Search
- Page Path
- HOME > Search
Original Articles
- National quality assurance program using digital cytopathology: a 5-year digital transformation experience by the Korean Society for Cytopathology
- Yosep Chong, Hyeong Ju Kwon, Soon Auck Hong, Sung Soon Kim, Bo-Sung Kim, Younghee Choi, Yoon Jung Choi, Jung-Soo Pyo, Ji Yun Jeong, Soo Jin Jung, Hoon Kyu Oh, Seung-Sook Lee
- J Pathol Transl Med. 2025;59(5):320-333. Published online September 15, 2025
- DOI: https://doi.org/10.4132/jptm.2025.06.27
- 2,711 View
- 102 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Digital cytopathology (DC) is emerging as a transformative approach in quality assurance programs (QAP), though its comprehensive evaluation remains limited. Since 2020, the Korean Society for Cytopathology has progressively incorporated DC into its national QAP, including digital proficiency testing (PT), sample adequacy testing (SAT), a customizable PT module, and a self-assessment module (SAM), aiming for full digital implementation by 2026. Methods: This 5-year study assessed diagnostic concordance between conventional and digital PT formats and analyzed participant feedback on service quality and digital image usability across PT, SAT, and SAM. Parallel testing was conducted during the transitional phase, and satisfaction was measured through structured surveys. Results: Participation in digital PT increased from 48 institutions in 2020 to 93 in 2024, while digital SAT participation rose from 29 to 71 between 2022 and 2024. In 2023, 56 institutions joined SAM. Diagnostic concordance rates were comparable between digital and conventional PTs (78.6%–84.6% vs. 82.0%–85.1%), including similar category C (major discordance) rates. Satisfaction with digital PT services and image quality exceeded 85%, and over 90% of institutions reported positive feedback on SAT and SAM. Over 80% were satisfied with the customizable PT module. Conclusions: DC is a reliable and effective modality for cytopathology QAP. It demonstrates diagnostic equivalence to conventional methods and high user satisfaction, supporting its broader implementation in national quality assurance frameworks. -
Citations
Citations to this article as recorded by- Practice of Cytopathology in Korea: A 40‐Year Evolution Through Standardization, Digital Transformation, and Global Partnership
Yosep Chong, Ran Hong, Hyeong Ju Kwon, Haeryoung Kim, Lucia Kim, Soon Jae Kim, Yoon Jung Choi
Diagnostic Cytopathology.2025;[Epub] CrossRef
- Practice of Cytopathology in Korea: A 40‐Year Evolution Through Standardization, Digital Transformation, and Global Partnership
- Diagnostic proficiency test using digital cytopathology and comparative assessment of whole slide images of cytologic samples for quality assurance program in Korea
- Yosep Chong, Soon Auck Hong, Hoon Kyu Oh, Soo Jin Jung, Bo-Sung Kim, Ji Yun Jeong, Ho-Chang Lee, Gyungyub Gong
- J Pathol Transl Med. 2023;57(5):251-264. Published online August 24, 2023
- DOI: https://doi.org/10.4132/jptm.2023.07.17
- 7,688 View
- 339 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
The Korean Society for Cytopathology introduced a digital proficiency test (PT) in 2021. However, many doubtful opinions remain on whether digitally scanned images can satisfactorily present subtle differences in the nuclear features and chromatin patterns of cytological samples.
Methods
We prepared 30 whole-slide images (WSIs) from the conventional PT archive by a selection process for digital PT. Digital and conventional PT were performed in parallel for volunteer institutes, and the results were compared using feedback. To assess the quality of cytological assessment WSIs, 12 slides were collected and scanned using five different scanners, with four cytopathologists evaluating image quality through a questionnaire.
Results
Among the 215 institutes, 108 and 107 participated in glass and digital PT, respectively. No significant difference was noted in category C (major discordance), although the number of discordant cases was slightly higher in the digital PT group. Leica, 3DHistech Pannoramic 250 Flash, and Hamamatsu NanoZoomer 360 systems showed comparable results in terms of image quality, feature presentation, and error rates for most cytological samples. Overall satisfaction was observed with the general convenience and image quality of digital PT.
Conclusions
As three-dimensional clusters are common and nuclear/chromatin features are critical for cytological interpretation, careful selection of scanners and optimal conditions are mandatory for the successful establishment of digital quality assurance programs in cytology. -
Citations
Citations to this article as recorded by- Sensitivity, Specificity, and Cost–Benefit Effect Between Primary Human Papillomavirus Testing, Primary Liquid‐Based Cytology, and Co‐Testing Algorithms for Cervical Lesions
Chang Gok Woo, Seung‐Myoung Son, Hye‐Kyung Hwang, Jung‐Sil Bae, Ok‐Jun Lee, Ho‐Chang Lee
Diagnostic Cytopathology.2025; 53(1): 35. CrossRef - Integration of AI‐Assisted in Digital Cervical Cytology Training: A Comparative Study
Yihui Yang, Dongyi Xian, Lihua Yu, Yanqing Kong, Huaisheng Lv, Liujing Huang, Kai Liu, Hao Zhang, Weiwei Wei, Hongping Tang
Cytopathology.2025; 36(2): 156. CrossRef - National quality assurance program using digital cytopathology: a 5-year digital transformation experience by the Korean Society for Cytopathology
Yosep Chong, Hyeong Ju Kwon, Soon Auck Hong, Sung Soon Kim, Bo-Sung Kim, Younghee Choi, Yoon Jung Choi, Jung-Soo Pyo, Ji Yun Jeong, Soo Jin Jung, Hoon Kyu Oh, Seung-Sook Lee
Journal of Pathology and Translational Medicine.2025; 59(5): 320. CrossRef - Integration of Digital Cytology in Quality Assurance Programs for Cytopathology
Yosep Chong, Maria Jesús Fernández Aceñero, Zaibo Li, Andrey Bychkov
Acta Cytologica.2025; : 1. CrossRef - Practice of Cytopathology in Korea: A 40‐Year Evolution Through Standardization, Digital Transformation, and Global Partnership
Yosep Chong, Ran Hong, Hyeong Ju Kwon, Haeryoung Kim, Lucia Kim, Soon Jae Kim, Yoon Jung Choi
Diagnostic Cytopathology.2025;[Epub] CrossRef - Quantitative Assessment of Focus Quality in Whole-Slide Imaging of Thyroid Liquid-Based Cytology Using Laplacian Variance
Chan Kwon Jung, Chankyung Kim, Sora Jeon, Andrey Bychkov
Endocrine Pathology.2025;[Epub] CrossRef - Validation of digital image slides for diagnosis in cervico-vaginal cytology
Francisco Tresserra, Gemma Fabra, Olga Luque, Miriam Castélla, Carla Gómez, Carmen Fernández-Cid, Ignacio Rodríguez
Revista Española de Patología.2024; 57(3): 182. CrossRef - Improved Diagnostic Accuracy of Thyroid Fine-Needle Aspiration Cytology with Artificial Intelligence Technology
Yujin Lee, Mohammad Rizwan Alam, Hongsik Park, Kwangil Yim, Kyung Jin Seo, Gisu Hwang, Dahyeon Kim, Yeonsoo Chung, Gyungyub Gong, Nam Hoon Cho, Chong Woo Yoo, Yosep Chong, Hyun Joo Choi
Thyroid®.2024; 34(6): 723. CrossRef
- Sensitivity, Specificity, and Cost–Benefit Effect Between Primary Human Papillomavirus Testing, Primary Liquid‐Based Cytology, and Co‐Testing Algorithms for Cervical Lesions
- Development of quality assurance program for digital pathology by the Korean Society of Pathologists
- Yosep Chong, Jeong Mo Bae, Dong Wook Kang, Gwangil Kim, Hye Seung Han
- J Pathol Transl Med. 2022;56(6):370-382. Published online November 15, 2022
- DOI: https://doi.org/10.4132/jptm.2022.09.30
- 6,622 View
- 162 Download
- 5 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Digital pathology (DP) using whole slide imaging is a recently emerging game changer technology that can fundamentally change the way of working in pathology. The Digital Pathology Study Group (DPSG) of the Korean Society of Pathologists (KSP) published a consensus report on the recommendations for pathologic practice using DP. Accordingly, the need for the development and implementation of a quality assurance program (QAP) for DP has been raised.
Methods
To provide a standard baseline reference for internal and external QAP for DP, the members of the Committee of Quality Assurance of the KSP developed a checklist for the Redbook and a QAP trial for DP based on the prior DPSG consensus report. Four leading institutes participated in the QAP trial in the first year, and we gathered feedback from these institutes afterwards.
Results
The newly developed checklists of QAP for DP contain 39 items (216 score): eight items for quality control of DP systems; three for DP personnel; nine for hardware and software requirements for DP systems; 15 for validation, operation, and management of DP systems; and four for data security and personal information protection. Most participants in the QAP trial replied that continuous education on unfamiliar terminology and more practical experience is demanding.
Conclusions
The QAP for DP is essential for the safe implementation of DP in pathologic practice. Each laboratory should prepare an institutional QAP according to this checklist, and consecutive revision of the checklist with feedback from the QAP trial for DP needs to follow. -
Citations
Citations to this article as recorded by- An equivalency and efficiency study for one year digital pathology for clinical routine diagnostics in an accredited tertiary academic center
Viola Iwuajoku, Kübra Ekici, Anette Haas, Mohammed Zaid Khan, Azar Kazemi, Atsuko Kasajima, Claire Delbridge, Alexander Muckenhuber, Elisa Schmoeckel, Fabian Stögbauer, Christine Bollwein, Kristina Schwamborn, Katja Steiger, Carolin Mogler, Peter J. Schüf
Virchows Archiv.2025; 487(1): 3. CrossRef - Quality Assurance of the Whole Slide Image Evaluation in Digital Pathology: State of the Art and Development Results
Miklós Vincze, Béla Molnár, Miklós Kozlovszky
Electronics.2025; 14(10): 1943. CrossRef - Integration of Digital Cytology in Quality Assurance Programs for Cytopathology
Yosep Chong, Maria Jesús Fernández Aceñero, Zaibo Li, Andrey Bychkov
Acta Cytologica.2025; : 1. CrossRef - Diagnostic proficiency test using digital cytopathology and comparative assessment of whole slide images of cytologic samples for quality assurance program in Korea
Yosep Chong, Soon Auck Hong, Hoon Kyu Oh, Soo Jin Jung, Bo-Sung Kim, Ji Yun Jeong, Ho-Chang Lee, Gyungyub Gong
Journal of Pathology and Translational Medicine.2023; 57(5): 251. CrossRef
- An equivalency and efficiency study for one year digital pathology for clinical routine diagnostics in an accredited tertiary academic center
- Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice
- Soon Auck Hong, Haeyoen Jung, Sung Sun Kim, Min-Sun Jin, Jung-Soo Pyo, Ji Yun Jeong, Younghee Choi, Gyungyub Gong, Yosep Chong
- J Pathol Transl Med. 2022;56(6):361-369. Published online October 27, 2022
- DOI: https://doi.org/10.4132/jptm.2022.09.21
- 4,850 View
- 105 Download
- 7 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
The Continuous Quality Improvement program for cytopathology in 2020 was completed during the coronavirus pandemic. In this study, we report the result of the quality improvement program.
Methods
Data related to cytopathology practice from each institute were collected and processed at the web-based portal. The proficiency test was conducted using glass slides and whole-slide images (WSIs). Evaluation of the adequacy of gynecology (GYN) slides from each institution and submission of case glass slides and WSIs for the next quality improvement program were performed.
Results
A total of 214 institutions participated in the annual cytopathology survey in 2020. The number of entire cytopathology specimens was 8,220,650, a reduction of 19.0% from the 10,111,755 specimens evaluated in 2019. Notably, the number of respiratory cytopathology specimens, including sputum and bronchial washing/ brushing significantly decreased by 86.9% from 2019, which could be attributed to the global pandemic of coronavirus disease. The ratio of cases with atypical squamous cells to squamous intraepithelial lesions was 4.10. All participating institutions passed the proficiency test and the evaluation of adequacy of GYN slides.
Conclusions
Through the Continuous Quality Improvement program, the effect of coronavirus disease 2019 pandemic, manifesting with a reduction in the number of cytologic examinations, especially in respiratory-related specimen has been identified. The Continuous Quality Improvement Program of the Korean Society for Cytopathology can serve as the gold standard to evaluate the current status of cytopathology practice in Korea. -
Citations
Citations to this article as recorded by- A Study on the Workload of Cytotechnologists: Focus on Commercial Laboratories
Eun-Suk PARK
Korean Journal of Clinical Laboratory Science.2025; 57(2): 228. CrossRef - Integration of Digital Cytology in Quality Assurance Programs for Cytopathology
Yosep Chong, Maria Jesús Fernández Aceñero, Zaibo Li, Andrey Bychkov
Acta Cytologica.2025; : 1. CrossRef - National quality assurance program using digital cytopathology: a 5-year digital transformation experience by the Korean Society for Cytopathology
Yosep Chong, Hyeong Ju Kwon, Soon Auck Hong, Sung Soon Kim, Bo-Sung Kim, Younghee Choi, Yoon Jung Choi, Jung-Soo Pyo, Ji Yun Jeong, Soo Jin Jung, Hoon Kyu Oh, Seung-Sook Lee
Journal of Pathology and Translational Medicine.2025; 59(5): 320. CrossRef - Commercially Available Artificial Intelligence Solutions for Gynaecologic Cytology Screening and Their Integration Into Clinical Workflow
Yosep Chong, Andrey Bychkov
Cytopathology.2025;[Epub] CrossRef - Practice of Cytopathology in Korea: A 40‐Year Evolution Through Standardization, Digital Transformation, and Global Partnership
Yosep Chong, Ran Hong, Hyeong Ju Kwon, Haeryoung Kim, Lucia Kim, Soon Jae Kim, Yoon Jung Choi
Diagnostic Cytopathology.2025;[Epub] CrossRef - A stepwise approach to fine needle aspiration cytology of lymph nodes
Yosep Chong, Gyeongsin Park, Hee Jeong Cha, Hyun-Jung Kim, Chang Suk Kang, Jamshid Abdul-Ghafar, Seung-Sook Lee
Journal of Pathology and Translational Medicine.2023; 57(4): 196. CrossRef - Diagnostic proficiency test using digital cytopathology and comparative assessment of whole slide images of cytologic samples for quality assurance program in Korea
Yosep Chong, Soon Auck Hong, Hoon Kyu Oh, Soo Jin Jung, Bo-Sung Kim, Ji Yun Jeong, Ho-Chang Lee, Gyungyub Gong
Journal of Pathology and Translational Medicine.2023; 57(5): 251. CrossRef
- A Study on the Workload of Cytotechnologists: Focus on Commercial Laboratories
Review
- Recommendations for pathologic practice using digital pathology: consensus report of the Korean Society of Pathologists
- Yosep Chong, Dae Cheol Kim, Chan Kwon Jung, Dong-chul Kim, Sang Yong Song, Hee Jae Joo, Sang-Yeop Yi
- J Pathol Transl Med. 2020;54(6):437-452. Published online October 8, 2020
- DOI: https://doi.org/10.4132/jptm.2020.08.27
- 12,370 View
- 339 Download
- 24 Web of Science
- 30 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Digital pathology (DP) using whole slide imaging (WSI) is becoming a fundamental issue in pathology with recent advances and the rapid development of associated technologies. However, the available evidence on its diagnostic uses and practical advice for pathologists on implementing DP remains insufficient, particularly in light of the exponential growth of this industry. To inform DP implementation in Korea, we developed relevant and timely recommendations. We first performed a literature review of DP guidelines, recommendations, and position papers from major countries, as well as a review of relevant studies validating WSI. Based on that information, we prepared a draft. After several revisions, we released this draft to the public and the members of the Korean Society of Pathologists through our homepage and held an open forum for interested parties. Through that process, this final manuscript has been prepared. This recommendation contains an overview describing the background, objectives, scope of application, and basic terminology; guidelines and considerations for the hardware and software used in DP systems and the validation required for DP implementation; conclusions; and references and appendices, including literature on DP from major countries and WSI validation studies.
-
Citations
Citations to this article as recorded by- Commercially Available Artificial Intelligence Solutions for Gynaecologic Cytology Screening and Their Integration Into Clinical Workflow
Yosep Chong, Andrey Bychkov
Cytopathology.2026; 37(1): 24. CrossRef - The impact of AI on modern oncology from early detection to personalized cancer treatment
Jun Li, Lei Zhang, Zhenglun Yu, Zhiye Bao, Danyang Li, Liming Wang
npj Precision Oncology.2026;[Epub] CrossRef - An equivalency and efficiency study for one year digital pathology for clinical routine diagnostics in an accredited tertiary academic center
Viola Iwuajoku, Kübra Ekici, Anette Haas, Mohammed Zaid Khan, Azar Kazemi, Atsuko Kasajima, Claire Delbridge, Alexander Muckenhuber, Elisa Schmoeckel, Fabian Stögbauer, Christine Bollwein, Kristina Schwamborn, Katja Steiger, Carolin Mogler, Peter J. Schüf
Virchows Archiv.2025; 487(1): 3. CrossRef - An adapted & improved validation protocol for digital pathology implementation
Ying-Han R. Hsu, Iman Ahmed, Juliana Phlamon, Charlotte Carment-Baker, Joyce Yin Tung Chan, Ioannis Prassas, Karen Weiser, Shaza Zeidan, Blaise Clarke, George M. Yousef
Seminars in Diagnostic Pathology.2025; 42(4): 150905. CrossRef - Transforming pathology into digital pathology: highway to hell or stairway to heaven?
Rainer Grobholz, Andrew Janowczyk, Inti Zlobec
Diagnostic Histopathology.2025; 31(7): 410. CrossRef - The Evolution of Digital Pathology in Infrastructure, Artificial Intelligence and Clinical Impact
Chan Kwon Jung
International Journal of Thyroidology.2025; 18(1): 6. CrossRef - Current Trends and Future Directions of Digital Pathology and Artificial Intelligence in Dermatopathology: A Scientometric-Based Review
Iuliu Gabriel Cocuz, Raluca Niculescu, Maria-Cătălina Popelea, Maria Elena Cocuz, Adrian-Horațiu Sabău, Andreea-Cătălina Tinca, Andreea Raluca Cozac-Szoke, Diana Maria Chiorean, Corina Eugenia Budin, Ovidiu Simion Cotoi
Diagnostics.2025; 15(17): 2196. CrossRef - Integration of Digital Cytology in Quality Assurance Programs for Cytopathology
Yosep Chong, Maria Jesús Fernández Aceñero, Zaibo Li, Andrey Bychkov
Acta Cytologica.2025; : 1. CrossRef - Quantitative Assessment of Focus Quality in Whole-Slide Imaging of Thyroid Liquid-Based Cytology Using Laplacian Variance
Chan Kwon Jung, Chankyung Kim, Sora Jeon, Andrey Bychkov
Endocrine Pathology.2025;[Epub] CrossRef - Performance of externally validated machine learning models based on histopathology images for the diagnosis, classification, prognosis, or treatment outcome prediction in female breast cancer: A systematic review
Ricardo Gonzalez, Peyman Nejat, Ashirbani Saha, Clinton J.V. Campbell, Andrew P. Norgan, Cynthia Lokker
Journal of Pathology Informatics.2024; 15: 100348. CrossRef - Swiss digital pathology recommendations: results from a Delphi process conducted by the Swiss Digital Pathology Consortium of the Swiss Society of Pathology
Andrew Janowczyk, Inti Zlobec, Cedric Walker, Sabina Berezowska, Viola Huschauer, Marianne Tinguely, Joel Kupferschmid, Thomas Mallet, Doron Merkler, Mario Kreutzfeldt, Radivoje Gasic, Tilman T. Rau, Luca Mazzucchelli, Isgard Eyberg, Gieri Cathomas, Kirst
Virchows Archiv.2024; 485(1): 13. CrossRef - ChatGPT as an aid for pathological diagnosis of cancer
Shaivy Malik, Sufian Zaheer
Pathology - Research and Practice.2024; 253: 154989. CrossRef - Possible benefits, challenges, pitfalls, and future perspective of using ChatGPT in pathology
Durre Aden, Sufian Zaheer, Sabina Khan
Revista Española de Patología.2024; 57(3): 198. CrossRef - Remote Placental Sign-Out: What Digital Pathology Can Offer for Pediatric Pathologists
Casey P. Schukow, Jacqueline K. Macknis
Pediatric and Developmental Pathology.2024; 27(4): 375. CrossRef - Digital Validation in Breast Cancer Needle Biopsies: Comparison of Histological Grade and Biomarker Expression Assessment Using Conventional Light Microscopy, Whole Slide Imaging, and Digital Image Analysis
Ji Eun Choi, Kyung-Hee Kim, Younju Lee, Dong-Wook Kang
Journal of Personalized Medicine.2024; 14(3): 312. CrossRef - Pathologists light level preferences using the microscope—study to guide digital pathology display use
Charlotte Jennings, Darren Treanor, David Brettle
Journal of Pathology Informatics.2024; 15: 100379. CrossRef - Eye tracking in digital pathology: A comprehensive literature review
Alana Lopes, Aaron D. Ward, Matthew Cecchini
Journal of Pathology Informatics.2024; 15: 100383. CrossRef - Diagnostic Assessment of Deep Learning Algorithms for Frozen Tissue Section Analysis in Women with Breast Cancer
Young-Gon Kim, In Hye Song, Seung Yeon Cho, Sungchul Kim, Milim Kim, Soomin Ahn, Hyunna Lee, Dong Hyun Yang, Namkug Kim, Sungwan Kim, Taewoo Kim, Daeyoung Kim, Jonghyeon Choi, Ki-Sun Lee, Minuk Ma, Minki Jo, So Yeon Park, Gyungyub Gong
Cancer Research and Treatment.2023; 55(2): 513. CrossRef - Recent application of artificial intelligence on histopathologic image-based prediction of gene mutation in solid cancers
Mohammad Rizwan Alam, Kyung Jin Seo, Jamshid Abdul-Ghafar, Kwangil Yim, Sung Hak Lee, Hyun-Jong Jang, Chan Kwon Jung, Yosep Chong
Briefings in Bioinformatics.2023;[Epub] CrossRef - Sustainable development goals applied to digital pathology and artificial intelligence applications in low- to middle-income countries
Sumi Piya, Jochen K. Lennerz
Frontiers in Medicine.2023;[Epub] CrossRef - Diagnostic proficiency test using digital cytopathology and comparative assessment of whole slide images of cytologic samples for quality assurance program in Korea
Yosep Chong, Soon Auck Hong, Hoon Kyu Oh, Soo Jin Jung, Bo-Sung Kim, Ji Yun Jeong, Ho-Chang Lee, Gyungyub Gong
Journal of Pathology and Translational Medicine.2023; 57(5): 251. CrossRef - Real-World Implementation of Digital Pathology: Results From an Intercontinental Survey
Daniel Gomes Pinto, Andrey Bychkov, Naoko Tsuyama, Junya Fukuoka, Catarina Eloy
Laboratory Investigation.2023; 103(12): 100261. CrossRef - National digital pathology projects in Switzerland: A 2023 update
Rainer Grobholz, Andrew Janowczyk, Ana Leni Frei, Mario Kreutzfeldt, Viktor H. Koelzer, Inti Zlobec
Die Pathologie.2023; 44(S3): 225. CrossRef - Understanding the ethical and legal considerations of Digital Pathology
Cheryl Coulter, Francis McKay, Nina Hallowell, Lisa Browning, Richard Colling, Philip Macklin, Tom Sorell, Muhammad Aslam, Gareth Bryson, Darren Treanor, Clare Verrill
The Journal of Pathology: Clinical Research.2022; 8(2): 101. CrossRef - Current Trend of Artificial Intelligence Patents in Digital Pathology: A Systematic Evaluation of the Patent Landscape
Muhammad Joan Ailia, Nishant Thakur, Jamshid Abdul-Ghafar, Chan Kwon Jung, Kwangil Yim, Yosep Chong
Cancers.2022; 14(10): 2400. CrossRef - Recent Applications of Artificial Intelligence from Histopathologic Image-Based Prediction of Microsatellite Instability in Solid Cancers: A Systematic Review
Mohammad Rizwan Alam, Jamshid Abdul-Ghafar, Kwangil Yim, Nishant Thakur, Sung Hak Lee, Hyun-Jong Jang, Chan Kwon Jung, Yosep Chong
Cancers.2022; 14(11): 2590. CrossRef - Automated Hybrid Model for Detecting Perineural Invasion in the Histology of Colorectal Cancer
Jiyoon Jung, Eunsu Kim, Hyeseong Lee, Sung Hak Lee, Sangjeong Ahn
Applied Sciences.2022; 12(18): 9159. CrossRef - Development of quality assurance program for digital pathology by the Korean Society of Pathologists
Yosep Chong, Jeong Mo Bae, Dong Wook Kang, Gwangil Kim, Hye Seung Han
Journal of Pathology and Translational Medicine.2022; 56(6): 370. CrossRef - Improving quality control in the routine practice for histopathological interpretation of gastrointestinal endoscopic biopsies using artificial intelligence
Young Sin Ko, Yoo Mi Choi, Mujin Kim, Youngjin Park, Murtaza Ashraf, Willmer Rafell Quiñones Robles, Min-Ju Kim, Jiwook Jang, Seokju Yun, Yuri Hwang, Hani Jang, Mun Yong Yi, Anwar P.P. Abdul Majeed
PLOS ONE.2022; 17(12): e0278542. CrossRef - What is Essential is (No More) Invisible to the Eyes: The Introduction of BlocDoc in the Digital Pathology Workflow
Vincenzo L’Imperio, Fabio Gibilisco, Filippo Fraggetta
Journal of Pathology Informatics.2021; 12(1): 32. CrossRef
- Commercially Available Artificial Intelligence Solutions for Gynaecologic Cytology Screening and Their Integration Into Clinical Workflow
Original Articles
- Current status of cytopathology practices in Korea: annual report on the Continuous Quality Improvement program of the Korean Society for Cytopathology for 2018
- Yosep Chong, Haeyoen Jung, Jung-Soo Pyo, Soon Won Hong, Hoon Kyu Oh
- J Pathol Transl Med. 2020;54(4):318-331. Published online April 15, 2020
- DOI: https://doi.org/10.4132/jptm.2020.02.26
- 7,186 View
- 118 Download
- 9 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
The Korean Society for Cytopathology has conducted the Continuous Quality Improvement program for cytopathology laboratories in Korea since 1995. In 2018 as part of the program, an annual survey of cytologic data was administered to determine the current status of cytopathology practices in Korea. Methods: A questionnaire was administered to 211 cytopathology laboratories. Individual laboratories submitted their annual statistics regarding cytopathology practices, diagnoses of gynecologic samples, inadequacy rates, and gynecologic cytology-histology correlation review (CHCR) data for 2018. In addition, proficiency tests and sample adequacy assessments were conducted using five consequent gynecologic slides. Results: Over 10 million cytologic exams were performed in 2018, and this number has almost tripled since this survey was first conducted in 2004 (compounded annual growth rate of 7.2%). The number of non-gynecologic samples has increased gradually over time and comprised 24% of all exams. The overall unsatisfactory rate was 0.14%. The ratio of the cases with atypical squamous cells to squamous intraepithelial lesions accounted for up to 4.24. The major discrepancy rate of the CHCR in gynecologic samples was 0.52%. In the proficiency test, the major discrepancy rate was approximately 1%. In the sample adequacy assessment, a discrepancy was observed in 0.1% of cases. Conclusions: This study represents the current status of cytopathology practices in Korea, illustrating the importance of the Continuous Quality Improvement program for increasing the accuracy and credibility of cytopathologic exams as well as developing national cancer exam guidelines and government projects on the prevention and treatment of cancer. -
Citations
Citations to this article as recorded by- Commercially Available Artificial Intelligence Solutions for Gynaecologic Cytology Screening and Their Integration Into Clinical Workflow
Yosep Chong, Andrey Bychkov
Cytopathology.2026; 37(1): 24. CrossRef - Practice of Cytopathology in Korea: A 40‐Year Evolution Through Standardization, Digital Transformation, and Global Partnership
Yosep Chong, Ran Hong, Hyeong Ju Kwon, Haeryoung Kim, Lucia Kim, Soon Jae Kim, Yoon Jung Choi
Diagnostic Cytopathology.2026; 54(2): 146. CrossRef - Sensitivity, Specificity, and Cost–Benefit Effect Between Primary Human Papillomavirus Testing, Primary Liquid‐Based Cytology, and Co‐Testing Algorithms for Cervical Lesions
Chang Gok Woo, Seung‐Myoung Son, Hye‐Kyung Hwang, Jung‐Sil Bae, Ok‐Jun Lee, Ho‐Chang Lee
Diagnostic Cytopathology.2025; 53(1): 35. CrossRef - A Study on the Workload of Cytotechnologists: Focus on Commercial Laboratories
Eun-Suk PARK
Korean Journal of Clinical Laboratory Science.2025; 57(2): 228. CrossRef - Integration of Digital Cytology in Quality Assurance Programs for Cytopathology
Yosep Chong, Maria Jesús Fernández Aceñero, Zaibo Li, Andrey Bychkov
Acta Cytologica.2025; : 1. CrossRef - National quality assurance program using digital cytopathology: a 5-year digital transformation experience by the Korean Society for Cytopathology
Yosep Chong, Hyeong Ju Kwon, Soon Auck Hong, Sung Soon Kim, Bo-Sung Kim, Younghee Choi, Yoon Jung Choi, Jung-Soo Pyo, Ji Yun Jeong, Soo Jin Jung, Hoon Kyu Oh, Seung-Sook Lee
Journal of Pathology and Translational Medicine.2025; 59(5): 320. CrossRef - Diagnostic proficiency test using digital cytopathology and comparative assessment of whole slide images of cytologic samples for quality assurance program in Korea
Yosep Chong, Soon Auck Hong, Hoon Kyu Oh, Soo Jin Jung, Bo-Sung Kim, Ji Yun Jeong, Ho-Chang Lee, Gyungyub Gong
Journal of Pathology and Translational Medicine.2023; 57(5): 251. CrossRef - Recent Application of Artificial Intelligence in Non-Gynecological Cancer Cytopathology: A Systematic Review
Nishant Thakur, Mohammad Rizwan Alam, Jamshid Abdul-Ghafar, Yosep Chong
Cancers.2022; 14(14): 3529. CrossRef - Re-Increasing Trends in Thyroid Cancer Incidence after a Short Period of Decrease in Korea: Reigniting the Debate on Ultrasound Screening
Chan Kwon Jung, Ja Seong Bae, Young Joo Park
Endocrinology and Metabolism.2022; 37(5): 816. CrossRef - Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice
Soon Auck Hong, Haeyoen Jung, Sung Sun Kim, Min-Sun Jin, Jung-Soo Pyo, Ji Yun Jeong, Younghee Choi, Gyungyub Gong, Yosep Chong
Journal of Pathology and Translational Medicine.2022; 56(6): 361. CrossRef
- Commercially Available Artificial Intelligence Solutions for Gynaecologic Cytology Screening and Their Integration Into Clinical Workflow
- Continuous quality improvement program and its results of Korean Society for Cytopathology
- Yoo-Duk Choi, Hoon-Kyu Oh, Su-Jin Kim, Kyung-Hee Kim, Yun-Kyung Lee, Bo-Sung Kim, Eun-Jeong Jang, Yoon-Jung Choi, Eun-Kyung Han, Dong-Hoon Kim, Younghee Choi, Chan-Kwon Jung, Sung-Nam Kim, Kyueng-Whan Min, Seok-Jin Yoon, Hun-Kyung Lee, Kyung Un Choi, Hye Kyoung Yoon
- J Pathol Transl Med. 2020;54(3):246-252. Published online April 15, 2020
- DOI: https://doi.org/10.4132/jptm.2020.02.22
- 7,044 View
- 131 Download
- 6 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Since 1995, the Korean Society for Cytopathology has overseen the Continuous Quality Improvement program for cytopathology laboratories. The Committee of Quality Improvement has carried out an annual survey of cytology data for each laboratory and set standards for proficiency tests. Methods: Evaluations were conducted four times per year from 2008 to 2018 and comprised statistics regarding cytology diagnoses of previous years, proficiency tests using cytology slides provided by the committee, assessment of adequacy of gynecology (GYN) cytology slides, and submission of cytology slides for proficiency tests. Results: A total of 206 institutes participated in 2017, and the results were as follows. The number of cytology tests increased from year to year. The ratio of liquid-based cytology in GYN gradually decreased, as most of the GYN cytology had been performed at commercial laboratories. The distribution of GYN diagnoses demonstrated nearly 3.0% as atypical squamous cells. The rate for squamous cell carcinoma was less than 0.02%. The atypical squamous cell/squamous intraepithelial lesion ratio was about 3:1 and showed an upward trend. The major discordant rate of cytology-histology in GYN cytology was less than 1%. The proficiency test maintained a major discordant rate less than 2%. The rate of inappropriate specimens for GYN cytology slides gradually decreased. Conclusions: The Continuous Quality Improvement program should be included in quality assurance programs. Moreover, these data can contribute to development of national cancer examination guidelines and facilitate cancer prevention and treatment. -
Citations
Citations to this article as recorded by- Practice of Cytopathology in Korea: A 40‐Year Evolution Through Standardization, Digital Transformation, and Global Partnership
Yosep Chong, Ran Hong, Hyeong Ju Kwon, Haeryoung Kim, Lucia Kim, Soon Jae Kim, Yoon Jung Choi
Diagnostic Cytopathology.2026; 54(2): 146. CrossRef - A Study on the Workload of Cytotechnologists: Focus on Commercial Laboratories
Eun-Suk PARK
Korean Journal of Clinical Laboratory Science.2025; 57(2): 228. CrossRef - Integration of Digital Cytology in Quality Assurance Programs for Cytopathology
Yosep Chong, Maria Jesús Fernández Aceñero, Zaibo Li, Andrey Bychkov
Acta Cytologica.2025; : 1. CrossRef - National quality assurance program using digital cytopathology: a 5-year digital transformation experience by the Korean Society for Cytopathology
Yosep Chong, Hyeong Ju Kwon, Soon Auck Hong, Sung Soon Kim, Bo-Sung Kim, Younghee Choi, Yoon Jung Choi, Jung-Soo Pyo, Ji Yun Jeong, Soo Jin Jung, Hoon Kyu Oh, Seung-Sook Lee
Journal of Pathology and Translational Medicine.2025; 59(5): 320. CrossRef - Diagnostic proficiency test using digital cytopathology and comparative assessment of whole slide images of cytologic samples for quality assurance program in Korea
Yosep Chong, Soon Auck Hong, Hoon Kyu Oh, Soo Jin Jung, Bo-Sung Kim, Ji Yun Jeong, Ho-Chang Lee, Gyungyub Gong
Journal of Pathology and Translational Medicine.2023; 57(5): 251. CrossRef - Re-Increasing Trends in Thyroid Cancer Incidence after a Short Period of Decrease in Korea: Reigniting the Debate on Ultrasound Screening
Chan Kwon Jung, Ja Seong Bae, Young Joo Park
Endocrinology and Metabolism.2022; 37(5): 816. CrossRef - Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice
Soon Auck Hong, Haeyoen Jung, Sung Sun Kim, Min-Sun Jin, Jung-Soo Pyo, Ji Yun Jeong, Younghee Choi, Gyungyub Gong, Yosep Chong
Journal of Pathology and Translational Medicine.2022; 56(6): 361. CrossRef
- Practice of Cytopathology in Korea: A 40‐Year Evolution Through Standardization, Digital Transformation, and Global Partnership
- Current Cytology Practices in Korea: A Nationwide Survey by the Korean Society for Cytopathology
- Eun Ji Oh, Chan Kwon Jung, Dong-Hoon Kim, Han Kyeom Kim, Wan Seop Kim, So-Young Jin, Hye Kyoung Yoon
- J Pathol Transl Med. 2017;51(6):579-587. Published online September 27, 2017
- DOI: https://doi.org/10.4132/jptm.2017.08.11
- 9,396 View
- 173 Download
- 13 Web of Science
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Limited data are available on the current status of cytology practices in Korea. This nationwide study presents Korean cytology statistics from 2015.
Methods
A nationwide survey was conducted in 2016 as a part of the mandatory quality-control program by the Korean Society for Cytopathology. The questionnaire was sent to 208 medical institutions performing cytopathologic examinations in Korea. Individual institutions were asked to submit their annual cytology statistical reports and gynecologic cytology-histology correlation data for 2015.
Results
Responses were obtained from 206 medical institutions including 83 university hospitals, 87 general hospitals, and 36 commercial laboratories. A total of 8,284,952 cytologic examinations were performed in 2015, primarily in commercial laboratories (74.9%). The most common cytology specimens were gynecologic samples (81.3%). Conventional smears and liquid-based cytology were performed in 6,190,526 (74.7%) and 2,094,426 (25.3%) cases, respectively. The overall diagnostic concordance rate between cytologic and histologic diagnoses of uterine cervical samples was 70.5%. Discordant cases were classified into three categories: category A (minimal clinical impact, 17.4%), category B (moderate clinical impact, 10.2%), and category C (major clinical impact, 1.9%). The ratio of atypical squamous cells of undetermined significance to squamous intraepithelial lesion was 1.6 in university hospitals, 2.9 in general hospitals, and 4.9 in commercial laboratories.
Conclusions
This survey reveals the current status and trend of cytology practices in Korea. The results of this study can serve as basic data for the establishment of nationwide cytopathology policies and quality improvement guidelines in Korean medical institutions. -
Citations
Citations to this article as recorded by- A Study on the Workload of Cytotechnologists: Focus on Commercial Laboratories
Eun-Suk PARK
Korean Journal of Clinical Laboratory Science.2025; 57(2): 228. CrossRef - Practice of Cytopathology in Korea: A 40‐Year Evolution Through Standardization, Digital Transformation, and Global Partnership
Yosep Chong, Ran Hong, Hyeong Ju Kwon, Haeryoung Kim, Lucia Kim, Soon Jae Kim, Yoon Jung Choi
Diagnostic Cytopathology.2025;[Epub] CrossRef - Current state of cytopathology residency training: a Korean national survey of pathologists
Uiju Cho, Tae Jung Kim, Wan Seop Kim, Kyo Young Lee, Hye Kyoung Yoon, Hyun Joo Choi
Journal of Pathology and Translational Medicine.2023; 57(2): 95. CrossRef - Diagnostic proficiency test using digital cytopathology and comparative assessment of whole slide images of cytologic samples for quality assurance program in Korea
Yosep Chong, Soon Auck Hong, Hoon Kyu Oh, Soo Jin Jung, Bo-Sung Kim, Ji Yun Jeong, Ho-Chang Lee, Gyungyub Gong
Journal of Pathology and Translational Medicine.2023; 57(5): 251. CrossRef - Recent Application of Artificial Intelligence in Non-Gynecological Cancer Cytopathology: A Systematic Review
Nishant Thakur, Mohammad Rizwan Alam, Jamshid Abdul-Ghafar, Yosep Chong
Cancers.2022; 14(14): 3529. CrossRef - Re-Increasing Trends in Thyroid Cancer Incidence after a Short Period of Decrease in Korea: Reigniting the Debate on Ultrasound Screening
Chan Kwon Jung, Ja Seong Bae, Young Joo Park
Endocrinology and Metabolism.2022; 37(5): 816. CrossRef - Diagnostic distribution and pitfalls of glandular abnormalities in cervical cytology: a 25-year single-center study
Jung-A Sung, Ilias P. Nikas, Haeryoung Kim, Han Suk Ryu, Cheol Lee
Journal of Pathology and Translational Medicine.2022; 56(6): 354. CrossRef - Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice
Soon Auck Hong, Haeyoen Jung, Sung Sun Kim, Min-Sun Jin, Jung-Soo Pyo, Ji Yun Jeong, Younghee Choi, Gyungyub Gong, Yosep Chong
Journal of Pathology and Translational Medicine.2022; 56(6): 361. CrossRef - Systematic screening for cervical cancer in Dakar region: prevalence and correlation with biological and socio-demographic parameters
Dominique Diouf, Gora Diop, Cheikh Ahmadou Tidian Diarra, Aminata Issa Ngom, Khadija Niane, Moussa Ndiaye, Sidy Ka, Oumar Faye, Ahmadou Dem
Infectious Agents and Cancer.2020;[Epub] CrossRef - Continuous quality improvement program and its results of Korean Society for Cytopathology
Yoo-Duk Choi, Hoon-Kyu Oh, Su-Jin Kim, Kyung-Hee Kim, Yun-Kyung Lee, Bo-Sung Kim, Eun-Jeong Jang, Yoon-Jung Choi, Eun-Kyung Han, Dong-Hoon Kim, Younghee Choi, Chan-Kwon Jung, Sung-Nam Kim, Kyueng-Whan Min, Seok-Jin Yoon, Hun-Kyung Lee, Kyung Un Choi, Hye
Journal of Pathology and Translational Medicine.2020; 54(3): 246. CrossRef - Current status of cytopathology practices in Korea: annual report on the Continuous Quality Improvement program of the Korean Society for Cytopathology for 2018
Yosep Chong, Haeyoen Jung, Jung-Soo Pyo, Soon Won Hong, Hoon Kyu Oh
Journal of Pathology and Translational Medicine.2020; 54(4): 318. CrossRef - Current Status of and Perspectives on Cervical Cancer Screening in Korea
Sung-Chul Lim, Chong Woo Yoo
Journal of Pathology and Translational Medicine.2019; 53(4): 210. CrossRef - Cytomorphological Features of Hyperchromatic Crowded Groups in Liquid-Based Cervicovaginal Cytology: A Single Institutional Experience
Youngeun Lee, Cheol Lee, In Ae Park, Hyoung Jin An, Haeryoung Kim
Journal of Pathology and Translational Medicine.2019; 53(6): 393. CrossRef - Comparison Study of the Adequacy and Pain Scale of Ultrasound-Guided Fine-Needle Aspiration of Solid Thyroid Nodules with a 21- or 23-Gauge Needle for Liquid-Based Cytology: a Single-Center Study
Soo Jin Jung, Dong Wook Kim, Hye Jin Baek
Endocrine Pathology.2018; 29(1): 30. CrossRef - Thyroid Fine-Needle Aspiration Cytology Practice in Korea
Yoon Jin Cha, Ju Yeon Pyo, SoonWon Hong, Jae Yeon Seok, Kyung-Ju Kim, Jee-Young Han, Jeong Mo Bae, Hyeong Ju Kwon, Yeejeong Kim, Kyueng-Whan Min, Soonae Oak, Sunhee Chang
Journal of Pathology and Translational Medicine.2017; 51(6): 521. CrossRef
- A Study on the Workload of Cytotechnologists: Focus on Commercial Laboratories
Review
- Good Laboratory Standards for Clinical Next-Generation Sequencing Cancer Panel Tests
- Jihun Kim, Woong-Yang Park, Nayoung K. D. Kim, Se Jin Jang, Sung-Min Chun, Chang-Ohk Sung, Jene Choi, Young-Hyeh Ko, Yoon-La Choi, Hyo Sup Shim, Jae-Kyung Won
- J Pathol Transl Med. 2017;51(3):191-204. Published online May 10, 2017
- DOI: https://doi.org/10.4132/jptm.2017.03.14
- 29,164 View
- 1,118 Download
- 36 Web of Science
- 37 Crossref
-
 Abstract
Abstract
 PDF
PDF - Next-generation sequencing (NGS) has recently emerged as an essential component of personalized cancer medicine due to its high throughput and low per-base cost. However, no sufficient guidelines for implementing NGS as a clinical molecular pathology test are established in Korea. To ensure clinical grade quality without inhibiting adoption of NGS, a taskforce team assembled by the Korean Society of Pathologists developed laboratory guidelines for NGS cancer panel testing procedures and requirements for clinical implementation of NGS. This consensus standard proposal consists of two parts: laboratory guidelines and requirements for clinical NGS laboratories. The laboratory guidelines part addressed several important issues across multistep NGS cancer panel tests including choice of gene panel and platform, sample handling, nucleic acid management, sample identity tracking, library preparation, sequencing, analysis and reporting. Requirements for clinical NGS tests were summarized in terms of documentation, validation, quality management, and other required written policies. Together with appropriate pathologist training and international laboratory standards, these laboratory standards would help molecular pathology laboratories to successfully implement NGS cancer panel tests in clinic. In this way, the oncology community would be able to help patients to benefit more from personalized cancer medicine.
-
Citations
Citations to this article as recorded by- Tumour purity assessment with deep learning in colorectal cancer and impact on molecular analysis
Lydia A Schoenpflug, Aikaterini Chatzipli, Korsuk Sirinukunwattana, Susan Richman, Andrew Blake, James Robineau, Kirsten D Mertz, Clare Verrill, Simon J Leedham, Claire Hardy, Celina Whalley, Keara Redmond, Philip Dunne, Steven Walker, Andrew D Beggs, Ult
The Journal of Pathology.2025; 265(2): 184. CrossRef - Clinical Validation of Local Versus Commercial Genomic Testing in Cancer: A Comparison of Tissue and Plasma Concordance
Lucy G. Faulkner, Lynne Howells, Susann Lehman, Caroline Cowley, Zahirah Sidat, Jacqui Shaw, Anne L. Thomas
Cancer Investigation.2025; 43(2): 119. CrossRef - Diagnostic Implications of NGS-Based Molecular Profiling in Mature B-Cell Lymphomas with Potential Bone Marrow Involvement
Bernhard Strasser, Sebastian Mustafa, Josef Seier, Erich Wimmer, Josef Tomasits
Diagnostics.2025; 15(6): 727. CrossRef - Pragmatic nationwide master observational trial based on genomic alterations in advanced solid tumors: KOrean Precision Medicine Networking Group Study of MOlecular profiling guided therapy based on genomic alterations in advanced Solid tumors (KOSMOS)-II
Sun Young Kim, Jee Hyun Kim, Tae-Yong Kim, Sook Ryun Park, Shinkyo Yoon, Soohyeon Lee, Se-Hoon Lee, Tae Min Kim, Sae-Won Han, Hye Ryun Kim, Hongseok Yun, Sejoon Lee, Jihun Kim, Yoon-La Choi, Kui Son Choi, Heejung Chae, Hyewon Ryu, Gyeong-Won Lee, Dae Youn
BMC Cancer.2024;[Epub] CrossRef - Reporting of somatic variants in clinical cancer care: recommendations of the Swiss Society of Molecular Pathology
Yann Christinat, Baptiste Hamelin, Ilaria Alborelli, Paolo Angelino, Valérie Barbié, Bettina Bisig, Heather Dawson, Milo Frattini, Tobias Grob, Wolfram Jochum, Ronny Nienhold, Thomas McKee, Matthias Matter, Edoardo Missiaglia, Francesca Molinari, Sacha Ro
Virchows Archiv.2024; 485(6): 1033. CrossRef - Acute myeloid leukemia and myelodysplastic neoplasms: clinical implications of myelodysplasia-related genes mutations and TP53 aberrations
Hyunwoo Kim, Ja Young Lee, Shinae Yu, Eunkyoung Yoo, Hye Ran Kim, Sang Min Lee, Won Sik Lee
Blood Research.2024;[Epub] CrossRef - Validation and Clinical Application of ONCOaccuPanel for Targeted Next-Generation Sequencing of Solid Tumors
Moonsik Kim, Changseon Lee, Juyeon Hong, Juhee Kim, Ji Yun Jeong, Nora Jee-Young Park, Ji-Eun Kim, Ji Young Park
Cancer Research and Treatment.2023; 55(2): 429. CrossRef - Establishing molecular pathology curriculum for pathology trainees and continued medical education: a collaborative work from the Molecular Pathology Study Group of the Korean Society of Pathologists
Jiwon Koh, Ha Young Park, Jeong Mo Bae, Jun Kang, Uiju Cho, Seung Eun Lee, Haeyoun Kang, Min Eui Hong, Jae Kyung Won, Youn-La Choi, Wan-Seop Kim, Ahwon Lee
Journal of Pathology and Translational Medicine.2023; 57(5): 265. CrossRef - Clinical applications of next-generation sequencing in the diagnosis of genetic disorders in Korea: a narrative review
Jihoon G. Yoon, Man Jin Kim, Yong Jin Kwon, Jong-Hee Chae
Journal of the Korean Medical Association.2023; 66(10): 613. CrossRef - Obtaining spatially resolved tumor purity maps using deep multiple instance learning in a pan-cancer study
Mustafa Umit Oner, Jianbin Chen, Egor Revkov, Anne James, Seow Ye Heng, Arife Neslihan Kaya, Jacob Josiah Santiago Alvarez, Angela Takano, Xin Min Cheng, Tony Kiat Hon Lim, Daniel Shao Weng Tan, Weiwei Zhai, Anders Jacobsen Skanderup, Wing-Kin Sung, Hwee
Patterns.2022; 3(2): 100399. CrossRef - Update on Molecular Diagnosis in Extranodal NK/T-Cell Lymphoma and Its Role in the Era of Personalized Medicine
Ka-Hei (Murphy) Sun, Yin-Ting (Heylie) Wong, Ka-Man (Carmen) Cheung, Carmen (Michelle) Yuen, Yun-Tat (Ted) Chan, Wing-Yan (Jennifer) Lai, Chun (David) Chao, Wing-Sum (Katie) Fan, Yuen-Kiu (Karen) Chow, Man-Fai Law, Ho-Chi (Tommy) Tam
Diagnostics.2022; 12(2): 409. CrossRef - Defining Novel DNA Virus-Tumor Associations and Genomic Correlates Using Prospective Clinical Tumor/Normal Matched Sequencing Data
Chad M. Vanderbilt, Anita S. Bowman, Sumit Middha, Kseniya Petrova-Drus, Yi-Wei Tang, Xin Chen, Youxiang Wang, Jason Chang, Natasha Rekhtman, Klaus J. Busam, Sounak Gupta, Meera Hameed, Maria E. Arcila, Marc Ladanyi, Michael F. Berger, Snjezana Dogan, Ahm
The Journal of Molecular Diagnostics.2022; 24(5): 515. CrossRef - Performance Evaluation of Three DNA Sample Tracking Tools in a Whole Exome Sequencing Workflow
Gertjan Wils, Céline Helsmoortel, Pieter-Jan Volders, Inge Vereecke, Mauro Milazzo, Jo Vandesompele, Frauke Coppieters, Kim De Leeneer, Steve Lefever
Molecular Diagnosis & Therapy.2022; 26(4): 411. CrossRef - Clinical Quality Considerations when Using Next-Generation Sequencing (NGS) in Clinical Drug Development
Timothé Ménard, Alaina Barros, Christopher Ganter
Therapeutic Innovation & Regulatory Science.2021; 55(5): 1066. CrossRef - Fast Healthcare Interoperability Resources (FHIR)–Based Quality Information Exchange for Clinical Next-Generation Sequencing Genomic Testing: Implementation Study
Donghyeong Seong, Sungwon Jung, Sungchul Bae, Jongsuk Chung, Dae-Soon Son, Byoung-Kee Yi
Journal of Medical Internet Research.2021; 23(4): e26261. CrossRef - Status of Next-Generation Sequencing-Based Genetic Diagnosis in Hematologic Malignancies in Korea (2017-2018)
JinJu Kim, Ja Young Lee, Jungwon Huh, Myung-Hyun Nam, Myungshin Kim, Young-Uk Cho, Sun-Young Kong, Seung-Tae Lee, In-Suk Kim
Laboratory Medicine Online.2021; 11(1): 25. CrossRef - MSI-Testung
Josef Rüschoff, Gustavo Baretton, Hendrik Bläker, Wolfgang Dietmaier, Manfred Dietel, Arndt Hartmann, Lars-Christian Horn, Korinna Jöhrens, Thomas Kirchner, Ruth Knüchel, Doris Mayr, Sabine Merkelbach-Bruse, Hans-Ulrich Schildhaus, Peter Schirmacher, Mark
Der Pathologe.2021; 42(4): 414. CrossRef - Molecular biomarker testing for non–small cell lung cancer: consensus statement of the Korean Cardiopulmonary Pathology Study Group
Sunhee Chang, Hyo Sup Shim, Tae Jung Kim, Yoon-La Choi, Wan Seop Kim, Dong Hoon Shin, Lucia Kim, Heae Surng Park, Geon Kook Lee, Chang Hun Lee
Journal of Pathology and Translational Medicine.2021; 55(3): 181. CrossRef - MSI testing
Josef Rüschoff, Gustavo Baretton, Hendrik Bläker, Wolfgang Dietmaier, Manfred Dietel, Arndt Hartmann, Lars-Christian Horn, Korinna Jöhrens, Thomas Kirchner, Ruth Knüchel, Doris Mayr, Sabine Merkelbach-Bruse, Hans-Ulrich Schildhaus, Peter Schirmacher, Mark
Der Pathologe.2021; 42(S1): 110. CrossRef - 16S rDNA microbiome composition pattern analysis as a diagnostic biomarker for biliary tract cancer
Huisong Lee, Hyeon Kook Lee, Seog Ki Min, Won Hee Lee
World Journal of Surgical Oncology.2020;[Epub] CrossRef - Risk Stratification Using a Novel Genetic Classifier IncludingPLEKHS1Promoter Mutations for Differentiated Thyroid Cancer with Distant Metastasis
Chan Kwon Jung, Seung-Hyun Jung, Sora Jeon, Young Mun Jeong, Yourha Kim, Sohee Lee, Ja-Seong Bae, Yeun-Jun Chung
Thyroid.2020; 30(11): 1589. CrossRef - Biomarker testing for advanced lung cancer by next-generation sequencing; a valid method to achieve a comprehensive glimpse at mutational landscape
Anurag Mehta, Smreti Vasudevan, Sanjeev Kumar Sharma, Manoj Panigrahi, Moushumi Suryavanshi, Mumtaz Saifi, Ullas Batra
Applied Cancer Research.2020;[Epub] CrossRef - Application Areas of Traditional Molecular Genetic Methods and NGS in relation to Hereditary Urological Cancer Diagnosis
Dmitry S. Mikhaylenko, Alexander S. Tanas, Dmitry V. Zaletaev, Marina V. Nemtsova
Journal of Oncology.2020; 2020: 1. CrossRef - Assembling and Validating Bioinformatic Pipelines for Next-Generation Sequencing Clinical Assays
Jeffrey A SoRelle, Megan Wachsmann, Brandi L. Cantarel
Archives of Pathology & Laboratory Medicine.2020; 144(9): 1118. CrossRef - Standard operating procedure for somatic variant refinement of sequencing data with paired tumor and normal samples
Erica K. Barnell, Peter Ronning, Katie M. Campbell, Kilannin Krysiak, Benjamin J. Ainscough, Lana M. Sheta, Shahil P. Pema, Alina D. Schmidt, Megan Richters, Kelsy C. Cotto, Arpad M. Danos, Cody Ramirez, Zachary L. Skidmore, Nicholas C. Spies, Jasreet Hun
Genetics in Medicine.2019; 21(4): 972. CrossRef - A DNA pool of FLT3-ITD positive DNA samples can be used efficiently for analytical evaluation of NGS-based FLT3-ITD quantitation - Testing several different ITD sequences and rates, simultaneously
Zoltán A. Mezei, Dávid Tornai, Róza Földesi, László Madar, Andrea Sümegi, Mária Papp, Péter Antal-Szalmás
Journal of Biotechnology.2019; 303: 25. CrossRef - Pharmacogenomic Testing: Clinical Evidence and Implementation Challenges
Catriona Hippman, Corey Nislow
Journal of Personalized Medicine.2019; 9(3): 40. CrossRef - Cancer Panel Assay for Precision Oncology Clinic: Results from a 1-Year Study
Dohee Kwon, Binnari Kim, Hyeong Chan Shin, Eun Ji Kim, Sang Yun Ha, Kee-Taek Jang, Seung Tae Kim, Jeeyun Lee, Won Ki Kang, Joon Oh Park, Kyoung-Mee Kim
Translational Oncology.2019; 12(11): 1488. CrossRef - Analytical Evaluation of an NGS Testing Method for Routine Molecular Diagnostics on Melanoma Formalin-Fixed, Paraffin-Embedded Tumor-Derived DNA
Irene Mancini, Lisa Simi, Francesca Salvianti, Francesca Castiglione, Gemma Sonnati, Pamela Pinzani
Diagnostics.2019; 9(3): 117. CrossRef - Benchmark Database for Process Optimization and Quality Control of Clinical Cancer Panel Sequencing
Donghyeong Seong, Jongsuk Chung, Ki-Wook Lee, Sook-Young Kim, Byung-Suk Kim, Jung-Keun Song, Sungwon Jung, Taeseob Lee, Donghyun Park, Byoung-Kee Yi, Woong-Yang Park, Dae-Soon Son
Biotechnology and Bioprocess Engineering.2019; 24(5): 793. CrossRef - Use of the Ion PGM and the GeneReader NGS Systems in Daily Routine Practice for Advanced Lung Adenocarcinoma Patients: A Practical Point of View Reporting a Comparative Study and Assessment of 90 Patients
Simon Heeke, Véronique Hofman, Elodie Long-Mira, Virginie Lespinet, Salomé Lalvée, Olivier Bordone, Camille Ribeyre, Virginie Tanga, Jonathan Benzaquen, Sylvie Leroy, Charlotte Cohen, Jérôme Mouroux, Charles Marquette, Marius Ilié, Paul Hofman
Cancers.2018; 10(4): 88. CrossRef - Use of the Ion AmpliSeq Cancer Hotspot Panel in clinical molecular pathology laboratories for analysis of solid tumours: With emphasis on validation with relevant single molecular pathology tests and the Oncomine Focus Assay
Ahwon Lee, Sung-Hak Lee, Chan Kwon Jung, Gyungsin Park, Kyo Young Lee, Hyun Joo Choi, Ki Ouk Min, Tae Jung Kim, Eun Jung Lee, Youn Soo Lee
Pathology - Research and Practice.2018; 214(5): 713. CrossRef - Recent Advancement of the Molecular Diagnosis in Pediatric Brain Tumor
Jeong-Mo Bae, Jae-Kyung Won, Sung-Hye Park
Journal of Korean Neurosurgical Society.2018; 61(3): 376. CrossRef - The long tail of molecular alterations in non-small cell lung cancer: a single-institution experience of next-generation sequencing in clinical molecular diagnostics
Caterina Fumagalli, Davide Vacirca, Alessandra Rappa, Antonio Passaro, Juliana Guarize, Paola Rafaniello Raviele, Filippo de Marinis, Lorenzo Spaggiari, Chiara Casadio, Giuseppe Viale, Massimo Barberis, Elena Guerini-Rocco
Journal of Clinical Pathology.2018; 71(9): 767. CrossRef - Clinical laboratory utilization management and improved healthcare performance
Christopher Naugler, Deirdre L. Church
Critical Reviews in Clinical Laboratory Sciences.2018; 55(8): 535. CrossRef - Development of HLA-A, -B and -DR Typing Method Using Next-Generation Sequencing
Dong Hee Seo, Jeong Min Lee, Mi Ok Park, Hyun Ju Lee, Seo Yoon Moon, Mijin Oh, So Young Kim, Sang-Heon Lee, Ki-Eun Hyeong, Hae-Jin Hu, Dae-Yeon Cho
The Korean Journal of Blood Transfusion.2018; 29(3): 310. CrossRef - Value-based genomics
Jun Gong, Kathy Pan, Marwan Fakih, Sumanta Pal, Ravi Salgia
Oncotarget.2018; 9(21): 15792. CrossRef
- Tumour purity assessment with deep learning in colorectal cancer and impact on molecular analysis
Original Article
- Quality Control Program for Fresh Frozen Tissue and Its Results of Chonbuk National University Hospital National Biobank of Korea.
- Shin Young Park, Hyun Ah Baek, Hyoung Jong Kwak, Sang Hyun Hong, Ho Sung Park, Kyu Yun Jang, Woo Sung Moon, Myoung Jae Kang, Dong Geun Lee, Myoung Ja Chung
- Korean J Pathol. 2010;44(3):295-301.
- DOI: https://doi.org/10.4132/KoreanJPathol.2010.44.3.295
- 5,312 View
- 59 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Molecular tools for tissue profiling generally require collection of fresh frozen tissues (FFT) as sources of high-quality DNA and RNA. Nowadays, researchers carry out large-scale, multi-center studies and they request inter-institutional minimal intrinsic bias, some fundamental similarities, and the same standardized and validated procedures.
METHODS
This study reports standardized quality control procedure for fresh frozen tissue of the National Biobank of Korea.
RESULTS
The main procedures for quality control for FFT are as follows: records related to sample collection such as labeling of samples, transport temperature, lag time from excision of tissue to freezing, and sample size were reviewed for all fresh frozen samples. The stability of RNA and DNA in fresh frozen tissue was evaluated for 3% of collected samples and purity was assessed (ratio of the absorbance at 260 and 280 nm) as was integrity (agarose gel electrophoresis). Stained hematoxylin and eosin sections were reviewed by a pathologist to confirm the diagnosis and to assess how representative the frozen sample was.
CONCLUSIONS
We introduced that the quality-control criteria for fresh frozen tissue of the NBK. We expect that this study contributes to standardization of collection, storage, and quality control of fresh frozen tissue. -
Citations
Citations to this article as recorded by- Influence of Cold Ischemia Time and Storage Period on DNA Quality and Biomarker Research in Biobanked Colorectal Cancer Tissues
Min Gyoung Pak, Mee Sook Roh
Kosin Medical Journal.2020; 35(1): 26. CrossRef
- Influence of Cold Ischemia Time and Storage Period on DNA Quality and Biomarker Research in Biobanked Colorectal Cancer Tissues
Review
- Quality Control Program and Its Results of Korean Society for Cytopathologists.
- Hye Kyung Lee, Sung Nam Kim, Shin Kwang Khang, Chang Suk Kang, Hye Kyoung Yoon
- J Pathol Transl Med. 2008;19(2):65-71.
- DOI: https://doi.org/10.3338/kjc.2008.19.2.65
- 3,556 View
- 23 Download
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF - In Korea, the quality control(QC) program forcytopathology was introduced in 1995. The program consists of a checklist for the cytolopathology departments, analysis data on all the participating institutions' QC data, including the annual data on cytologic examinations, the distribution of the gynecological cytologic diagnoses, as based on The Bethesda System 2001, and the data on cytologic-histolgical correlation of the gynecological field, and an evaluation for diagnostic accuracy. The diagnostic accuracy program has been performed 3 times per year with using gynecological, body fluid and fine needle aspiration cytologic slides. We report here on the institutional QC data and the evaluation for diagnostic accuracy since 2004, and also on the new strategy for quality control and assurance in the cytologic field. The diagnostic accuracy results of both the participating institutions and the QC committee were as follows; Category 0 and A: about 94%, Category B: 4~5%, Category C: less than 2%. As a whole, the cytologic daignostic accuracy is relatively satisfactory. In 2008, on site evaluation for pathology and cytology laboratories, as based on the "Quality Assurance Program for Pathology Services" is now going on, and a new method using virtual slides or image files for determining the diagnostic accuracy will be performed in November 2008.
-
Citations
Citations to this article as recorded by- A Study on the Workload of Cytotechnologists: Focus on Commercial Laboratories
Eun-Suk PARK
Korean Journal of Clinical Laboratory Science.2025; 57(2): 228. CrossRef - Diagnostic proficiency test using digital cytopathology and comparative assessment of whole slide images of cytologic samples for quality assurance program in Korea
Yosep Chong, Soon Auck Hong, Hoon Kyu Oh, Soo Jin Jung, Bo-Sung Kim, Ji Yun Jeong, Ho-Chang Lee, Gyungyub Gong
Journal of Pathology and Translational Medicine.2023; 57(5): 251. CrossRef - Usefulness of p16INK4a Immunocytochemical staining for the Differentiation between Atrophy and ASCUS in Diagnosis of Uterine Cervical Cancer
Hye Ryoung Shin, Taekil Eom, Wan-Su Choi
Biomedical Science Letters.2023; 29(3): 144. CrossRef - Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice
Soon Auck Hong, Haeyoen Jung, Sung Sun Kim, Min-Sun Jin, Jung-Soo Pyo, Ji Yun Jeong, Younghee Choi, Gyungyub Gong, Yosep Chong
Journal of Pathology and Translational Medicine.2022; 56(6): 361. CrossRef - Current status of cytopathology practices in Korea: annual report on the Continuous Quality Improvement program of the Korean Society for Cytopathology for 2018
Yosep Chong, Haeyoen Jung, Jung-Soo Pyo, Soon Won Hong, Hoon Kyu Oh
Journal of Pathology and Translational Medicine.2020; 54(4): 318. CrossRef - Continuous quality improvement program and its results of Korean Society for Cytopathology
Yoo-Duk Choi, Hoon-Kyu Oh, Su-Jin Kim, Kyung-Hee Kim, Yun-Kyung Lee, Bo-Sung Kim, Eun-Jeong Jang, Yoon-Jung Choi, Eun-Kyung Han, Dong-Hoon Kim, Younghee Choi, Chan-Kwon Jung, Sung-Nam Kim, Kyueng-Whan Min, Seok-Jin Yoon, Hun-Kyung Lee, Kyung Un Choi, Hye
Journal of Pathology and Translational Medicine.2020; 54(3): 246. CrossRef - Current Status of and Perspectives on Cervical Cancer Screening in Korea
Sung-Chul Lim, Chong Woo Yoo
Journal of Pathology and Translational Medicine.2019; 53(4): 210. CrossRef - Current Cytology Practices in Korea: A Nationwide Survey by the Korean Society for Cytopathology
Eun Ji Oh, Chan Kwon Jung, Dong-Hoon Kim, Han Kyeom Kim, Wan Seop Kim, So-Young Jin, Hye Kyoung Yoon
Journal of Pathology and Translational Medicine.2017; 51(6): 579. CrossRef - Comparison of Unsatisfactory Samples from Conventional Smear versus Liquid-Based Cytology in Uterine Cervical Cancer Screening Test
Hoiseon Jeong, Sung Ran Hong, Seoung-Wan Chae, So-Young Jin, Hye Kyoung Yoon, Juhie Lee, Eun Kyung Kim, Sook Tai Ha, Sung Nam Kim, Eun-Jung Park, Jong Jae Jung, Sun Hee Sung, Sung-chul Lim
Journal of Pathology and Translational Medicine.2017; 51(3): 314. CrossRef - The Usefulness of p16INK4aImmunocytochemical Staining in ASC-H Patients
Kwang Il Yim, Yeo-Ju Kang, Tae Eun Kim, Gyeongsin Park, Eun Sun Jung, Yeong-Jin Choi, Kyo-Young Lee, Chang Seok Kang, Ahwon Lee
The Korean Journal of Pathology.2011; 45(3): 290. CrossRef
- A Study on the Workload of Cytotechnologists: Focus on Commercial Laboratories
Original Articles
- Quality Assurance of Intraoperative Consultation Review Analysis of 2,392 frozen sections.
- Dong Hae Chung, Jae Hee Suh, On Ja Kim
- Korean J Pathol. 1997;31(4):332-341.
- 2,694 View
- 48 Download
-
 Abstract
Abstract
 PDF
PDF - A retrospective quality assurance study of intraoperative consultation (frozen section) was carried out to assess the accuracy and to determine the reasons of discordance. Of 14,977 surgical pathology cases accessioned over a 6-month period in Asan Medical Center, frozen sections were done on 1,270 (8.5%) patients and 2,392 frozen sections (1.88 frozen sections/case) were performed. Discordance was noted in 106 cases (4.4%) and diagnosis was deferred in 26 cases (1.1%). All deferred cases were reviewed with the result of 53.8% justified and 46.2% unjustified. The discordant cases were divided into three categories as to their clinical significances: category A (no affect on patient care) 61.3%, B (minimal affect) 9.4%, and C (major affect) 29.2%. Of 31 category C cases, 7 cases were false positive and 24 cases were false negative. Misinterpretation (70.8%) was the leading cause of discordance, followed by sampling error (15.1%), failure to identify lesion (8.5%), and technical problem (5.7%). More than one-third (35.8%) of all discordances were of central nervous system cases. Total central nervous system cases were 403 (16.8%) with a significantly higher disordance rate (9.8%) and deferral rate (2.5%) in comparison to the other cases with 3.4% discordance rate and 0.8% deferral rate. There were 43 colorectal cancer cases of intraoperative consultation for adequacy of resectional margins. The surgical margins were between 0.4 cm and 28 cm (mean: 6.7 cm) away from the tumor and there was no tumor-positive case. The study indicates surgical pathology should 1) promote interpretative skills in cases involving minute fragments of neurosurgical cases, 2) defer the diagnosis and ask for more tissue on inadequate or inappropriate specimens and 3) give only gross opinions without unnecessary frozen section procedures in the event of simple, clear-cut cases.
- Cytologic and Histologic Correlation for Quality Assurance in Aspiration Cytology.
- Ho Jung Lee, Young Mee Cho, So Young Park, Joo ryung Huh, On Ja Kim, Gyung Yub Gong
- Korean J Pathol. 1997;31(11):1214-1221.
- 2,691 View
- 11 Download
-
 Abstract
Abstract
- For quality assurance purposes, the authors correlated aspiration cytology and subsequent tissue findings and examined the reasons for discrepancies. In three months from Jan. to Mar. 1996, 1,383 aspirations were performed, of which 285 (20.6%) had subsequent tissue diagnoses within two months. The aspiration sites were thyroid (483), lymph node (LN) (290), breast (250), soft tissue (154), liver (89), lung (49), salivary gland (26), pancreas (22), gallbladder (3), bone (3), joint (2), adrenal gland (2), scrotum (2), mediastinum (2), omentum (2), oral cavity (1), chest wall (1), and intraabdominal (1) and pelvic cavities (1). A total of 68 discrepancies were identified, and biopsies and smears from these cases were reviewed monthly. In 27 cases (40%), the discrepancy was attributed to sampling error. In five cases (9%), aspiration gave superior results with better sampling and preservation than biopsy. Thirty six cases (53% of discrepant cases) were errors in cytologic diagnosis. We categorized these discrepancies into "A", "B", and "C" ("A": minor disagreement with no affect on patient care, "B": minimal affect on patient care, "C": major affect on patient care), which were 9 (13%), 14 (21%) and 13 (19%) cases, respectively. In thirteen cases of category "C", there were eleven false negative and two false positive diagnoses. Eleven false negative cases included thyroid (3), lymph node (2), breast (2), bone (1), salivary gland (1), lung (1), and liver (1). Three cases of thyroid were papillary carcinomas diagnosed as nodular hyperplasia (1), occasional pleomorphic cells (1), and cystic change (1). Two breast cases of invasive ductal carcinomas were diagnosed as ductal hyperplasia. A malignant lymphoma was diagnosed as reactive hyperplasia and a metastatic carcinoma of LN was diagnosed as tuberculosis. Other cases were malignant tumors of bone, salivary gland, lung, and liver those were misinterpreted as benign lesion or normal. Of two false positive cases, one was nodular hyperplasia of thyroid diagnosed as papillary carcinoma and the other was normal islet cell of pancreas diagnosed as islet cell tumor. A continuous monitoring of laboratory performance is an essential component of the quality control and assurance, and the review of discrepant cases provides useful information for improvement of diagnosis.
- Quality Assuarance on Fine Needle Aspiration Cytology of Malignant Salivary Gland Neoplasms.
- Young Hyeh Ko, Young Lyun Oh
- J Pathol Transl Med. 2004;15(1):40-44.
- 2,008 View
- 11 Download
-
 Abstract
Abstract
 PDF
PDF - To evaluate the quality of fine needle aspiration cytology diagnosis on malignant salivary gland neoplasms, cytologic findings were correlated with histologic diagnosis of 56 surgically removed malignant salivary gland tumors. Seven cases (12.5%) were insufficient, 23 cases (41.1%) were diagnosed as malignant, 17 (30.4%) cases were accurately diagnosed by histologic subtype, and 9 cases (16%) were diagnosed as benign. Five out of 9 false negative cases were misdiagnosed as pleomorphic adenomas. Except the cases with insufficient specimen, overall sensitivity was 81.6%, and the sensitivity varied according to the histologic subtype; 91% in salivary duct carcinoma, 100% in carcinoma ex pleomorphic adenoma, 50% in mucoepidermoid carcinoma, 63% in adenoid cystic carcinoma, and 50% in acinic cell carcinoma. The diagnostic accuracy differed among cytopathologists irrespective of periods after acquisition of board of pathologists. These results confirm that salivary gland neoplasm can be easily misdiagnosed in fine needle aspiration cytology and a great caution should be given in diagnosing the benign appearing salivary aspirates to avoid under-diagnosis of malignant neoplasm with low grade cytologic atypia.
- Quality Assurance of Frozen Section Diagnosis An analysis of 5,273 consecutive cases .
- Sang Yong Song, Geunghwan Ahn
- Korean J Pathol. 1999;33(12):1182-1190.
- 2,550 View
- 43 Download
-
 Abstract
Abstract
 PDF
PDF - Quality assurance analysis of frozen section diagnosis is very important for the pathologists to improve the diagnostic ability and the quality of medical service. We analysed 5,273 consecutive cases of frozen section diagnosis which were done in Samsung Medical Center during 10 months from June 1, 1998 to March 31. 1999 with special reference to the discordance between frozen section diagnosis and final diagnosis. The concordance rate was 97.65%, discordance rate 1.34%, and deferred diagnosis (type 1) rate 1.01%. Category A (discordant diagnosis without any effect on the patients) was 53 cases (1.01%), category B (discordant diagnosis with minimal but no serious effect on the patients) was 10 cases (0.19%), and category C (discordant diagnosis with serious effect on the patients) was 8 cases (0.15%). Type 2 (discordant diagnosis by extra-pathologist problem) was 22 cases (0.42%) and type 3 (discordant diagnosis by pathologist problem) was 49 cases (0.93%). The most frequent causes of type 2 and 3 discordant diagnosis were presence of new lesions on deeper sections and the misinterpretation of lesions. Discordant diagnosis was noted in lymphoreticular system, central nervous system, thyroid, gastric resection margin, breast, female genital organs, intestine, hepatobiliary system, upper aerodigestive tract, urinary tract, lung, and soft tissue in descending order of frequency. Frozen section diagnosis was deferred in central nervous system, lymphoreticular system, gastric resection margin, female genital organs, thyroid, intestine, upper aerodigestive tract, lung, and soft tissue in descending order of frequency. The most important cause of discordant diagnosis was a misinterpretation of the lesions. Based on our results, a continuous and careful follow-up of quality assurance analysis of frozen section diagnosis and a share of experience of problematic cases are mandatory for the pathologists to improve the quality of medical services.
- The Current Practice of the Autopsy Services and the Autopsy Records at the Seoul National University Hospital.
- Jeong Wook Seo, Yoon Sung Lee, Je Geun Chi, Ghee Young Choe, Soong Deok Lee, Chong Jai Kim, In Ae Park, Woo Ho Kim, Ja June Jang, Chul Woo Kim, Seong Hoe Park, Jung Bin Lee, Hyun Soon Lee, Yong Il Kim, Eui Keun Ham, Sang Kook Lee
- Korean J Pathol. 1998;32(6):453-459.
- 2,254 View
- 10 Download
-
 Abstract
Abstract
- This study outlines the current status of the autopsy practice and the medical records for autopsies at the Department of Pathology, Seoul National University Hospital. Total number of autopsy cases from 1954 to 1995 was 3,131. Adults aged over 17 were 371 cases and children were 2,515 cases. The demographic data in 245 cases was not available. The number of adult autopsies and its proportion among total number of autopsies during 10-year periods decreased from 144 cases (40%) during the 10-year-period from 1956 to 52 cases (3%) during the 10-year-period from 1986. The number of children cases during the same period groups increased slightly from 210 cases (58%) to 393 cases (25%). But the number of fetal cases increased rapidly from 7 cases (2%) to 1,146 cases (72%). Among fetal autopsies the proportion of fetuses died earlier than 24 weeks of gestation increased and this figure exceeds that of fetuses that died later than 24 weeks of gestation from 1992. Forty percent of the cases were submitted from the clinical departments of the Seoul National University Hospital but the remainders were referred from 73 hospitals. Final autopsy diagnoses were analysed according to the Korean Standard Classification of Disease (KCD)-3 coding system and by searching key words for all cases. Common diagnoses as coded among cases from 1990 were P9, P0, P2, Q2 and Q0. Common diseases by key words for adult cases were liver disease, tuberculosis and pneumonia. Common diseases for children cases were pneumonia, hyaline membrane disease, meningitis and tuberculosis. Through this study we could show the importance of autopsy services for fetuses. We could also establish a regular registration system for autopsies at general hospitals.
- Image Standardization and Determination of Gray Level Threshold in the Assessment of the Myocardial Fibrosis by the Computerized Image Analysis.
- Nam Young Lee, Young Sik Park, Jin Haeng Chung, Jeong Wook Seo
- Korean J Pathol. 1998;32(7):494-503.
- 1,969 View
- 10 Download
-
 Abstract
Abstract
- The computerized image analysis is a useful tool for the quantitative assessment of histopathologic findings. In contrast to the usual microscopic examination by pathologists, the computerization should be accompanied with the standardization process of the image. We developed an algorithm to standardize images and to determine the optimal gray level threshold, using a myocardial fibrosis model. Sirius red staining was more convenient for the image analysis than Masson's trichrome staining because of a better contrast with the surrounding structures. To get an optimal measurement, light intensity was standardized at each of the fibrosis, myocardium and background. In this study, the most promising method to determine the degree of fibrosis was that of revising the background without tissue to a gray level of 200, obtaining a green component of the color image, revising the myocardial fiber to 163, and defining a partial ratio as fibrosis index when the gray level threshold was 120. These threshold levels and parameters were determined after drawing the binarization index curves according to the change of the gray level threshold and by the morphological examination of the actual binarization figures overlaid to the original color image. Through these processes we could get a consistent result on the myocardial fibrosis and we expect a similar principle applies when we analyze color images in the histopathologic quantitation by computerized image analysis.
- Second Opinion Diagnostic Discrepancy in Surgical Pathology: Asan Medical Center Experience.
- Young Min Kim, Kyung Ja Cho, Sun Young Jun, Mi Sun Choe, Shin Kwang Khang, Jae Y Ro
- Korean J Pathol. 2003;37(5):301-306.
- 2,751 View
- 40 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Review of the outside pathology material is an important practice that provides useful information on patient managements and improves the diagnostic quality in surgical pathology. We report our experience with the frequency and types of diagnostic discrepancies in patients referred to the Asan Medical Center for treatment or a second opinion.
METHODS
All referral pathology diagnoses (867 surgical cases) made from October 2001 to July 2002 at Asan Medical Center were compared with outside pathology diagnoses.
RESULTS
Of the 867 surgical cases reviewed, 231 (26.7%) cases had a diagnostic discrepancy, which included 49 (5.7%) major and 182 (21.0%) minor discrepancies. The contents of the major discrepancies were a change in the diagnosis (34 cases), a change in the type of malignancy including small cell carcinoma and non-small cell carcinoma of the lung (10), a diagnosis of a metastasis as the primary lesion (4), and errors in interpreting the invasiveness (1). The causes or reasons for the major discrepancy were a difference in interpretation (81.6%), the availability of special studies (10.2%), a failure to identify the lesions (4.1%), and a lack of clinical information (4.1%).
CONCLUSIONS
The major discrepancy rate (5.7%) was comparable to that of the other reports from western countries. Among the major discrepancies, a change in diagnosis was most commonly observed and difference in interpretation was the most common reason. A routine review of all the patients pathology material is recommended for all referral patients for an improvement in the pathologic diagnoses and to provide better medical care.
- Quality Improvement Methods in Cervico-vaginal Cytology: Cytologic/Histologic Correlation vs. 10% Random Rescreening .
- Ghil Suk Yoon, Jooryung Huh, Kyung Hee Son, On Ja Kim, Gyungyub Gong
- J Pathol Transl Med. 1998;9(2):129-138.
- 1,965 View
- 14 Download
-
 Abstract
Abstract
 PDF
PDF - Although the success of the Papanicolaou test as a screening tool of cervical cancer is evident, there still exists 2-5% of discrepancy rate by both human and machine. To improve the qualilty of cervico-vaginal cytology, the authors compared cervicovaginal smear with cervical biopsy diagnoses, and analysed the causes of discrepancies. Among 30,922 cervicovaginal smears from June 1996 to April 1997 at our hospital, there were 271 cases of cervicovaginal smear with subsequent cervical punch or LEEP cone biopsies within several months. The biopsies and smears from a total of 98 discordant cases were reviewed. The discrepancy was attributed to sampling errors in 43 cases(43.9%), and to cytologic diagnosis in 49 cases(50.0%). Among these, 43 cases were interpretative errors(categories A;19, B;16 and C;8), whereas six cases were screening errors(categories B;2 and C;4). Among cervical biopsy cases, errors were present in four. As for 10% random rescreening, cytote chnologists reviewed 3,196 of 30,922 smears during the same period. There were 43 cases of screening error(categories A;27, B;16). Cytologic/histologic correlation was superior to 10% random rescreening of negative cases. The most effective method for quality improvement in cervicovaginal cytology was to implement both quality control(rescreening) and quality assurance(cytologic/histologic correlation) programs.

 E-submission
E-submission
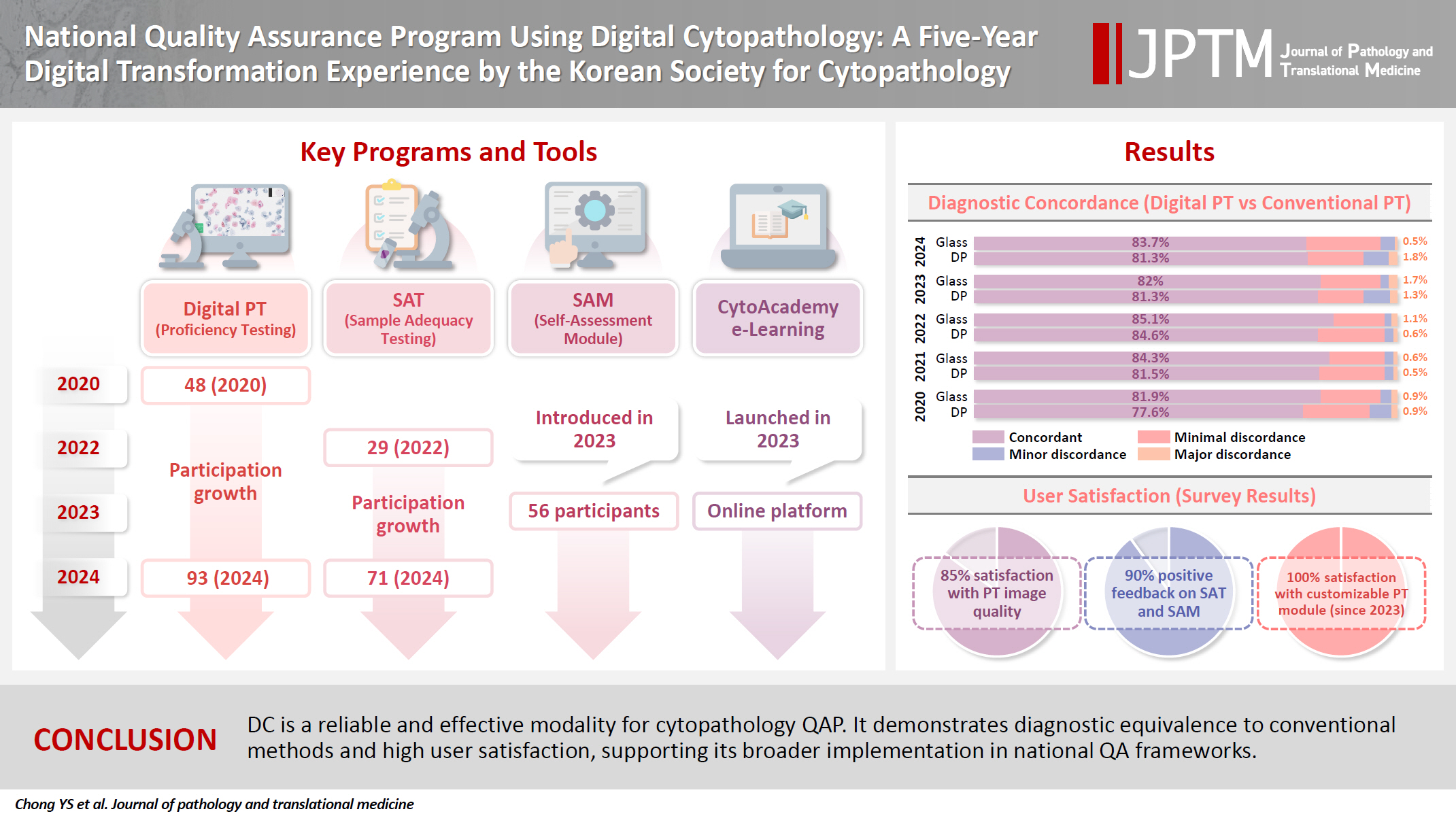

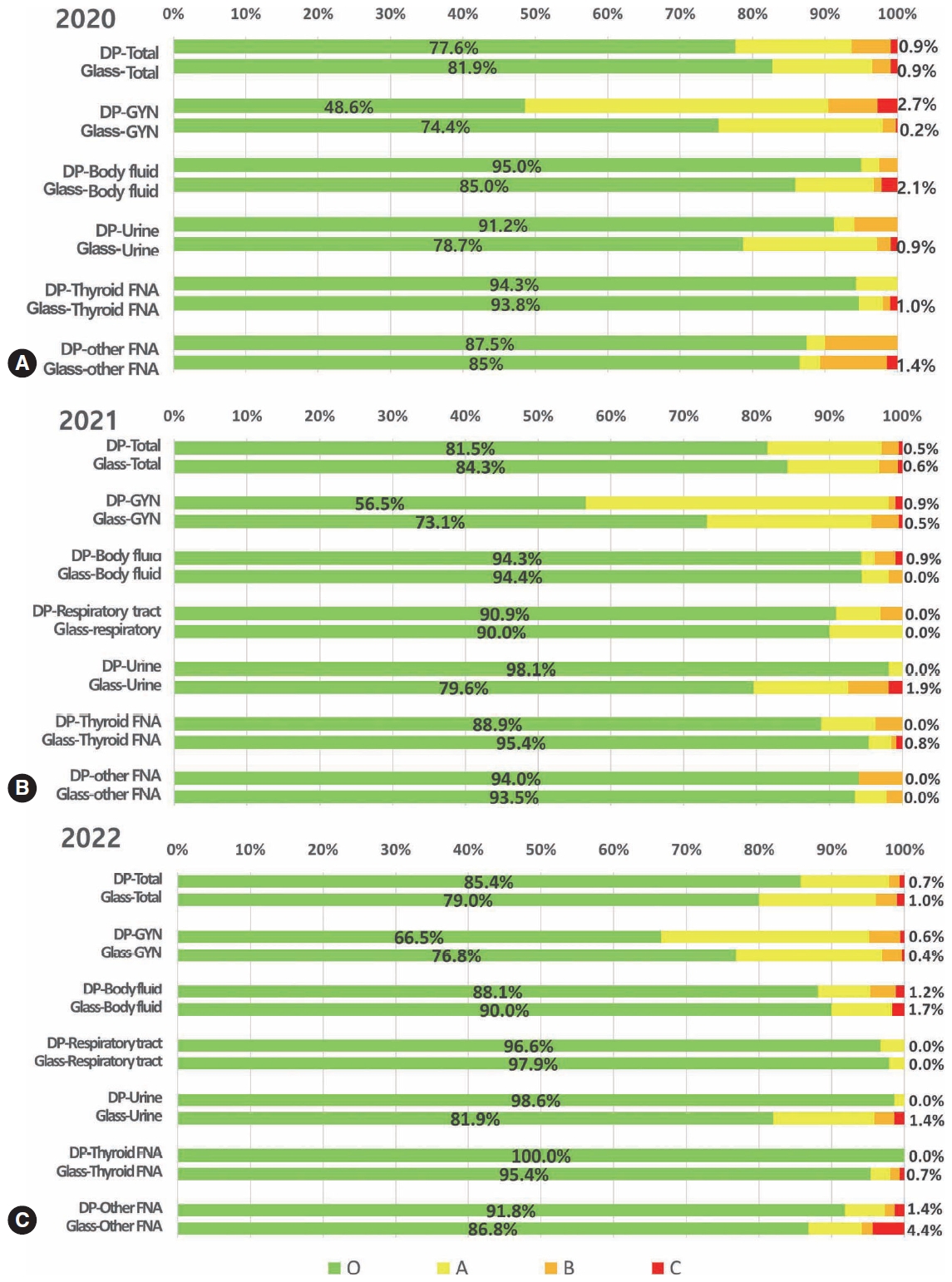


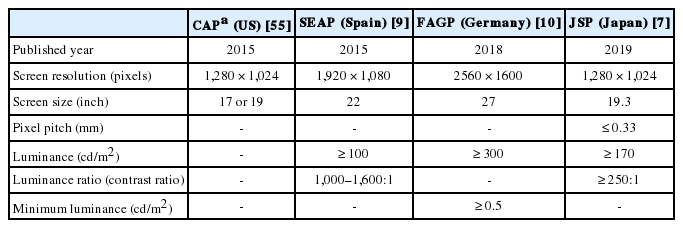


 First
First Prev
Prev



