mucinous tumor is classified into adenoma, borderline tumor, and carcinoma. We recently experienced a case of fine needle aspiration cytology of the intraductal papillary mucinous adenoma in a 69-year-old male. The fine needle aspiration cytology yielded flat sheets of columnar, mucin containing epithelial cells in the background of dense mucin containing degenerated cellular material and histiocytes.
- Combined Mucinous Tumor and Carcinoid of Appendix Associated with Mucinous Tumor of Ovary and Pseudomyxoma Peritonei: A case report .
- Hye Jeong Choi, Mi Jin Kim
- Korean J Pathol. 1999;33(11):1094-1096.
- 2,206 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF - We report a case of mucinous cystadenoma of uncertain malignant potential and carcinoid of appendix associated with bilateral mucinous cystadenoma of ovary and pseudomyxoma peritonei. The patient was a 46-year-old female. She suffered from dyspnea and lower abdominal palpable masses for several months. Ultrasonogram showed multilocular huge ovarian cysts. Appendectomy, transabdominal hysterectomy, bilateral salphingo-oophorectomy and biopsy of omentum were performed. The bilateral ovaries measured 16 11 cm and 7X5 cm in size, respectively. The both ovaries showed multilocular cysts filled with thick mucus material. The ovarian cysts were covered by a single layer of columnar epithelium with focal proliferation. Mucus materials dissected through the ovarian stroma (pseudomyxoma ovarii). The tip of appendix was dilated and covered by mucus material. The cut surface showed a cyst and a yellowish solid mass proximal to the cyst. Microscopically, the appendiceal cyst was lined by stratified columnar epithelium with moderate cytologic atypia. Mucus material dissected through the wall. In the proximal portion of the appendix, a classic carcinoid with focal tubular form was present in submucosa and muscle layer. The omentum was covered by thick mucus material. Microscopically, the omentum showed mucinous epithelium and mucus material (pseudomyxoma peritonei).
- Ovarian Borderline Epithelial Tumors.
- Geunghwan Ahn
- Korean J Pathol. 2005;39(5):291-300.
- 2,377 View
- 27 Download
-
 Abstract
Abstract
 PDF
PDF - Ovarian borderline epithelial tumors are abnormal proliferative epithelial lesions without obvious invasion of the stroma of the ovary, a finding distinguishing between borderline tumors and carcinoma. There have been controversies regarding the terminology and diagnostic feature of the tumors, even though these tumors have been accepted as a distinct entity in WHO classification of ovarian epithelial tumors. This review is limited to serous and mucinous borderline tumors which are the most common and about which many clinicopathological studies have been undertaken. It has been agreed that "micropapillary carcinoma" espoused by a group of pathologists is a micropapillary variant of serous borderline tumor in the borderline ovarian tumor workshop. Diagnostic criteria of invasive implants needs further study but invasion of underlying normal tissue was reported to be correlated well with prognosis. Other issues such as diagnostic criteria of microinvasion and multiplicity of serous borderline tumors have been presented. The sole diagnostic criteria agreed upon for the diagnosis of intraepithelial carcinoma in the mucinous borderline tumor was the presence of severe cytological atypia. It was also agreed that the ovarian tumors associated with pseudomyxoma peritonei are almost invariably from gastrointestinal tract, usually appendix. Stratification and complex intracystic growth without severe cytological atypia are considered to be characteristics of mucinous borderline tumors. Diagnostic criteria of microinvasion and two types of invasion, expansile and infiltrative invasion, have also been discussed.
- Morphometric Study on Mucinous Tumors of the Ovary.
- Joo Seub Keum, Jung Dal Lee
- Korean J Pathol. 1991;25(4):305-317.
- 1,800 View
- 12 Download
-
 Abstract
Abstract
 PDF
PDF - Mucinous tumors of the ovary are the most common tumors arising from the common epithelium of the ovary in Korean. Distinguishing the tumor with borderline malignancy from mucinous cystadenocarcinoma is very important in determining proper therapeutic modalities and prognosis. Authors have undertaken morphometric analysis of various parameters from both borderline lesions and carcinomas of mucinous nature of the ovary. In each, five cases of the borderline and malignant tumors were subjected to be evaluated. Various cytologic and histologic parameters were analyzed using Kontron IBAS-I. 1) The most helpful parameter-for differentiation between borderline and malignant mucinous tumors is cell concentration (sensitivity 80%, specificity 80%). The discrimination value is more than 35 cells per 100 micrometer of the basement membrane length. If the cellular concentration is higher than the discriminating value, that indicates malignancy. 2) Tumor cell height, though it is other parameter of stratification, is not helpful for differentiation of the two lesions. 3) Cytologic atypia, either in size or in form, can not be a criterion distinguishing the borderline from malignancy. 4) Papillary growths can not be a criterion of either borderline and malignant lesions. 5) The degree of irregularity at tumor-stroma interface is not helpful for differentiation between borderline and malignancy.
- Mucinous Tumors of the Appendix Associated with Mucinous Tumors of the Ovary and Pseudomyxoma Peritonei: A Clinicopathologic Analysis of 5 Cases Supporting an Appendiceal Origin.
- Eung Seok Lee, Han Kyeom Kim, In Sun Kim
- Korean J Pathol. 1998;32(2):131-137.
- 2,751 View
- 53 Download
-
 Abstract
Abstract
 PDF
PDF - Pseudomyxoma peritonei often have synchronous appendiceal and ovarian mucinous tumors. There has been considerable debate as to whether the ovarian tumors are secondary to the appendiceal tumor or they are independent primary ovarian tumors. It is important to reveal the primary site for treatment and prognosis of a patient. Five cases of synchronous mucinous tumors of the ovary and appendix were studied. Four cases had pseudomyxoma peritonei and pseudomyxoma ovarii. The ovarian tumors were bilateral in two cases, right in two, and left in one. The ovarian tumors were four mucinous cystadenoma of borderine malignancy and one mucinous cystadenocarcinoma, and the appendiceal tumors consisted of four mucinous tumors of borderline malignancy and one mucinous adenocarcinoma. The histology of the ovarian and appendiceal tumors was similar. Rupture of the tumor was seen in all appendiceal tumors and two ovarian tumors. It has been reported that cytokeratin 7 is a useful marker for distinguishing primary ovarian neoplasms from metastases of intestinal origin. All ovarian and appendiceal tumors showed positive reaction for broad-spectrum cytokeratin, but negative for cytokeratin 7. Based on the clinicopathologic and immunohistochemical features, it should be considered that the appendiceal tumors are primary and ovarian tumors are secondary in the synchronous presentation of the ovarian and appendiceal mucinous tumors.
- Aberrant Crypt Foci: Histopathologic Classification and Profiles of Mucin Secretion.
- Aeree Kim, Jong Sang Choi, Won Jun Choi, Hong Young Moon
- Korean J Pathol. 2000;34(1):50-55.
- 2,324 View
- 36 Download
-
 Abstract
Abstract
 PDF
PDF - Aberrant crypt foci (ACF) are grossly unidentifiable lesions of the colon and visible only with low-power microscopic examinations after methylene blue stain. To establish the role of ACF in colorectal carcinogenesis, we evaluated the distribution, frequency, histopathological classification, and patterns of mucin secretion of ACF in the colon. A total of 142 aberrant crypt foci were found in 41 colectomy specimen for adenocarcinoma (36 cases) and benign diseases of colon (5 cases). Ten of 142 ACFs were in the ascending and transverse colon, 39 in the descending and sigmoid colon, and 93 in the rectum. The mean number of ACFs in the rectum (0.13 0.11/cm2) was higher than in the ascending and transverse colons (0.019 0.018/ cm2) and descending and sigmoid colon (0.10 0.14/cm2). ACFs were found only in cancer patients. One hundred and twenty ACFs among 142 ACFs identified by topology, were identified on histological examination. We classified ACFs into simple (48.3%), hyperplastic (42.5%), and dysplastic (9.2%) types. All ACFs were infiltrated by the lymphocytes in the stroma and 18 of these accompanied the lymphoid follicles. ACFs have variable histopathologic features and mucin profiles. Some variants of ACFs are at the early stage of the spectrum between benign and malignant.
- Fine Needle Aspiration Cytology of Mucinous Cystic Carcinoma of the Pancreas: A Case Report.
- Kyungji Lee, Ahwon Lee, Kyo Young Lee, Chang Suk Kang, Sang In Shim
- J Pathol Transl Med. 2005;16(2):88-92.
- 1,945 View
- 15 Download
-
 Abstract
Abstract
 PDF
PDF - Mucious cystic neoplasm of pancreas is a cystic neoplasm composed of columnar, mucin-producing epithelium and is supported by ovarian-type stroma. The key to the cytologic evaluation of pancreatic cystic lesions is to recognize the cytologic components as being diagnostic of a mucin-producing cystic neoplasm, as all of these neoplasms need to be resected. We report the use of fine needle aspiration cytology in the diagnosis of an invasive mucinous cystic carcinoma confirmed by partial pancreatectomy. The cytologic specimen showed a abundant mucin background and sheets or papillae of neoplastic cells. There are mucin-containing columnar cells that show a variable degree of cytologic atypia.
- Fine Needle Aspiration Cytology of Chronic Sclerosing Sialadenitis with Mucinous Metaplasia in Parotid Gland: A Case Report.
- Jae Yeon Seok, Woo Hee Jung, Xu Xiang Fan, Jin Kim, Soon Won Hong
- J Pathol Transl Med. 2005;16(2):102-105.
- 2,864 View
- 72 Download
-
 Abstract
Abstract
 PDF
PDF - Chronic sclerosing sialadenitis, also known as Kuttner tumor, is a benign chronic inflammatory lesion of the salivary gland. Here, we describe a case of chronic sclerosing sialadenitis with mucinous ductal metaplasia in a parotid gland, which was confused with low-grade mucoepidermoid carcinoma on aspiration cytology.
- Peanut Agglutinin Binding Activity in Overian Malignant Mucinous Tumors.
- Hyung Geun Song, Chul Woo Kim
- Korean J Pathol. 1987;21(4):249-256.
- 1,947 View
- 11 Download
-
 Abstract
Abstract
 PDF
PDF - The positive binding activity of lectin, peanut agglutinin (PNA), against the mucinous malignancies of the ovary was studied in order to clarify biologic differences among those lesions using immunoperoxidase method (ABC). A total of 23 cases were included in this study and they were classified as 10 cases of mucinous cystadenocarcinoma, 9 mucinous tumors of borderline malignancy and 4 pseudomyxoma peritonei, histologically. Nine of 10 cystadenocarcinomas and all cases of pseuomyxoma peritonei showed more than moderate degree of positive binding activity (>2+) with PNA in the neoplastic epithelial cells. In the cases of borderline malignancy, only 3 of 9 revealed as much similar binding pattern with PNA as cystadenocarcinoma group, in contrast, minimal degree of positivity (1+) was noted in the remainder. These findings may suggest heterogeneity in the biochemical characteristics among the cases of borderline lesion. And it is proposed that the higher PNA binding cases in ovarian mucinous borderline malignancy require extensive sampling by multiple sections and further careful follow-up study.
- Evaluation of DNA Ploidy and Other Morphometric Parameters of Ovarian Mucinous Tumors.
- Yun Mee Kim, Sang Woo Juhng, Joo Yong Yoo, Kyu Hyuk Cho
- Korean J Pathol. 1991;25(5):397-406.
- 1,957 View
- 10 Download
-
 Abstract
Abstract
- Biological behavior of malignant tumors has been assessed by morphological grading, clinical staging, and estimating other tumor markers. Recently DNA ploidy measured by flow cytometry and image analyser has been suggested as an additional useful indicator of the tumor behavior. In order to extract useful tumor cell-specific information in ovarian mucinous tumors, DNA contents and other morphologic parameters were measured by image analysis and DNA ploidy was also measured by flow cytometry. In all cases of cystadenoma, DNA diploidies were observed. In borderline malignancy, DNA diploidies were chiefly observed except one case of polyploidy. In true malignancy, DNA aneuploidies were observed except one case of polyploidy and two cases of diploidies by image analysis, and except four cases of diploides and one cas of polyploidy by flow cytometry. The statistical significance were observed in DNA ploidy pattern by image analysis. In nuclear areas, perimeters and major axis, statistical significance were not observed. These results suggest that DNA ploidy pattern are more or less independent parameter as an additional useful indicator of the histological grade of malignancy and that image analysis are better than flow cytometry in detecting DNA aneuploidy.
- Salivary Duct Carcinoma with Mucin Containing Cells: Report of a Case Misdiagnosed as Mucoepidermoid Carcinoma on Fine Needle Aspiration Cytology.
- Haeryoung Kim, Hyunki Kim, Hoguen Kim, Jin Kim, Soon Won Hong, Se Hoon Kim
- J Pathol Transl Med. 2006;17(1):56-62.
- 2,333 View
- 25 Download
-
 Abstract
Abstract
 PDF
PDF - Salivary duct carcinoma (SDC) is a rare primary salivary gland malignancy characterized by histological features similar to those of ductal carcinomas of the breast. It is regarded as a high-grade malignancy associated with frequent local recurrences and early distant metastases that require aggressive treatment. The typical fine needle aspiration cytology (FNAC) findings in SDC include cellular smears showing tumor cells with eccentric pleomorphic nuclei and a granular cytoplasm arranged in flat sheets or cribriform patterns against a necrotic background. However, the presence of mucin-containing cells in SDC has been rarely described. We report the FNAC findings in a patient with histologically confirmed SDC that demonstrated numerous mucin-containing cells and was subsequently misdiagnosed as a high-grade mucoepidermoid carcinoma. Here we discuss the problems involved in distinguishing SDC from high-grade mucoepidermoid carcinoma on the basis of cytologic findings alone.
- Intraductal Papillary-Mucinous Neoplasm of the Pancreas: A case report.
- Ji Eun Kim, Young Hyeh Ko, Howe Jung Ree, Yong Il Kim, Poong Ryul Lee
- Korean J Pathol. 1996;30(8):726-732.
- 2,398 View
- 24 Download
-
 Abstract
Abstract
 PDF
PDF - Intraductal papillary mucinous neoplasm (IPMN) is a recently recognized clinicopathologic entity characterized by dilatation of pancreatic duct filled with copious mucin and papillary ductal epithelial proliferation ranging from simple hyperplasia to invasive carcinoma. The exact clinicopathologic identification of this tumor is important because of favorable prognosis contrast to that of conventional ductal adenocarcinoma. Herein we report a case of surgically resected typical IPMN. A 59-year-old man had a long history of diabetes mellitus with epigastric pain of 4 months duration. Ultrasonography and computed tomographic examination revealed cystic dilatations of main pancreatic duct in the head. The patient underwent total pancreatectomy. The gross appearance showed diffuse dilatation of main pancreatic duct associated with cystic dilatation of subbranches in the uncinate process. Histologic examination revealed diffuse papillary proliferations lined by mucinous epithelium with mild atypism within ectatic ducts. No invasive carcinoma was noted. Histochemically, the papillary epithelium contained mostly neutral and acid sialomucin.
- Value of Fine Needle Aspiration Cytology of Mucinous Carcinoma of the Breast.
- Yoon Jung Kim, Gyung Yub Gong, Joo Ryung Huh, Jeong Mi Park, Sei Hyun Ahn, On Ja Kim
- J Pathol Transl Med. 1996;7(2):157-162.
- 2,162 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - Fine needle aspiration(FNA) is an effective tool in diagnosing mammary carcinoma, We experienced 7 cases of histologically confirmed mammary mucinous carcinoma among 3,052 aspirated cases of breast from 1992 to 1996 in Asan Medical Center" The average age of the patient was 48(33-64) years. The mean size of the lesions was 1.6(0.7-3) cm, and they were palpated as well-defined, firm to hard masses. The cytologic features that may be useful in making a FNA diagnosis of mucinous carcinoma of the breast were analysed. Mucinous background and tumor cell, clusters with occasional single cells were observed in all cases. Among them, two, cases showed abundant scattered single cells, whereas only few single cells were seen in the other two cases. Tumor cells exhibited mild pleomorphism in four cases, and moderate pleomorphism in three cases. Nucleoli tended to be not prominen and are observed in three cases, rarely noted in other three cases and not seen in one. There was microcalcification in four cases(57%). In conclusion, mucinous background and clustered tumor cells showing mild td moderate pleomorphism with characteristic clinical findings allow us to diagnose, mucinous carcinoma of the breast.
- Goblet Cell Carcinoid of the Appendix: A case report.
- Joo Heon Kim, Ho Lee, So Young Oh, Myoung Jae Kang, Ho Yeul Choi, Dong Geun Lee
- Korean J Pathol. 1996;30(9):839-842.
- 2,233 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF - Carcinoid tumors of the appendix are common incidental findings, but appendiceal tumors with histologic features of both carcinoids and adenocarcinomas are rare, and their biologic behavior and histogenesis are still unclear. We report a case of goblet cell carcinoid of the appendix in a 54-year-old male, who exhibited pain in the right lower abdomen. Microscopically, the tumor contained smooth-bordered, widely separated nests composed of tumor cells with abundant mucin. The principal tumor cell type had a close resemblance to the normal goblet cell. Histochemically, the tumor cells revealed positive reaction for PAS and alcian blue stain. Immunohistochemically, the tumor showed strong reactivity for carcinoembryonic antigen, chromogranin and, neuron specific enolase but none for cytokeratin and epithelial membrane antigen.
- Mucinous Adenocarcinoma of Anal Ducts.
- Young Ha Oh, Wan Seop Kim, Eun Kyung Hong, Moon Hyang Park, Jung Dal Lee
- Korean J Pathol. 1996;30(9):843-850.
- 2,423 View
- 44 Download
-
 Abstract
Abstract
 PDF
PDF - Anal duct carcinoma is a rare tumor, and accounts for less than 5 percent of all anal cancers, which typically present a long-standing perianal fistulas. Some authors suggest that the fistulous tracts are congenital duplications of the lower end of the hind gut lined by rectal mucosa which is prone to malignant change to mucinous adenocarcinoma. It is usually a well differentiated mucinous (colloid) adenocarcinoma. The prognosis after wide excision of the rectum is relatively good. Since 1985, we have had three cases of anal duct carcinoma with well differentiated mucinous adenocarcinoma involving the posterior wall of the anus. Two patients had a long history of perianal fistula with mucinous discharge. There was no spread to the regional lymph node except one patient who had regional lymph node metastasis, and post-operative chemotherapy and radiation therapy were then given. All patients have no evidence of any recurrent problem at 16 months to 3 years following the surgical treatment. Because of their rarity and the failure of recognition at an early stage, we are presenting three cases to emphasize the characteristic features of this insidious, slow-growing carcinoma.
- Histochemical and Immunohistochemical Properties of Endometrial and Endocervical Adenocarcinoma.
- Kyu Rae Kim, In Joon Choi
- Korean J Pathol. 1988;22(3):259-267.
- 2,012 View
- 30 Download
-
 Abstract
Abstract
 PDF
PDF - The histologic differentiation of endometrial and endocervical adenocarcinomas is a common diagnostic problum of clinical importance, because the staging, treatment and prognosis of these lesions are quite different. First, we examined the distribution of acid mucin in endometrial and endocervical adenocarcinoma (23 cases and 25 cases repectively), but distinguishing differences between endometrial and endocervical adenocarcinoma, especially of endometrioid type, were not observed. Secondly, the distribution of low-molecular weight cytokeratin, vimentin and carcino-embryonic antigen (CEA) by immunohistochemistry were examined in formalin-fixed tissues. CEA was present in 88% of endocervical adenocarcinomas and 34.8% of endometrial adenocarcinoma. vimentin was found in 91.3% of endometrial adenocarcinomas, in contrast with only in 16% of endocervical adenocarcinomas. This study showed that the presence of vimentin in neoplastic glands, in which CEA is negative, may be helpful in the differential diagnosis of endometrial from endocervical adenocarcinomas.

 E-submission
E-submission



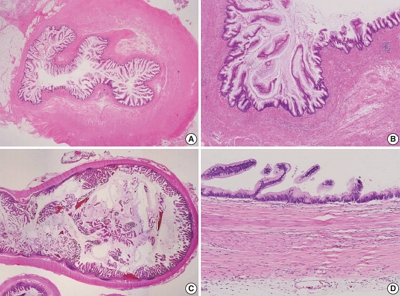
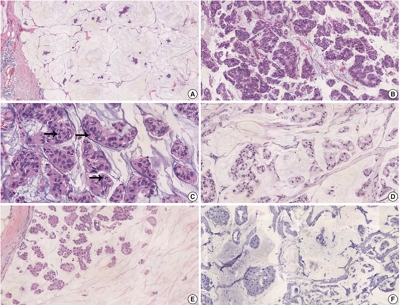
 First
First Prev
Prev



