Search
- Page Path
- HOME > Search
Case Study
- Primary carcinoid tumor in the external auditory canal
- Dong Hae Chung, Gyu Cheol Han, Na Rae Kim
- J Pathol Transl Med. 2020;54(2):184-187. Published online November 13, 2019
- DOI: https://doi.org/10.4132/jptm.2019.11.07
- 9,036 View
- 167 Download
- 4 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
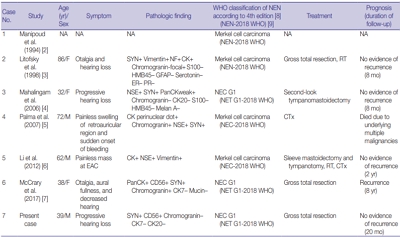
PDF - A 39-year-old man visited the department of otolaryngology due to an ongoing hearing disturbance that had lasted for 1 year. Temporal bone computed tomography revealed soft tissue density nearly obliterating the left external auditory canal (EAC). The mass was composed of sheets of round tumor cells containing moderate amounts of fine granular cytoplasm and salt and pepper chromatin. Neither mitosis nor necrosis was found. The Ki-67 proliferation index was less than 2%. Cells were positive for CD56 and synaptophysin but negative for chromogranin, cytokeratin (CK) 20, and CK7. Based on these findings, the tumor was diagnosed as a carcinoid tumor, well differentiated neuroendocrine carcinoma, grade 1 (G1) according to current World Health Organization (WHO) classification of head and neck tumors; and a neuroendocrine tumor, G1 according to neuroendocrine neoplasm (NEN)-2018 WHO standard classification. He remained free of local recurrence and metastasis after 20 months of follow up. To date, only six cases of primary NENs in the EAC have been reported. Metastatic tumor should be included in the differential diagnoses. Because of its rarity, the prognosis and treatment have not yet been clarified.
-
Citations
Citations to this article as recorded by- First Report on a Rare Poorly Differentiated Neuroendocrine Tumour of the External Auditory Canal Involving Pinna
Akash Varshney, Amit Kumar Tyagi, Prashant Durgapal, Kajal Mahto, Akhilesh Chandra Yadav, Ankita Semwal
Indian Journal of Otolaryngology and Head & Neck Surgery.2025; 77(4): 1922. CrossRef - Incidental finding of a neuroendocrine neoplasm in a suspected ear canal exostosis
Alexander Wieck Fjaeldstad, Gerda Elisabeth Villadsen, Gitte Dam, Stephen Jacques Hamilton-Dutoit, Thomas Winther Frederiksen
Otolaryngology Case Reports.2022; 22: 100394. CrossRef - 68Ga-DOTATATE Uptake in Well-Differentiated Neuroendocrine Tumor of the External Auditory Canal
Özge Erol Fenercioğlu, Ediz Beyhan, Rahime Şahin, Mehmet Can Baloğlu, Tevfik Fikret Çermik
Clinical Nuclear Medicine.2022; 47(8): e552. CrossRef
- First Report on a Rare Poorly Differentiated Neuroendocrine Tumour of the External Auditory Canal Involving Pinna
Original Article
- SSTR2A Protein Expression in Neuroendocrine Neoplasms of the Colorectum.
- Young Eun Kim, Jeeyun Lee, Young Suk Park, Kyoung Mee Kim
- Korean J Pathol. 2011;45(3):276-280.
- DOI: https://doi.org/10.4132/KoreanJPathol.2011.45.3.276
- 4,199 View
- 24 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Expression studies of somatostatin receptor type 2A (SSTR2A) in neuroendocrine neoplasms (NENs) led to the development of clinically relevant diagnostic and therapeutic strategies. However, most of these strategies used in-house-developed antibodies and were focused on lung tumors. We evaluated commercially available SSTR2A antibodies in NENs of the colorectum to observe their subcellular localization and distribution within the resected tumor.
METHODS
The immunohistochemistry of 77 NENs located in the colorectum were studied using a commercially available antibody against SSTR2A.
RESULTS
Most neuroendocrine tumors (NET) grade (G)1 and G2 expressed the SSTR2A in the cytoplasm with apical or luminal localization. However, all neuroendocrine carcinomas (NEC) G3 were negative for SSTR2A.
CONCLUSIONS
Our data indicate that SSTR2A immunohistochemistry shows cytoplasmic staining with distinct subcellular localization in most NET G1 in the colorectum using a commercially available antibody. Low or no expression of SSTR2A in NET G2 and NEC G3 raises the possibility that SSTR2A may correlate with histologic differentiation and proliferative activity. Further validation studies in large case series are needed.

 E-submission
E-submission

 First
First Prev
Prev



