Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 50(4); 2016 > Article
-
Original Article
Transformation to Small Cell Lung Cancer of Pulmonary Adenocarcinoma: Clinicopathologic Analysis of Six Cases - Soomin Ahn1, Soo Hyun Hwang1, Joungho Han1, Yoon-La Choi1, Se-Hoon Lee2, Jin Seok Ahn2, Keunchil Park2, Myung-Ju Ahn2, Woong-Yang Park3,4
-
Journal of Pathology and Translational Medicine 2016;50(4):258-263.
DOI: https://doi.org/10.4132/jptm.2016.04.19
Published online: May 10, 2016
1Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
2Division of Hematology and Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
3Department of Molecular Cell Biology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
4Samsung Genomic Institute, Samsung Medical Center, Seoul, Korea
- Corresponding Author Joungho Han, MD Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea Tel: +82-2-3410-2765 Fax: +82-2-3410-0025 E-mail: hanjho@skku.edu
© 2016 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background:
- Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are considered the first line treatment for a subset of EGFR-mutated non-small cell lung cancer (NSCLC) patients. Although transformation to small cell lung cancer (SCLC) is one of the known mechanisms of resistance to EGFR TKIs, it is not certain whether transformation to SCLC is exclusively found as a mechanism of TKI resistance in EGFR-mutant tumors.
-
Methods:
- We identified six patients with primary lung adenocarcinoma that showed transformation to SCLC on second biopsy (n = 401) during a 6-year period. Clinicopathologic information was analyzed and EGFR mutation results were compared between initial and second biopsy samples.
-
Results:
- Six patients showed transformation from adenocarcinoma to SCLC, of which four were pure SCLCs and two were combined adenocarcinoma and SCLCs. Clinically, four cases were EGFR-mutant tumors from non-smoking females who underwent TKI treatment, and the EGFR mutation was retained in the transformed SCLC tumors. The remaining two adenocarcinomas were EGFR wild-type, and one of these patients received EGFR TKI treatment.
-
Conclusions:
- NSCLC can acquire a neuroendocrine phenotype with or without EGFR TKI treatment.
- Cases
- During a 6-year period (2010–2015), there were a total of 2,310 diagnoses of pulmonary ADC in our institute. Of 2,310 patients, 401 patients underwent a second biopsy or resection for recurrent or metastatic tumors. Out of 401 patients, a total of six patients (1.5%) with primary lung ADC showed transformed SCLC morphology in second biopsy. Two experienced pathologists reviewed the histological slides (S.A and J.H). All patients were treated in the Department of Oncology, Samsung Medical Center (Seoul, Korea). Clinical and follow-up data were obtained through a retrospective analysis of the medical records, including age, sex, smoking history, treatment, clinical course and follow-ups. All patients were followed until March 2016 with median follow-up period of 39.2 months. The study was approved by the Institutional Review Board at Samsung Medical Center (2014-14-08610).
- EGFR mutation test
- DNA was extracted from sections of formalin-fixed, paraffin-embedded (FFPE) tissue that was also used for histologic diagnosis. Manual microdissection was performed if tumor cell percentages were less than 70% in available samples. Genomic DNA was extracted using Qiagen DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. In cases of lung ADC, routine testing for the EGFR mutation was performed in the pathology laboratory using peptide nucleic acid–mediated clamping polymerase chain reaction (PCR) mutation detection kit as previously described [13], and results were retrieved from electronic medical records. For one SCLC sample, the EGFR mutation was detected using targeted sequencing via Illumina HiSeq 2500 (Illumina Inc., San Diego, CA, USA), which was performed for clinical trial enrollment. For the rest of SCLC samples, the EGFR mutation was newly evaluated using Cobas test, a real-time PCR test as previously described [14]. EGFR mutation results were available for all samples except for one that had no residual tumor.
- Immunohistochemistry
- In the current study, we used representative FFPE tissue sections for immunohistochemical staining (IHC). IHC for CD56 and thyroid transcription factor 1 (TTF-1) was performed for SCLC or combined tumors. Staining was performed on 3-μm-thick sections from each case using a biotin-avidin-peroxidase method on a BOND-MAX autostainer (Leica, Wetzlar, Germany) after retrieval with T/E buffer (CD56) or citrate buffer (TTF-1). We used primary antibodies to CD56 (1:200, Novocastra, Newcastle upon Tyne, UK) and TTF-1 (1:100, Dako, Glostrup, Denmark).
MATERIALS AND METHODS
- Sample information and histologic features
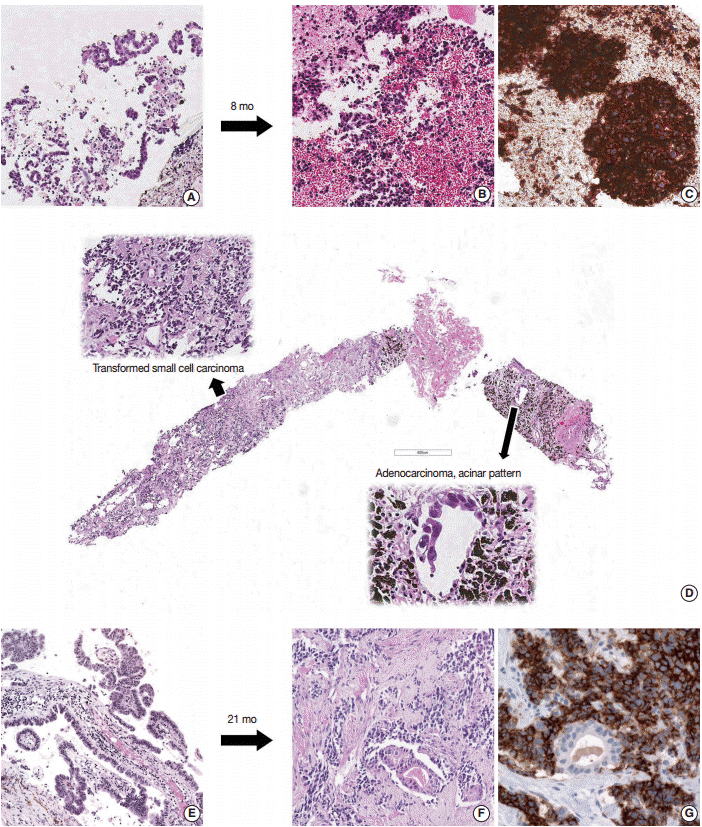
- Six patients showed transformation from ADC to SCLC. Sample information and pathologic features are summarized in Table 1. Of the initial samples with diagnosis of ADC, four were obtained using needle biopsy and two were surgically resected specimens. All second biopsies were obtained using needle biopsy. The histology of the six ADCs was acinar (n=4), mixed acinar and papillary (n=1), and mixed acinar and solid (n=1). Of samples that showed transformation to SCLC upon second biopsy, four showed pure SCLC morphology and two showed combined ADC and SCLC morphology. In two cases, ADC components demonstrated acinar morphology (Fig. 1). For small cell components, TTF-1 was expressed in four of six cases and CD56 was expressed in all five available cases. CD56 was not expressed in ADC components.
- Clinical information and EGFR status
- The clinical information and EGFR mutation status of the six patients are summarized in Table 2. Initial treatments included complete resection and adjuvant chemotherapy for case 1 (cT2NO), palliative chemotherapy for case 2 (cT3N1M1) and case 4 (cT1N0M1), EGFR TKI for case 3 (cT2N3M1), incomplete resection and palliative chemotherapy for case 5 (cT1N0M1), and complete resection for case 6 (cT1NO). The sites of distant metastasis were as follows; brain (cases 2 and 4), pleura (case 3, 4, and 5), bone (case 4), and liver (case 4).
- Of the six patients with ADC in initial biopsy, four (cases 1–4) harbored an EGFR mutation (L858R mutation, n=1; exon 19 deletion, n=3). All four, along with case 5 who had wild-type EGFR but was enrolled in a clinical trial of gefetinib, were treated with EGFR TKIs. Cases 1 and 3 were treated with irressa, and afatinib was also added for case 1. Case 2 was treated with afatinib only, and cases 4 and 5 were treated with gefetinib only. All five patients who underwent TKI treatment were female non-smokers (Table 2). Despite TKI treatment, all five patients showed disease progression, upon which a second biopsy was performed. The interval between initial biopsy and second biopsy ranged from 5 to 50 months with mean of 25.3 months.
- After confirmation of transformation of SCLC on second biopsy, four patients received further treatment. The treatment option for two patients (cases 1 and 3) was switched to etoposide and cisplatin, and one of them (case 3) showed partial response. Case 5 died due to disease progression and the other patients were alive in the short-term follow-up period.
- EGFR mutation status was compared between initial and second samples, and all pairs showed the same EGFR status. The original EGFR mutation in cases 1–4 was retained in all transformed SCLC samples, while cases 5 and 6 showed no EGFR mutation in transformed SCLC samples.
RESULTS
- Transformation of NSCLC to SCLC was recently proposed as a mechanism of resistance to TKI therapy [2,6]. Identification of histologic transformation may be an important factor in determining a patient’s treatment plan due to the differences between NSCLC and SCLC. While most reports of transformation of ADC to SCLC were identified in EGFR mutant patients related to TKI treatment, it is not certain whether transformation is exclusively related to EGFR mutation or EGFR TKI treatment [10]. Here, we report six cases of ADC which showed histologic transformation to SCLC over a 6-year period at a single institute. Similar to previous reports, four cases in our series were ADC with EGFR activating mutations that underwent TKI treatment and were subsequently found to have SCLC transformation on second biopsy. However, we also identified two additional cases of SCLC transformation that had no EGFR mutation, and one of these cases underwent initial TKI treatment.
- EGFR TKIs are now being used worldwide for first-line treatment in a subset of lung cancers bearing EGFR-activating mutations, and they have demonstrated dramatic therapeutic efficacy [15]. However, acquired resistance through multiple mechanisms has become a major problem [6]. One of mechanism of resistance to EGFR inhibitors is the histological transformation of ADC to SCLC [6]. Although the presence of combined ADC and SCLC histology at initial diagnosis is a possibility, genomic sequencing of EGFR mutations shows that both the original tumor and transformed SCLC at the time of resistance share the original EGFR-activating mutation, thus supporting the conclusion that these were not independent tumors [6-11]. However, the small biopsy size represents only a portion of tumors, and SCLC components may become dominant at the time of disease progression. Of the six patients with initially diagnosed with ADC in our report, two were diagnosed using surgically resected samples rather than needle biopsies. For these two cases, the possibility of combined histology at the initial biopsy can be excluded. In case 6, it was difficult to distinguish between SCLC transformation and second primary SCLC considering the early stage of initial ADC.
- In our series, two of six cases were EGFR–wild-type ADC. This suggests that transformation to SCLC is not unique to tumors bearing EGFR mutations, nor does it exclusively result from TKI treatment. Transformation to SCLC is also reported as a mechanism of acquired resistance to crizotinib in ALK rearranged lung tumors [16]. In addition, transformation to large cell neuroendocrine carcinoma was identified as an acquired resistance mechanism to EGFR TKIs and crizotinib [17,18]. Recent studies suggest that alveolar type II cells can give rise to both ADC and SCLC [19], so EGFR-mutant lung cancers derived from alveolar type II cells may have the potential to transform into SCLC during the disease progression [2]. In sum, it seems that acquisition of neuroendocrine phenotype, which includes SCLC transformation, can occur in the progression of disease in both EGFR-mutant and EGFR–wild-type NSCLCs.
- Second biopsies are not routinely performed for lung cancer patients when patients showed resistance to TKI treatment. Therefore, the incidence of transformation to SCLC in NSCLCs cannot be accurately calculated. An acquired TKI resistance arising from the histological transformation to SCLC has been reported to be as high as 3% [5]. In our institute, the incidence of transformation to SCLC identified in the second biopsy of total ADCs was 1.5%. Recently in our institute, there were two cases of SCLC transformation in EGFR mutant ADC during treatment with AZD9291, an oral irreversible EGFR TKI with selectivity for activating EGFR mutations and the T790M resistance mutation [20]. These cases were not included in this report. In clinical practice, identification of small cell component is important as the treatment option can be switched to etoposide and cisplatin against SCLC [12].
- In conclusion, we report six cases of lung cancer demonstrating transformation from ADC to SCLC. Four cases were EGFR-mutant tumors from female non-smokers who underwent TKI treatment, and the EGFR mutation was retained in the transformed SCLC tumors. The other two ADCs were EGFR–wild-type, and one of these patients received EGFR TKI treatment. The neuroendocrine phenotype can thus be acquired during ADC disease progression independent of EGFR TKI treatment.
DISCUSSION
| Case No. | Initial tumor | Sample type | Sample acquisition site | Subtype | Interval between biopsy (mo) | Transformed tumor | Sample type | Sample acquisition site | IHC TTF-1/CD56 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ADC | Biopsy | Lung | Acinar | 37 | SCLC | Biopsy | Celiac LN | –/+ |
| 2 | ADC | Biopsy | Lung, brain | Acinar and papillary | 21 | Combined SCLC and ADC | Biopsy | Lung | –/+a |
| 3 | ADC | Biopsy | LN 4 | Acinar | 8 | SCLC | Biopsy | LN 7 | +/+ |
| 4 | ADC | Biopsy | Lung | Acinar | 5 | Combined SCLC and ADC | Biopsy | Lung (same site) | +/NA |
| 5 | ADC | Resection | Lung | Acinar | 31 | SCLC | Biopsy | Pleura | +/+ |
| 6 | ADC | Resection | Lung | Acinar and solid | 50 | SCLC | Biopsy | Neck LN | +/+ |
| Case No. | Sex | Age (yr) | Smoking history (pack years) | Tumor histology in initial sample | Clinical tage | Initial treatment | EGFR mutation in initial sample | Treatment related to TKI | Duration of TKI treatment before transformation (mo) | Response to TKI | Tumor histology in second biopsy sample | EGFR mutation in second biopsy ample | Treatment after second biopsy | Progression or recurrance | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 57 | 0 | ADC | T2N0 | Operation, adjuvant CTx (paclitaxel/carboplatin) | L858R | Irressa, afatinib | 10 | PD | SCLC | L858R | CTx (etoposide/cisplatin) | F/U loss | F/U loss |
| 2 | F | 54 | 0 | ADC | T3N1M1 | CTx (Alimta/cisplatin) | Del19 | Afatinib | 11 | PD | Combined SCLC and ADC | del19 | CTx (gemcitabine/cisplatin) | PR | Alive |
| 3 | F | 55 | 0 | ADC | T2N3M1 | Iressa | Del19 | Iressa | 9 | PD | SCLC | del19 | CTx (etoposide/cisplatin) | PR | Alive |
| 4 | F | 59 | 0 | ADC | T1N0M1 | CTx (Alimta/cisplatin) | Del19 | Gefetinib | 11 | PD | Combined SCLC and ADC | del19 | Rociletinib | PD | Alive |
| 5 | F | 68 | 0 | ADC | T1N0M1 | Operation, palliative CTx (Alimta/cisplatin) | No mutation | Gefetinib | 2 | PD | SCLC | No mutation | No treatmenta | PD | Deadb |
| 6 | M | 67 | 35 | ADC | T1NO | Operation | No mutation | No TKI | NA | PD | SCLC | No mutation | No treatmenta | F/U loss | F/U loss |
EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; F, female; ADC, adenocarcinoma; CTx, chemotherapy; PD, progressive disease; SCLC, small cell lung cancer; F/U, follow-up; PR, partial response; M, male; NA, not-applicable.
aNo further treatment due to poor condition;
bDead due to disease progression.
- 1. Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet 2011; 378: 1727-40. ArticlePubMed
- 2. Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol 2015; 16: e165-72. ArticlePubMedPMC
- 3. Moiseenko VM, Protsenko SA, Semenov II, et al. Effectiveness of gefitinib (Iressa) as first-line therapy for inoperable non-small-cell lung cancer with mutated EGFR gene (phase II study). Vopr Onkol 2010; 56: 20-3. PubMed
- 4. Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res 2008; 14: 2895-9. ArticlePubMedPDF
- 5. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013; 19: 2240-7. ArticlePubMedPMCPDF
- 6. Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011; 3: 75ra26.
- 7. Zakowski MF, Ladanyi M, Kris MG; Memorial Sloan-Kettering Cancer Center Lung Cancer OncoGenome Group. EGFR mutations in small-cell lung cancers in patients who have never smoked. N Engl J Med 2006; 355: 213-5. Article
- 8. van Riel S, Thunnissen E, Heideman D, Smit EF, Biesma B. A patient with simultaneously appearing adenocarcinoma and small-cell lung carcinoma harbouring an identical EGFR exon 19 mutation. Ann Oncol 2012; 23: 3188-9. ArticlePubMed
- 9. Morinaga R, Okamoto I, Furuta K, et al. Sequential occurrence of non-small cell and small cell lung cancer with the same EGFR mutation. Lung Cancer 2007; 58: 411-3. ArticlePubMed
- 10. Norkowski E, Ghigna MR, Lacroix L, et al. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: new insights in the era of molecular pathology. J Thorac Oncol 2013; 8: 1265-71. ArticlePubMed
- 11. Watanabe S, Sone T, Matsui T, et al. Transformation to small-cell lung cancer following treatment with EGFR tyrosine kinase inhibitors in a patient with lung adenocarcinoma. Lung Cancer 2013; 82: 370-2. ArticlePubMed
- 12. Kim WJ, Kim S, Choi H, et al. Histological transformation from non-small cell to small cell lung carcinoma after treatment with epidermal growth factor receptor-tyrosine kinase inhibitor. Thorac Cancer 2015; 6: 800-4. ArticlePubMed
- 13. Lee B, Han G, Kwon MJ, Han J, Choi YL. KRAS mutation detection in non-small cell lung cancer using a peptide nucleic acid-mediated polymerase chain reaction clamping method and comparative validation with next-generation sequencing. Korean J Pathol 2014; 48: 100-7. ArticlePubMedPMC
- 14. Ahn S, Lee J, Sung JY, et al. Comparison of three BRAF mutation tests in formalin-fixed paraffin embedded clinical samples. Korean J Pathol 2013; 47: 348-54. ArticlePubMedPMC
- 15. Pao W, Miller VA. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J Clin Oncol 2005; 23: 2556-68. ArticlePubMed
- 16. Miyamoto S, Ikushima S, Ono R, et al. Transformation to small-cell lung cancer as a mechanism of acquired resistance to crizotinib and alectinib. Jpn J Clin Oncol 2016; 46: 170-3. ArticlePubMed
- 17. Kogo M, Shimizu R, Uehara K, et al. Transformation to large cell neuroendocrine carcinoma as acquired resistance mechanism of EGFR tyrosine kinase inhibitor. Lung Cancer 2015; 90: 364-8. ArticlePubMed
- 18. Caumont C, Veillon R, Gros A, Laharanne E, Bégueret H, Merlio JP. Neuroendocrine phenotype as an acquired resistance mechanism in ALK-rearranged lung adenocarcinoma. Lung Cancer 2016; 92: 15-8. ArticlePubMed
- 19. Sutherland KD, Proost N, Brouns I, Adriaensen D, Song JY, Berns A. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 2011; 19: 754-64. ArticlePubMed
- 20. Ham JS, Kim S, Kim HK, et al. Two cases of small cell lung cancer transformation from EGFR mutant adenocarcinoma during AZD9291 treatment. J Thorac Oncol 2016; 11: e1-4. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Patients outcomes in lung adenocarcinoma transforming to small-cell lung cancer after tyrosine kinase inhibitor therapy
Shuai Wang, Yongsen Wang, Xuan Wu, Li Yang, Xiaoju Zhang
World Journal of Surgical Oncology.2025;[Epub] CrossRef - Baseline retinoblastoma transcriptional corepressor 1 (Rb1) functional inactivation is a pre-requisite but not sufficient for small-cell histological transformation in epidermal growth factor receptor (EGFR) mutant lung adenocarcinomas post-tyrosine kinas
Aruna Nambirajan, Amber Rathor, Hemavathi Baskarane, Anju GS, Sachin Khurana, Somagattu Sushmitha, Aparna Sharma, Prabhat Singh Malik, Deepali Jain
Virchows Archiv.2025; 487(3): 639. CrossRef - Case Report: Transforming small cell lung cancer: two cases report and literature review
Jinlong Liu, Jing Ai, Lize Zhao, Yimeng Qian, Qingxin Zhao, Chunling Ma, Yu Zhao, Jing Zhao
Frontiers in Oncology.2025;[Epub] CrossRef - Exploration of CT-based discrimination and diagnosis of various pathological types of ground glass nodules in the lungs
Haihui Wu, Xiong Zhang, Zheng Zhong
BMC Medical Imaging.2025;[Epub] CrossRef - Correlation between treatments and outcomes of patients with EGFR-mutated non-small-cell lung cancer that transitioned into small-cell lung cancer: an international retrospective study
C. Catania, S.V. Liu, M. Garassino, A. Delmonte, V. Scotti, F. Cappuzzo, C. Genova, A. Russo, M. Russano, C. Bennati, I. Colantonio, S. Martini, M. Pino, F. Conforti, L. Pala, G. Minuti, F. Citarella, E. Olmetto, A. Esposito, P. Cascetta, A. Di Lello, T.
ESMO Open.2025; 10(7): 105326. CrossRef - TPM3–NTRK1 fusion confers resistance to osimertinib in lung adenocarcinoma: a model in a continuous cell line
Fang Cao, Jiayin Dai, Kun Dong, Zhenli Yang, Yanli Zhu, Changsong Qi, Dongmei Lin, Xiaocui Bian, Yuqin Liu
Human Cell.2025;[Epub] CrossRef - Small cell lung cancer transdifferentiation: not a negligible phenomenon
Lilla Horvath, Kristiina Boettiger, Zsolt Megyesfalvi, Balázs Döme, Clemens Aigner, Anita Horváth-Rózsás, Judit Berta
Current Opinion in Oncology.2025;[Epub] CrossRef - Transformation to small cell lung cancer is irrespective of EGFR and accelerated by SMAD4-mediated ASCL1 transcription independently of RB1 in non-small cell lung cancer
Xi Ding, Min-xing Shi, Di Liu, Jing-xue Cao, Kai-xuan Zhang, Run-dong Zhang, Li-ping Zhang, Kai-xing Ai, Bo Su, Jie Zhang
Cell Communication and Signaling.2024;[Epub] CrossRef - The study of primary and acquired resistance to first-line osimertinib to improve the outcome of EGFR-mutated advanced Non-small cell lung cancer patients: the challenge is open for new therapeutic strategies
Alessandra Ferro, Gian Marco Marinato, Cristiana Mulargiu, Monica Marino, Giulia Pasello, Valentina Guarneri, Laura Bonanno
Critical Reviews in Oncology/Hematology.2024; 196: 104295. CrossRef - Comprehensive molecular and clinical insights into non-small cell lung cancer transformation to small cell lung cancer with an illustrative case report
Kresimir Tomic, Kristina Krpina, Lara Baticic, Miroslav Samarzija, Semir Vranic
Journal of Drug Targeting.2024; 32(5): 499. CrossRef - Transcriptomic Heterogeneity of EGFR-Mutant Non–Small Cell Lung Cancer Evolution Toward Small-Cell Lung Cancer
Songji Oh, Jaemoon Koh, Tae Min Kim, Soyeon Kim, Jeonghwan Youk, Miso Kim, Bhumsuk Keam, Yoon Kyung Jeon, Ja-Lok Ku, Dong-Wan Kim, Doo Hyun Chung, Dae Seog Heo
Clinical Cancer Research.2024; 30(20): 4729. CrossRef - Transformation of lung adenocarcinoma into small cell lung cancer after treatment with epidermal growth factor receptor tyrosine kinase inhibitors
Linwu Kuang, Yangkai Li
Oncology and Translational Medicine.2024; 10(6): 286. CrossRef - Exon-18-EGFR Mutated Transformed Small-Cell Lung Cancer: A Case Report and Literature Review
Nunzio Digiacomo, Tommaso De Pas, Giovanna Rossi, Paola Bossi, Erika Stucchi, Fabio Conforti, Emilia Cocorocchio, Daniele Laszlo, Laura Pala, Emma Zattarin, Chiara Catania
Current Oncology.2023; 30(3): 3494. CrossRef - Current knowledge of small cell lung cancer transformation from non-small cell lung cancer
Giuseppe Giaccone, Yongfeng He
Seminars in Cancer Biology.2023; 94: 1. CrossRef - Targeting the EGFR signaling pathway in cancer therapy: What’s new in 2023?
Sushanta Halder, Soumi Basu, Shobhit P. Lall, Apar K. Ganti, Surinder K. Batra, Parthasarathy Seshacharyulu
Expert Opinion on Therapeutic Targets.2023; 27(4-5): 305. CrossRef - Outcomes in Patients With Lung Adenocarcinoma With Transformation to Small Cell Lung Cancer After EGFR Tyrosine Kinase Inhibitors Resistance: A Systematic Review and Pooled Analysis
Jinhe Xu, Lihuan Xu, Baoshan Wang, Wencui Kong, Ying Chen, Zongyang Yu
Frontiers in Oncology.2022;[Epub] CrossRef - Small cell lung cancer transformation: From pathogenesis to treatment
Xiaomeng Yin, Yueyi Li, Hang Wang, Tingting Jia, Enli Wang, Yuling Luo, Yuhao Wei, Zeyi Qin, Xuelei Ma
Seminars in Cancer Biology.2022; 86: 595. CrossRef - Small Cell Lung Cancer Transformation following Treatment in EGFR-Mutated Non-Small Cell Lung Cancer
Isa Mambetsariev, Leonidas Arvanitis, Jeremy Fricke, Rebecca Pharaon, Angel R. Baroz, Michelle Afkhami, Marianna Koczywas, Erminia Massarelli, Ravi Salgia
Journal of Clinical Medicine.2022; 11(5): 1429. CrossRef - Morphologic-Molecular Transformation of Oncogene Addicted Non-Small Cell Lung Cancer
Fiorella Calabrese, Federica Pezzuto, Francesca Lunardi, Francesco Fortarezza, Sofia-Eleni Tzorakoleftheraki, Maria Vittoria Resi, Mariaenrica Tiné, Giulia Pasello, Paul Hofman
International Journal of Molecular Sciences.2022; 23(8): 4164. CrossRef - Genomic and Gene Expression Studies Helped to Define the Heterogeneity of Small-Cell Lung Cancer and Other Lung Neuroendocrine Tumors and to Identify New Therapeutic Targets
Ugo Testa, Elvira Pelosi, Germana Castelli
Onco.2022; 2(3): 186. CrossRef - Neuroendocrine transformation from EGFR/ALK-wild type or TKI-naïve non-small cell lung cancer: An under-recognized phenomenon
Xiao Chu, Yuyin Xu, Ye Li, Yue Zhou, Li Chu, Xi Yang, Jianjiao Ni, Yida Li, Tiantian Guo, Zhiqin Zheng, Qiang Zheng, Qianlan Yao, Yuan Li, Xiaoyan Zhou, Zhengfei Zhu
Lung Cancer.2022; 169: 22. CrossRef - Three Third-Generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer: Similarities and Differences
Ling Chen, Yangqingqing Zhou, Chaosheng Gan, XiaoLi Wang, Yihui Liu, Chunhui Dong, Ruiyuan He, Jin Yang
Cancer Investigation.2022; 40(7): 590. CrossRef - Histomorphological transformation from non-small cell lung carcinoma to small cell lung carcinoma after targeted therapy or immunotherapy: A report of two cases
Hao Liu, Li-Hong Chen, Zhi-Hui Zhang, Ning Wang, Si-Hui Zhuang, Hao Chen, Jin Du, Li-Juan Pang, Yan Qi
Frontiers in Oncology.2022;[Epub] CrossRef - Impressive response to dabrafenib, trametinib, and osimertinib in a metastatic EGFR-mutant/BRAF V600E lung adenocarcinoma patient
Maurício Fernando Silva Almeida Ribeiro, Franciele Hinterholz Knebel, Fabiana Bettoni, Rodrigo Saddi, Karina Perez Sacardo, Felipe Sales Nogueira Amorim Canedo, João Victor Machado Alessi, Andrea Kazumi Shimada, José Flávio Gomes Marin, Anamaria Aranha Ca
npj Precision Oncology.2021;[Epub] CrossRef - Histological transformation of non-small cell lung cancer: Clinical analysis of nine cases
Cai-Bao Jin, Ling Yang
World Journal of Clinical Cases.2021; 9(18): 4617. CrossRef - Lamellarin 14, a derivative of marine alkaloids, inhibits the T790M/C797S mutant epidermal growth factor receptor
Naoyuki Nishiya, Yusuke Oku, Chie Ishikawa, Tsutomu Fukuda, Shingo Dan, Tetsuo Mashima, Masaru Ushijima, Yoko Furukawa, Yuka Sasaki, Keishi Otsu, Tomoko Sakyo, Masanori Abe, Honami Yonezawa, Fumito Ishibashi, Masaaki Matsuura, Akihiro Tomida, Hiroyuki Sei
Cancer Science.2021; 112(5): 1963. CrossRef - Case Report: Transformation From Non-Small Cell Lung Cancer to Small Cell Lung Cancer During Anti-PD-1 Therapy: A Report of Two Cases
Qian Shen, Jingjing Qu, Lingyan Sheng, Qiqi Gao, Jianying Zhou
Frontiers in Oncology.2021;[Epub] CrossRef - Exploring the resistance mechanisms of second-line osimertinib and their prognostic implications using next-generation sequencing in patients with non-small-cell lung cancer
Kyoungmin Lee, Deokhoon Kim, Shinkyo Yoon, Dae Ho Lee, Sang-We Kim
European Journal of Cancer.2021; 148: 202. CrossRef - Comprehensive analysis of treatment modes and clinical outcomes of small cell lung cancer transformed from epidermal growth factor receptor mutant lung adenocarcinoma
Shouzheng Wang, Tongji Xie, Xuezhi Hao, Yan Wang, Xingsheng Hu, Lin Wang, Yan Li, Junling Li, Puyuan Xing
Thoracic Cancer.2021; 12(19): 2585. CrossRef - EGFR-Mutant SCLC Exhibits Heterogeneous Phenotypes and Resistance to Common Antineoplastic Drugs
Chih-An Lin, Sung-Liang Yu, Hsuan-Yu Chen, Huei-Wen Chen, Shr-Uen Lin, Chia-Ching Chang, Chong-Jen Yu, Pan-Chyr Yang, Chao-Chi Ho
Journal of Thoracic Oncology.2019; 14(3): 513. CrossRef - The clinicopathologic of pulmonary adenocarcinoma transformation to small cell lung cancer
Haiyan Yang, Li Liu, Chunhua Zhou, Yi Xiong, Yijuan Hu, Nong Yang, Jingjing Qu
Medicine.2019; 98(12): e14893. CrossRef - Chemistry and pharmacological diversity of quinoxaline motifs as anticancer agents
Olayinka O. Ajani, Martins T. Nlebemuo, Joseph A. Adekoya, Kehinde O. Ogunniran, Tolutope O. Siyanbola, Christiana O. Ajanaku
Acta Pharmaceutica.2019; 69(2): 177. CrossRef - Resistance to EGFR inhibitors in non-small cell lung cancer: Clinical management and future perspectives
Chiara Tomasello, Cinzia Baldessari, Martina Napolitano, Giulia Orsi, Giulia Grizzi, Federica Bertolini, Fausto Barbieri, Stefano Cascinu
Critical Reviews in Oncology/Hematology.2018; 123: 149. CrossRef - Small cell lung cancer transformation from EGFR-mutated lung adenocarcinoma: A case report and literatures review
Yangyang Liu
Cancer Biology & Therapy.2018; 19(6): 445. CrossRef - Anaplastic lymphoma kinase (ALK)-expressing Lung Adenocarcinoma with Combined Neuroendocrine Component or Neuroendocrine Transformation: Implications for Neuroendocrine Transformation and Response to ALK-tyrosine Kinase Inhibitors
Jongmin Sim, Hyunjin Kim, Jiyeon Hyeon, Yoon-La Choi, Joungho Han
Journal of Korean Medical Science.2018;[Epub] CrossRef - Assessment of Resistance Mechanisms and Clinical Implications in Patients WithEGFRT790M–Positive Lung Cancer and Acquired Resistance to Osimertinib
Geoffrey R. Oxnard, Yuebi Hu, Kathryn F. Mileham, Hatim Husain, Daniel B. Costa, Philip Tracy, Nora Feeney, Lynette M. Sholl, Suzanne E. Dahlberg, Amanda J. Redig, David J. Kwiatkowski, Michael S. Rabin, Cloud P. Paweletz, Kenneth S. Thress, Pasi A. Jänne
JAMA Oncology.2018; 4(11): 1527. CrossRef - Clinicopathological and genomic comparisons between different histologic components in combined small cell lung cancer and non-small cell lung cancer
Mong-Wei Lin, Kang-Yi Su, Te-Jen Su, Chia-Ching Chang, Jing-Wei Lin, Yi-Hsuan Lee, Sung-Liang Yu, Jin-Shing Chen, Min-Shu Hsieh
Lung Cancer.2018; 125: 282. CrossRef - Transformation to small‑cell lung cancer following treatment with icotinib in a patient with lung adenocarcinoma
Hongyang Lu, Bo Chen, Jing Qin, Fajun Xie, Na Han, Zhiyu Huang
Oncology Letters.2018;[Epub] CrossRef - Histological transformation of adenocarcinoma to small cell carcinoma lung as a rare mechanism of resistance to epidermal growth factor receptor-tyrosine kinase inhibitors: Report of a case with review of literature
Monalisa Hui, ShantveerG Uppin, BalaJoseph Stalin, G Sadashivudu
Lung India.2018; 35(2): 160. CrossRef - A rare case of squamous cell carcinoma lung with multiple locoregional recurrences and histological transformation
Ram Niwas, Shibdas Chakrabarti, Viswesvaran Balasubramanian, ManasKamal Sen, JagdishChander Suri
Lung India.2018; 35(6): 511. CrossRef - Small cell lung cancer transformation during immunotherapy with nivolumab: A case report
Takuma Imakita, Kohei Fujita, Osamu Kanai, Tsuyoshi Terashima, Tadashi Mio
Respiratory Medicine Case Reports.2017; 21: 52. CrossRef - Pulmonary neuroendocrine tumor in a female wolf (Canis lupus lupus)
Ayako SHIRAKI, Toshinori YOSHIDA, Masahi KAWASHIMA, Hirotada MURAYAMA, Rei NAGAHARA, Nanao ITO, Makoto SHIBUTANI
Journal of Veterinary Medical Science.2017; 79(3): 588. CrossRef - Secondary biopsy of non‐oncogenic‐driven lung cancer may reveal a clinically sensible histologic change. A brief report of two paradigmatic cases
Maria C. Mengoli, Giulia Orsi, Filippo Lococo, Giulia Grizzi, Fausto Barbieri, Federica Bertolini, Giulio Rossi, Silvia Novello
Thoracic Cancer.2017; 8(4): 359. CrossRef - Small-cell lung cancer in never smokers: The clinicopathological features including the prognosis
Masahiro Yamasaki, Naomi Saito, Wakako Daido, Sayaka Ishiyama, Naoko Deguchi, Masaya Taniwaki, Akio Sakatani, Megumu Fujihara, Nobuyuki Ohashi, Ken-ichi Arita
Cancer Treatment and Research Communications.2017; 12: 1. CrossRef - Clonal History and Genetic Predictors of Transformation Into Small-Cell Carcinomas From Lung Adenocarcinomas
June-Koo Lee, Junehawk Lee, Sehui Kim, Soyeon Kim, Jeonghwan Youk, Seongyeol Park, Yohan An, Bhumsuk Keam, Dong-Wan Kim, Dae Seog Heo, Young Tae Kim, Jin-Soo Kim, Se Hyun Kim, Jong Seok Lee, Se-Hoon Lee, Keunchil Park, Ja-Lok Ku, Yoon Kyung Jeon, Doo Hyun
Journal of Clinical Oncology.2017; 35(26): 3065. CrossRef - Australian recommendations for EGFR T790M testing in advanced non-small cell lung cancer
Thomas John, Jeffrey J Bowden, Stephen Clarke, Stephen B Fox, Kerryn Garrett, Keith Horwood, Christos S Karapetis
Asia-Pacific Journal of Clinical Oncology.2017; 13(4): 296. CrossRef - Metastatic Squamous Cell Carcinoma from Lung Adenocarcinoma after Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy
Hyung Kyu Park, Youjeong Seo, Yoon-La Choi, Myung-Ju Ahn, Joungho Han
Journal of Pathology and Translational Medicine.2017; 51(4): 441. CrossRef - Sequential occurrence of small cell and non-small lung cancer in a male patient: Is it a transformation?
Ahsan Wahab, Kavitha Kesari, Siddique Chaudhary, Mahin Khan, Hafiz Khan, Susan Smith, Yanis Boumber
Cancer Biology & Therapy.2017; 18(12): 940. CrossRef - The expression of S100B protein in serum of patients with brain metastases from small-cell lung cancer and its clinical significance
Shanling Mu, Hong Ma, Jun Shi, Dezhi Zhen
Oncology Letters.2017;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

Fig. 1.
| Case No. | Initial tumor | Sample type | Sample acquisition site | Subtype | Interval between biopsy (mo) | Transformed tumor | Sample type | Sample acquisition site | IHC TTF-1/CD56 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ADC | Biopsy | Lung | Acinar | 37 | SCLC | Biopsy | Celiac LN | –/+ |
| 2 | ADC | Biopsy | Lung, brain | Acinar and papillary | 21 | Combined SCLC and ADC | Biopsy | Lung | –/+ |
| 3 | ADC | Biopsy | LN 4 | Acinar | 8 | SCLC | Biopsy | LN 7 | +/+ |
| 4 | ADC | Biopsy | Lung | Acinar | 5 | Combined SCLC and ADC | Biopsy | Lung (same site) | +/NA |
| 5 | ADC | Resection | Lung | Acinar | 31 | SCLC | Biopsy | Pleura | +/+ |
| 6 | ADC | Resection | Lung | Acinar and solid | 50 | SCLC | Biopsy | Neck LN | +/+ |
| Case No. | Sex | Age (yr) | Smoking history (pack years) | Tumor histology in initial sample | Clinical tage | Initial treatment | EGFR mutation in initial sample | Treatment related to TKI | Duration of TKI treatment before transformation (mo) | Response to TKI | Tumor histology in second biopsy sample | EGFR mutation in second biopsy ample | Treatment after second biopsy | Progression or recurrance | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 57 | 0 | ADC | T2N0 | Operation, adjuvant CTx (paclitaxel/carboplatin) | L858R | Irressa, afatinib | 10 | PD | SCLC | L858R | CTx (etoposide/cisplatin) | F/U loss | F/U loss |
| 2 | F | 54 | 0 | ADC | T3N1M1 | CTx (Alimta/cisplatin) | Del19 | Afatinib | 11 | PD | Combined SCLC and ADC | del19 | CTx (gemcitabine/cisplatin) | PR | Alive |
| 3 | F | 55 | 0 | ADC | T2N3M1 | Iressa | Del19 | Iressa | 9 | PD | SCLC | del19 | CTx (etoposide/cisplatin) | PR | Alive |
| 4 | F | 59 | 0 | ADC | T1N0M1 | CTx (Alimta/cisplatin) | Del19 | Gefetinib | 11 | PD | Combined SCLC and ADC | del19 | Rociletinib | PD | Alive |
| 5 | F | 68 | 0 | ADC | T1N0M1 | Operation, palliative CTx (Alimta/cisplatin) | No mutation | Gefetinib | 2 | PD | SCLC | No mutation | No treatment |
PD | Dead |
| 6 | M | 67 | 35 | ADC | T1NO | Operation | No mutation | No TKI | NA | PD | SCLC | No mutation | No treatment |
F/U loss | F/U loss |
IHC, immunohistochemistry; TTF-1,thyroid transcription factor; ADC, adenocarcinoma; SCLC, small cell lung cancer; LN, lymph node; NA, not-applicable. CD56 was positive in only SCLC components.
EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; F, female; ADC, adenocarcinoma; CTx, chemotherapy; PD, progressive disease; SCLC, small cell lung cancer; F/U, follow-up; PR, partial response; M, male; NA, not-applicable. No further treatment due to poor condition; Dead due to disease progression.

 E-submission
E-submission