Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 51(1); 2017 > Article
-
Original Article
Evaluation of Pathologic Complete Response in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: Experience in a Single Institution over a 10-Year Period - Misun Choi, Yeon Hee Park1, Jin Seok Ahn1, Young-Hyuck Im1, Seok Jin Nam2, Soo Youn Cho, Eun Yoon Cho
-
Journal of Pathology and Translational Medicine 2017;51(1):69-78.
DOI: https://doi.org/10.4132/jptm.2016.10.05
Published online: December 25, 2016
Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
1Division of Hematology-Oncology,Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
2Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
-
Corresponding Author Eun Yoon Cho, MD, PhD Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea Tel: +82-2-3410-2796 Fax: +82-2-3410-0025 E-mail: eunyoon.cho@samsung.com
Soo Youn Cho, MD, PhD Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea Tel: +82-2-3410-2817 Fax: +82-2-3410-0025 E-mail: sooyoun.cho@samsung.com
© 2017 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Pathologic complete response (pCR) after neoadjuvant chemotherapy (NAC) has been associated with favorable clinical outcome in breast cancer patients. However, the possibility that the prognostic significance of pCR differs among various definitions has not been established.
-
Methods
- We retrospectively evaluated the pathologic response after NAC in 353 breast cancer patients and compared the prognoses after applying the following different definitions of pCR: ypT0/is, ypT0, ypT0/is ypN0, and ypT0 ypN0.
-
Results
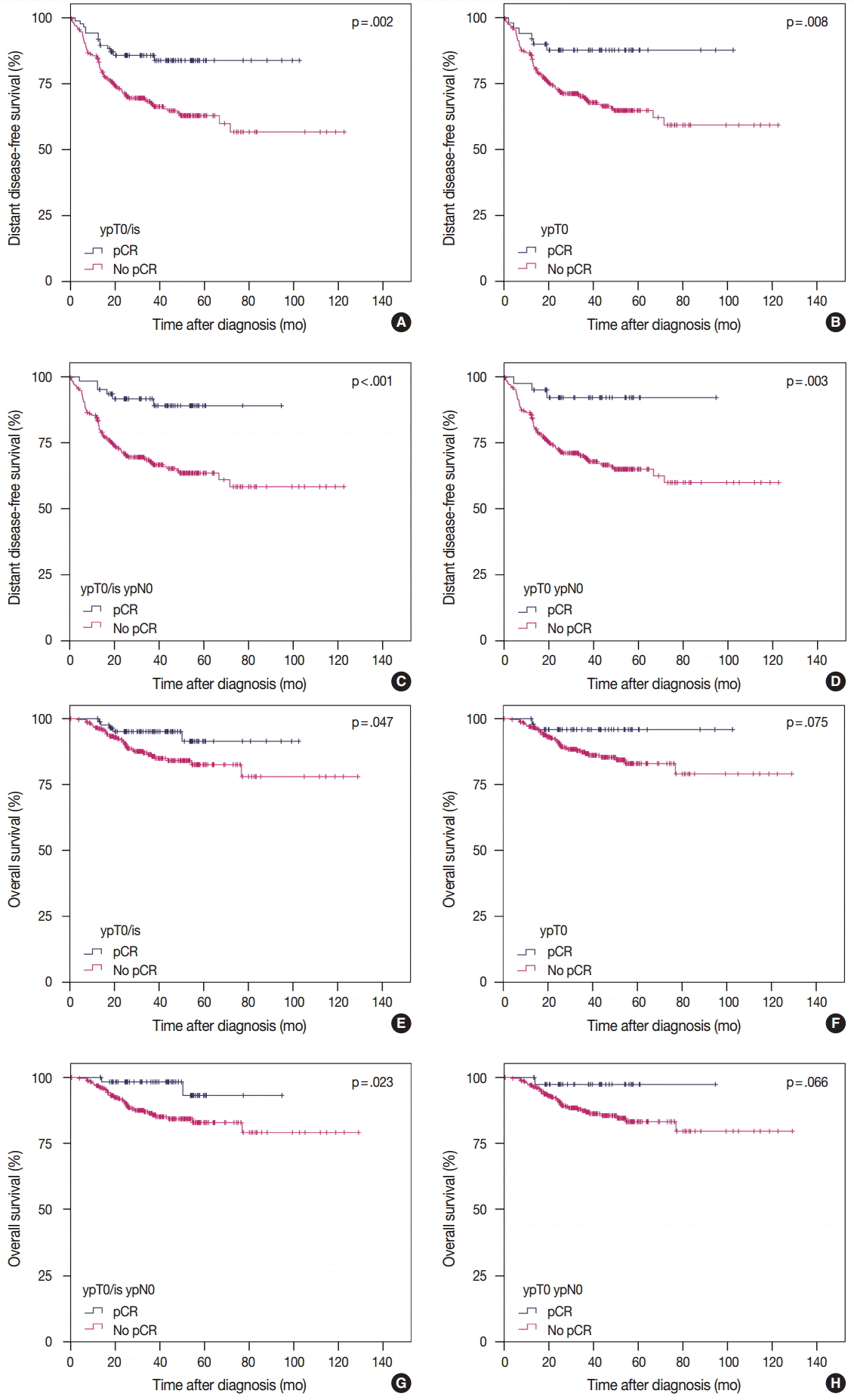
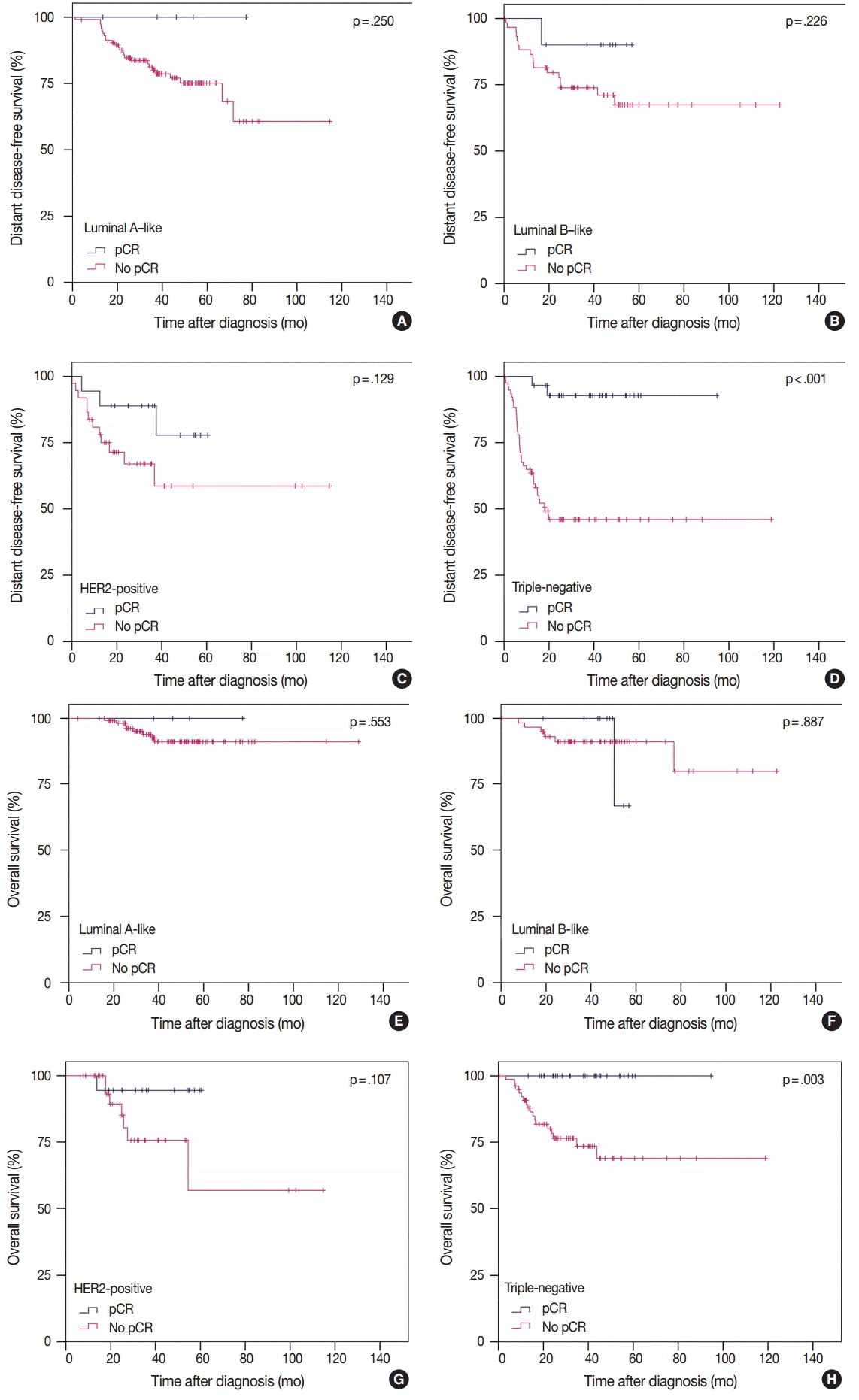
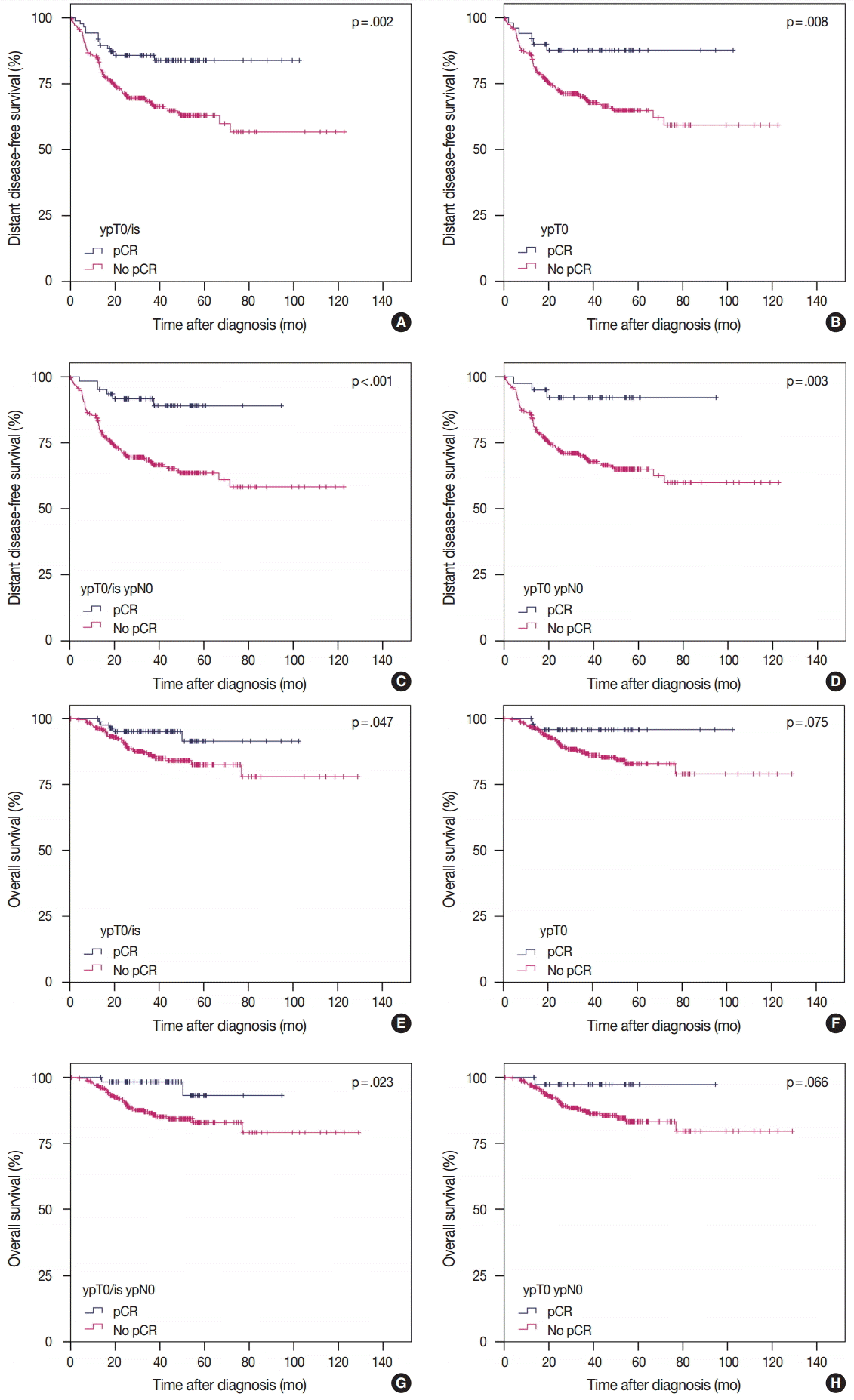
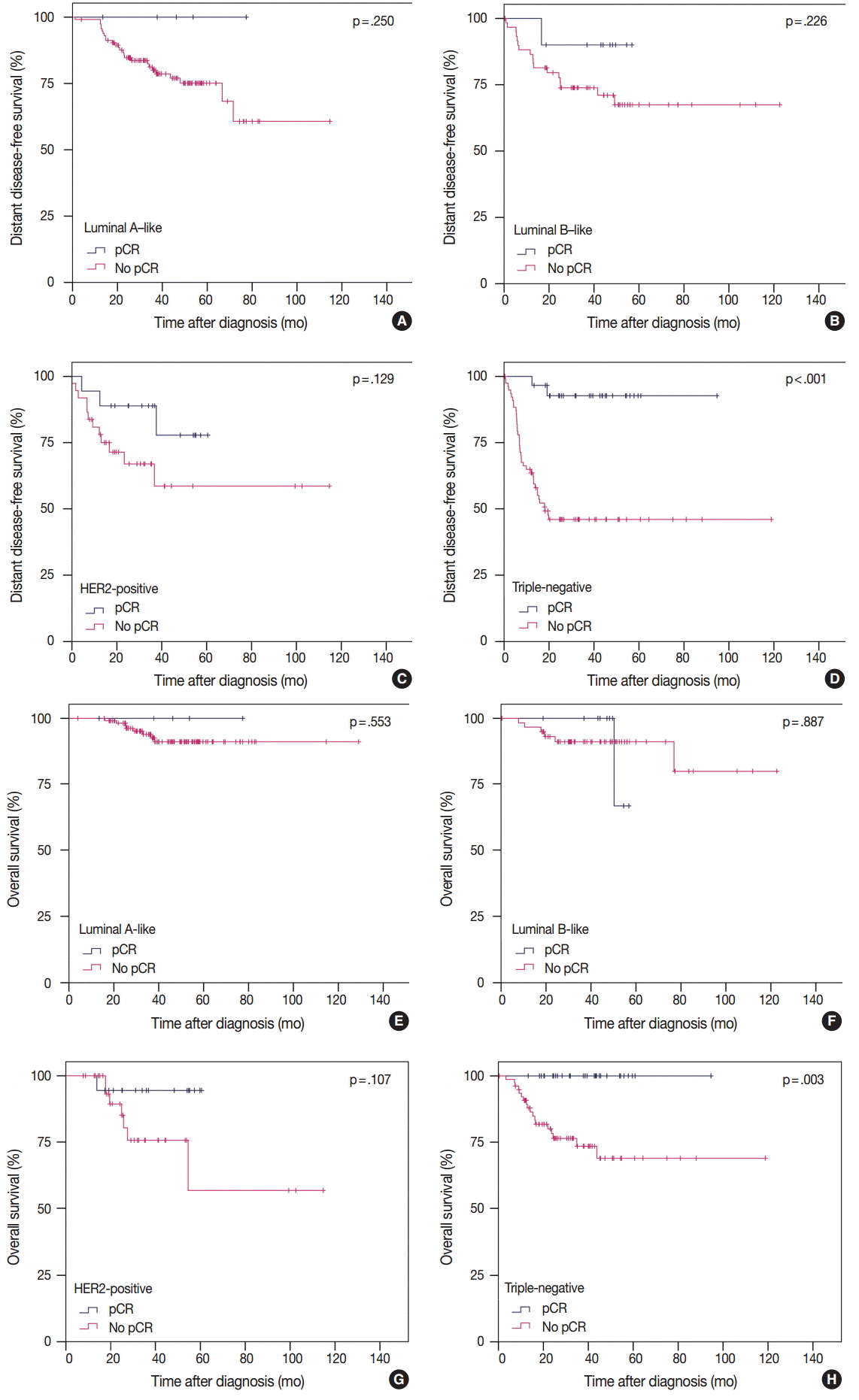
- pCR was significantly associated with improved distant disease-free survival (DDFS) regardless of the definition (ypT0/is, p = .002; ypT0, p = .008; ypT0/is ypN0, p < .001; ypT0 ypN0, p = .003). Presence of tumor deposits of any size in the lymph nodes (LNs; ypN ≥ 0(i+)) was associated with worse DDFS (ypT0 ypN0 vs ypT0 ypN ≥ 0(i+), p = .036 and ypT0/is ypN0 vs ypT0/is ypN ≥ 0(i+), p = .015), and presence of isolated tumor cells was associated with decreased overall survival (OS; ypT0/is ypN0 vs ypT0/is ypN0(i+), p = .013). Residual ductal carcinoma in situ regardless of LN status showed no significant difference in DDFS or OS (DDFS: ypT0 vs ypTis, p = .373 and ypT0 ypN0 vs ypTis ypN0, p = .462; OS: ypT0 vs ypTis, p = .441 and ypT0 ypN0 vs ypTis ypN0, p = .758). In subsequent analysis using ypT0/is ypN0, pCR was associated with improved DDFS and OS in triple-negative tumors (p < .001 and p = .003, respectively).
-
Conclusions
- Based on our study results, the prognosis and rate of pCR differ according to the definition of pCR and ypT0/is ypN0 might be considered a more preferable definition of pCR.
- Study population
- We retrospectively retrieved data from 353 individual patients from electronic medical records of Samsung Medical Center in Seoul, Korea, from January 2004 to December 2013. Patients treated with anthracycline and taxane-based NAC and who subsequently underwent surgery with curative intent for primary breast cancer were included. Patients who had histologically confirmed distant metastasis at the time of diagnosis and who were diagnosed with inflammatory carcinoma were excluded. This study was approved by the Institutional Review Board of Samsung Medical Center, and the requirement for informed consent was waived.
- Baseline studies including clinical examination, mammography, ultrasound, and magnetic resonance imaging were performed to assess the extent of primary tumor in the breast and LNs. All patients were diagnosed with breast cancer based on core needle biopsies, and LN metastasis was confirmed using core needle biopsy or fine-needle aspiration. All patients were treated with four or six cycles of anthracycline and taxane-based regimen at 3-week intervals, including adriamycin with docetaxel, adriamycin with cyclophosphamide plus docetaxel (AC-T), or AC-T plus trastuzumab. Patients with hormonal receptor–positive tumors received adjuvant endocrine therapy for at least 5 years after surgery. Patients with HER2-overexpressing and/or amplified tumors received neoadjuvant trastuzumab plus chemotherapy followed by adjuvant trastuzumab. Local and regional recurrence was confirmed either histologically or cytologically.
- Histologic review
- Core biopsies before NAC and surgical specimens obtained after NAC were reviewed. The largest size of tumors, histologic type, histologic grade, lymphovascular invasion (LVI), proportion of DCIS, number of positive LNs, size of the largest metastasis, and treatment response in breast and LN were evaluated. Tumor size and extent in breast and LNs were assessed according to the recommendation proposed by Provenzano et al. [23]. Histologic type was defined in accordance with the World Health Organization classification [24], and histologic grade was classified using the modified Scarff-Bloom-Richardson grading system [25].
- To compare the prognostic impact of the pCR components defined previously, all patients were subdivided into the following subgroups according to TNM [16]: (1) no residual invasive tumor cells in the breast, ypT0/is, (2) no residual invasive tumor cells in the breast or LNs, ypT0/is ypN0, (3) no residual invasive tumor cells or DCIS in the breast, ypT0, and (4) no residual invasive tumor cells or DCIS in the breast and LNs, ypT0 ypN0.
- ER, PR, and HER2 were assessed on both core biopsies and surgical specimens. ER and PR were considered positive only when greater than or equal to 1% of tumor cells showed nuclear staining. HER2 was positive if tumor cells showed 3+by IHC or 2+by IHC with amplification using silver in situ hybridization [26]. To assess the prognostic impact of pCR on intrinsic subtypes of breast cancer, all patients were classified into four subtypes according to the IHC results as follows: ER/PR+HER2- with histologic grade 1 or 2 tumors as luminal A-like; ER/PR+HER2– with histologic grade 3 tumors or ER/PR/HER2+ tumors as luminal B–like; ER/PR–HER2+tumors as HER2-positive; and ER/PR/HER2– tumors as TN [3,27].
- Statistical analysis
- Distant disease-free survival (DDFS) and OS were defined as the time between the date of initial diagnosis to the date of distant recurrence or the date of death from any cause, respectively. The significance of differences in baseline variables was analyzed using two-sided χ2, Fisher exact or Mann-Whitney U test as appropriate. DDFS and OS were plotted using the Kaplan-Meier product-limit method, and the log-lank p-value was calculated. To assess the prognostic impact of the pCR component, hazard ratios (HRs), 95% confidence intervals and p-value were calculated using Cox proportional hazards model. All tests were two sided, and a p-value less than .05 was considered statistically significant. Statistical analyses were performed using the SPSS statistical software ver. 20.0 (IBM Corp., Armonk, NY, USA).
MATERIALS AND METHODS
- Patient characteristics
- Median age at diagnosis was 44.0 years (range, 22 to 68 years). Median size of tumor was 4.6 cm (range, 0.7 to 11.0 cm) before NAC and 1.5 cm (range, 0.0 to 13.0 cm) after NAC. Most patients (282/353, 79.9%) had cN2 or N3 nodal status before NAC, and median number of positive LNs after NAC was 1 (range, 0 to 39). Baseline clinicopathologic characteristics and corresponding pCR rates according to definition of pCR are summarized in Table 1. In brief, histologic grade, LVI, presence of metastatic LN, and hormonal receptor status showed significant correlation with pCR rate according to definition. Patients with high histologic grade tumors, no LVI, no metastatic LN, and negative hormonal receptor status tended to have higher pCR rates. HER2-positive and TN tumors showed significantly higher pCR rates than luminal A-like and luminal B-like tumors regardless of the pCR definition. The median follow-up time of 353 patients was 36.5 months (range, 0.4 to 129.0 months). During this period, 101 patients (28.6%) had a relapse and 41 (11.6%) died. The 5-year DDFS was 68.0%, and OS was 84.8%.
- Correlation between pCR and survival according to definition
- According to the four definitions of pCR, 86 (24.4%) patients were diagnosed as ypT0/is, 50 (14.2%) as ypT0, 62 (17.6%) as ypT0/is ypN0, and 40 (11.3%) as ypT0 ypN0. Patients who achieved pCR showed significantly better DDFS than patients who did not (Fig. 1A, C, E, G). Similarly, patients who achieved pCR also tended to have better OS than patients who did not, but this difference was not statistically significant when ypT0 and ypT0 ypN0 were used as the pCR definitions (Fig. 1B, D, F, H). HRs for DDFS and OS increased sequentially as follows: ypT0/is, ypT0, ypT0/is ypN0, and ypT0 ypN0 (Table 2).
- Prognostic significance of LN status
- In the ypT0 subgroup (n=50), 10 patients (20.0%) had tumor deposits in the LNs (ypN≥0(i+)) and experienced worse DDFS than patients with no metastatic LNs (5-year DDFS: ypT0 ypN0, 92.1%; ypT0 ypN≥0(i+), 68.6%; p=.036). In the ypT0/is subgroup (n=86), 24 patients (27.9%) had residual tumor deposits in the LNs (ypN≥0(i+)) and experienced worse DDFS than patients with no metastatic LNs (5-year DDFS: ypT0/is ypN0, 89.0%; ypT0/is ypN≥0(i+), 70.2%; p =.015). The 5-year OS for patients with ypT0 ypN≥0(i+) and ypT0/is ypN≥0(i+) (88.9% and 86.5%, respectively) appeared worse than for patients with ypT0 ypN0 and ypT0/is ypN0 (97.4% and 93.2%, respectively), but the difference was not statistically significant (p=.236 and p=.095, respectively).
- Prognostic significance of ITCs in the LNs
- Among patients with ypT0 ypN≥0(i+) (n=10), 3 had ITCs in the LNs, and none relapsed or died. Among patients with ypT0/is ypN≥ 0(i+) (n=24), five had ITCs in the LNs, and showed worse OS compared to patients with ypT0/is ypN0 (5-year OS: ypT0/is ypN0(i+), 75.0% vs ypT0/is ypN0, 93.2%; p=.013). For DDFS, patients with ITCs in LNs tended to experience worse DDFS than patients with no tumor cells in the LNs (5-year DDFS: ypT0/is ypN0(i+), 80.0% vs ypT0/is ypN0, 89.0%; p=.336).
- Prognostic significance of residual DCIS
- Patients with residual DCIS in the breast tended to experience worse DDFS and OS compared with patients with no residual tumor cells in the breast (5-year DDFS: ypT0, 87.6% and ypTis, 78.3%; 5-year OS: ypT0, 95.8% and ypTis, 85.5%), but the difference was not statistically significant (p=.373 and p=.441, respectively). Considering LN status, patients with ypT0 ypN0 had 5-year DDFS of 92.1% and OS of 97.4%, which appeared better than those of patients with ypTis ypN0 (5-year DDFS, 83.3% and 5-year OS, 87.5%). However, these were not statistically significant (p=.462 and p=.758, respectively).
- Among patients who achieved pCR when ypT0/is was used as the definition of pCR, the proportion of patients with residual DCIS was significantly different among IHC subtypes (p=.001); highest with HER2-positive tumors (15/23, 65.2%) followed by luminal B-like (9/14, 64.3%), luminal A-like (3/6, 50%), and lowest with TN tumors (9/43, 20.9%).
- Prognostic significance of pCR in IHC subtypes
- To analyze the prognosis between patients with or without pCR according to IHC subtype, we defined pCR as ypT0/is ypN0 according to our study results. In TN tumors, pCR was significantly associated with improved survival in terms of both DDFS and OS. However, in luminal A-like, luminal B-like, and HER2-positive tumors, pCR showed no prognostic impact on survival (Fig. 2). In patients without pCR, HER2-positive and TN tumors showed poorer prognosis than in luminal A-like and luminal B-like tumors (Fig. 2).
RESULTS
- To the best of our knowledge, this study is the first analysis of the prognostic significance of different pCR definitions on long-term outcome in breast cancer patients treated homogeneously with anthracycline and taxane-based NAC regimens as well as neoadjuvant trastzumab plus chemotherapy at a single institute in Korea. We compared the following four definitions of pCR; ypT0/is, ypT0, ypT0/is ypN0, and ypT0 ypN0, and the corresponding pCR rates were 24.4%, 14.2%, 17.6%, and 11.3%, respectively (Table 1). These rates were similar to previous studies. In a study by JBCS (n=353), pCR rates of ypT0/is, ypT0, ypT0/is ypN0, and ypT0 ypN0 were 20.4%, 9.9%, 18.4%, and 8.2%, respectively [28]. In a meta-analysis by CTNeoBC (n=13,125), pCR rates of ypT0/is, ypT0/is ypN0, and ypT0 ypN0 were 22%, 18%, and 13%, respectively [12]. In the study by GBG and AGO-B (n=6,377), pCR rates of ypT0/is, ypT0/is ypN0, and ypT0 ypN0 were 22.8%, 19.8%, and 15.0%, respectively [3].
- In the present study, patients with pCR, regardless of definition, had significantly better DDFS than patients without pCR. Regarding OS, however, patients with pCR when ypT0/is and ypT0/is ypN0 were used as pCR definitions showed significantly better survival than patients without pCR (Fig. 1). Regarding LN status, even if tumor cells were not present in the breast including DCIS, patients with residual tumor cells of any size in the LNs experienced worse DDFS than patients with no metastatic LNs. Presence of ITCs in the LNs after NAC is regarded as non-pCR by the American Joint Committee on Cancer for TNM staging [16]; however, data supporting this recommendation is insufficient. Our study showed that patients with ITCs in the LNs might have poorer OS than patients with no tumor cells in the LNs. However, further studies with larger populations are warranted.
- Theoretically, ypT0 ypN0 represents the strictest definition of pCR, meaning complete eradication of all tumor cells in both the breast and LNs. Thus, we compared the prognosis between ypT0 and ypTis as well as between ypT0 ypN0 and ypT0/is ypN0 and found that presence of DCIS did not result in any difference. Thus, based on the results from this study, we considered ypT0/is ypN0 the more preferable definition of pCR. These results were consistent with previous studies by JBCS, CTNeoBC, and MD Anderson [12,17,28]. But not with those of GBG and AGO-B, which suggested ypT0 ypN0 as the best definition of pCR [3]. This discrepancy might be caused by the smaller number of patients and events in ypTis (n=36) and ypTis ypN0 (n=22), resulting in a much lower statistical significance to show prognostic differences in this study.
- Among the IHC subtypes, HER2-positive and TN tumors achieved high pCR rates. pCR was significantly correlated with DDFS and OS only in TN tumors (Fig. 2). However, in patients without pCR, HER2-positive and TN tumors showed poorer prognosis than in luminal A-like and luminal B-like tumors. These results are in agreement with the previously reported studies by Liedtke et al. [29] and Houssami et al. [30].
- The potential limitations of this study are as follows. First, due to the small number of patients and events, comparison between patients with pCR and without pCR using Cox proportional hazards model was not feasible. Second, because Ki-67 was not available for all patients, subtypes based on only ER, PR, and HER2 status and histologic grade might not be the same as molecular intrinsic subtypes.
- In conclusion, the prognosis and rate of pCR varied according to definition of pCR. In our study, pCR defined as ypT0/is ypN0 was considered the most preferable. pCR could be used as a surrogate of favorable clinical outcome in TN tumors but not in luminal A-like, luminal B-like, or HER2-positive tumors.
DISCUSSION
pCR, pathologic complete response; NAC, neoadjuvant chemotherapy; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; Luminal A-like, ER/PR+HER2– tumors with histologic grade 1 or 2; Luminal B-like, ER/PR+HER2– tumors with histologic grade 3 or ER/PR+HER2+ tumors; HER2-positive, ER/PR–HER2+ tumors; Triple-negative, ER/PR/HER2– tumors.
| Definitions of pCR | Total, n (%) | Distant metastasis, n (%) |
DDFS |
Died of disease, n (%) |
OS |
||
|---|---|---|---|---|---|---|---|
| HRa (95% CI) | p-valuea | HRa (95% CI) | p-valuea | ||||
| Breast only | |||||||
| ypT0/is | 86 (24.4) | 13 (15.1) | 2.472 (1.380–4.426) | .002 | 5 (5.9) | 2.497 (0.980–6.364) | .055 |
| ypT0 | 50 (14.2) | 6 (12.0) | 2.900 (1.270–6.618) | .011 | 2 (4.0) | 3.373 (0.814–13.969) | .094 |
| Breast and lymph nodes | |||||||
| ypT0/is ypN0 | 62 (17.6) | 6 (9.7) | 3.954 (1.732–9.026) | .001 | 2 (3.2) | 4.498 (1.086–18.638) | .038 |
| ypT0 ypN0 | 40 (11.3) | 3 (7.5) | 4.741 (1.502–14.958) | .008 | 1 (2.5) | 5.277 (0.725–38.398) | .100 |
- 1. Kaufmann M, von Minckwitz G, Bear HD, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol 2007; 18: 1927-34. ArticlePubMed
- 2. Kaufmann M, von Minckwitz G, Mamounas EP, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol 2012; 19: 1508-16. ArticlePubMedPDF
- 3. von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012; 30: 1796-804. ArticlePubMed
- 4. Cortazar P, Geyer CE Jr. Pathological complete response in neoadjuvant treatment of breast cancer. Ann Surg Oncol 2015; 22: 1441-6. ArticlePubMedPDF
- 5. Kuroi K, Toi M, Tsuda H, Kurosumi M, Akiyama F. Issues in the assessment of the pathologic effect of primary systemic therapy for breast cancer. Breast Cancer 2006; 13: 38-48. ArticlePubMed
- 6. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001; (30):96-102. ArticlePubMed
- 7. Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998; 16: 2672-85. ArticlePubMed
- 8. Robidoux A, Tang G, Rastogi P, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol 2013; 14: 1183-92. ArticlePubMed
- 9. Bear HD, Tang G, Rastogi P, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med 2012; 366: 310-20. PubMedPMC
- 10. Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (Neo-Sphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012; 13: 25-32. ArticlePubMed
- 11. Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 2012; 379: 633-40. ArticlePubMedPMC
- 12. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384: 164-72. ArticlePubMed
- 13. Corben AD, Abi-Raad R, Popa I, et al. Pathologic response and long-term follow-up in breast cancer patients treated with neoadjuvant chemotherapy: a comparison between classifications and their practical application. Arch Pathol Lab Med 2013; 137: 1074-82. ArticlePubMedPDF
- 14. Rouzier R, Extra JM, Klijanienko J, et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol 2002; 20: 1304-10. ArticlePubMed
- 15. McCready DR, Hortobagyi GN, Kau SW, Smith TL, Buzdar AU, Balch CM. The prognostic significance of lymph node metastases after preoperative chemotherapy for locally advanced breast cancer. Arch Surg 1989; 124: 21-5. ArticlePubMed
- 16. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer (AJCC) cancer staging manual. 7th ed. New York: Springer, 2009.
- 17. Mazouni C, Peintinger F, Wan-Kau S, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol 2007; 25: 2650-5. ArticlePubMed
- 18. Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007; 25: 4414-22. ArticlePubMed
- 19. Kurosumi M, Akiyama F, Iwase T, et al. Histopathological criteria for assessment of therapeutic response in breast cancer. Breast Cancer 2001; 8: 1-2. ArticlePubMedPDF
- 20. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000; 406: 747-52. ArticlePubMedPDF
- 21. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006; 295: 2492-502. ArticlePubMed
- 22. Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res 2009; 7: 4-13. ArticlePubMedPMC
- 23. Provenzano E, Bossuyt V, Viale G, et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod Pathol 2015; 28: 1185-201. ArticlePubMedPDF
- 24. Lakhani SR, Ellis IO, Schinitt SJ, Tan PH, van de Vijver MJ. WHO classification of tumours of the breast. 4th ed. Lyon: IARC Press, 2012.
- 25. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991; 19: 403-10. ArticlePubMed
- 26. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013; 31: 3997-4013. PubMed
- 27. Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011; 22: 1736-47. ArticlePubMedPMC
- 28. Kuroi K, Toi M, Ohno S, et al. Comparison of different definitions of pathologic complete response in operable breast cancer: a pooled analysis of three prospective neoadjuvant studies of JBCRG. Breast Cancer 2015; 22: 586-95. ArticlePubMedPDF
- 29. Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008; 26: 1275-81. ArticlePubMed
- 30. Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer 2012; 48: 3342-54. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Differential prognostic value of residual nodal burden in breast cancer subtypes
Christine Hong Ngoc Che Thai, Selena J. An, Conner R. Haase, Julia M. Selfridge, Chris B. Agala, Philip M. Spanheimer
Breast Cancer Research and Treatment.2025; 209(2): 315. CrossRef - Neoadjuvant Chemotherapy for Early Breast Cancer: A Study on Response Rate and Toxicity
Matt Galloway, Paula Barlow, Jody Jordan, Edward Lo
Journal of Clinical Medicine.2025; 14(20): 7362. CrossRef - Association of residual ductal carcinoma in situ with breast cancer treatment outcomes after neoadjuvant chemotherapy according to hormone receptor status
Eunju Shin, Tae-Kyung Yoo, Jisun Kim, Il Yong Chung, Beom Seok Ko, Hee Jeong Kim, Jong Won Lee, Byung Ho Son, Sae Byul Lee
Discover Oncology.2024;[Epub] CrossRef - Efficacy of Mammographic Artificial Intelligence-Based Computer-Aided Detection in Predicting Pathologic Complete Response to Neoadjuvant Chemotherapy
Ga Eun Park, Bong Joo Kang, Sung Hun Kim, Han Song Mun
Life.2024; 14(11): 1449. CrossRef - Pathology after neoadjuvant treatment – How to assess residual disease
Giuseppe Viale, Nicola Fusco
The Breast.2022; 62: S25. CrossRef - Pathological examination of breast cancer samples before and after neoadjuvant therapy: recommendations from the Italian Group for the Study of Breast Pathology - Italian Society of Pathology (GIPaM-SIAPeC)
Nicola Fusco, Antonio Rizzo, Leopoldo Costarelli, Alfredo Santinelli, Bruna Cerbelli, Cristian Scatena, Ettore Macrì, Francesca Pietribiasi, Giulia d’Amati, Anna Sapino, Isabella Castellano
Pathologica.2022; 114(2): 104. CrossRef - Pathological complete response as a surrogate to improved survival in human epidermal growth factor receptor-2-positive breast cancer: systematic review and meta-analysis
Matthew G. Davey, Ferdia Browne, Nicola Miller, Aoife J. Lowery, Michael J. Kerin
BJS Open.2022;[Epub] CrossRef - Neoadjuvant therapy with doxorubicin-cyclophosphamide followed by weekly paclitaxel in early breast cancer: a retrospective analysis of 200 consecutive patients treated in a single center with a median follow-up of 9.5 years
Lisi M. Dredze, Michael Friger, Samuel Ariad, Michael Koretz, Bertha Delgado, Ruthy Shaco-Levy, Margarita Tokar, Michael Bayme, Ravit Agassi, Maia Rosenthal, Victor Dyomin, Olga Belochitski, Shai Libson, Tamar Mizrahi, David B. Geffen
Breast Cancer Research and Treatment.2022; 193(3): 597. CrossRef - “No Ink on Tumor” in Breast-Conserving Surgery after Neoadjuvant Chemotherapy
Giulia Atzori, Marco Gipponi, Chiara Cornacchia, Raquel Diaz, Marco Sparavigna, Maurizio Gallo, Tommaso Ruelle, Federica Murelli, Simonetta Franchelli, Francesca Depaoli, Daniele Friedman, Piero Fregatti
Journal of Personalized Medicine.2022; 12(7): 1031. CrossRef - Machine Learning Models and Multiparametric Magnetic Resonance Imaging for the Prediction of Pathologic Response to Neoadjuvant Chemotherapy in Breast Cancer
Carmen Herrero Vicent, Xavier Tudela, Paula Moreno Ruiz, Víctor Pedralva, Ana Jiménez Pastor, Daniel Ahicart, Silvia Rubio Novella, Isabel Meneu, Ángela Montes Albuixech, Miguel Ángel Santamaria, María Fonfria, Almudena Fuster-Matanzo, Santiago Olmos Antó
Cancers.2022; 14(14): 3508. CrossRef - Applying artificial intelligence technology to assist with breast cancer diagnosis and prognosis prediction
Meredith A. Jones, Warid Islam, Rozwat Faiz, Xuxin Chen, Bin Zheng
Frontiers in Oncology.2022;[Epub] CrossRef - Chemotherapy response score as a prognostic tool in patients with advanced stage endometrial carcinoma treated with neoadjuvant chemotherapy
Ina Jani, Ricardo R Lastra, Katherine S Brito, Chuanhong Liao, Isabel Lazo, Nita Karnik Lee, S Diane Yamada, Katherine C Kurnit
International Journal of Gynecological Cancer.2021; 31(6): 852. CrossRef - Application of neoadjuvant chemotherapy combined with anlotinib in occult breast cancer: A case report and review of literature
Yu Zhang, Di Wu, Bo Zhao, Xue-Liang Tian, Tian-Cheng Yao, Feng Li, Wei-Fang Liu, Ai-Ping Shi
World Journal of Clinical Cases.2021; 9(4): 919. CrossRef - Pathologic Complete Response and Its Impact on Breast Cancer Recurrence and Patient’s Survival after Neoadjuvant Therapy: A Comprehensive Meta-Analysis
Hui Liu, Liqiong Lv, Hui Gao, Ming Cheng, Tao Huang
Computational and Mathematical Methods in Medicine.2021; 2021: 1. CrossRef - Impact of Surgical Margins in Breast Cancer After Preoperative Systemic Chemotherapy on Local Recurrence and Survival
K. Wimmer, M. Bolliger, Z. Bago-Horvath, G. Steger, D. Kauer-Dorner, R. Helfgott, C. Gruber, F. Moinfar, M. Mittlböck, F. Fitzal
Annals of Surgical Oncology.2020; 27(5): 1700. CrossRef - Predictive factors for omitting lymphadenectomy in patients with node‐positive breast cancer treated with neo‐adjuvant systemic therapy
Sergi Fernandez‐Gonzalez, Catalina Falo, Maria J. Pla, Paula Verdaguer, Diana Nuñez, Anna Guma, Teresa Soler, Andrea Vethencourt, Silvia Vázquez, Maria Eulalia Fernandez‐Montoli, Miriam Campos, Sonia Pernas, Miguel Gil, Jordi Ponce, Amparo Garcia‐Tejedor
The Breast Journal.2020; 26(5): 888. CrossRef - Is There a Role for Post-Mastectomy Radiotherapy for T1-2N1 Breast Cancers With Node-Positive Pathology After Patients Become Node-Negative Pathology Following Neoadjuvant Chemotherapy?
Qian Wang, Jingjing Zhao, Xiaowei Han, Puchun Er, Xiangying Meng, Jinyan Shi, Huiru Sun, Jingyang Zhu, Li Zhu, Shikai Wu, Wencheng Zhang, Bing Sun
Frontiers in Oncology.2020;[Epub] CrossRef - Prognostic role of microRNA 182 and microRNA 18a in locally advanced triple negative breast cancer
Rajat Bajaj, Rupal Tripathi, T. S. Sridhar, Aruna Korlimarla, Kumardeep Dutta Choudhury, Moushumi Suryavanshi, Anurag Mehta, Dinesh Chandra Doval, Elda Tagliabue
PLOS ONE.2020; 15(11): e0242190. CrossRef - Association of Pathologic Complete Response with Long-Term Survival Outcomes in Triple-Negative Breast Cancer: A Meta-Analysis
Min Huang, Joyce O'Shaughnessy, Jing Zhao, Amin Haiderali, Javier Cortés, Scott D. Ramsey, Andrew Briggs, Peter Hu, Vassiliki Karantza, Gursel Aktan, Cynthia Z. Qi, Chenyang Gu, Jipan Xie, Muhan Yuan, John Cook, Michael Untch, Peter Schmid, Peter A. Fasch
Cancer Research.2020; 80(24): 5427. CrossRef - Multiparametric MR imaging to assess response following neoadjuvant systemic treatment in various breast cancer subtypes: Comparison between different definitions of pathologic complete response
G Santamaría, X Bargalló, S Ganau, I Alonso, M Muñoz, M Mollà, PL Fernández, A Prat
European Journal of Radiology.2019; 117: 132. CrossRef - Prognostic significance of residual nodal burden using lymph node ratio in locally advanced breast cancer after neoadjuvant chemotherapy
Reshu Agarwal, Arun Philip, Keechilat Pavithran, Anupama Rajanbabu, Gaurav Goel, DK Vijaykumar
Indian Journal of Cancer.2019; 56(3): 228. CrossRef - Application of neoadjuvant chemotherapy in occult breast cancer
Haisong Yang, Ling Li, Mengmeng Zhang, Shiyong Zhang, Shu Xu, Xiaoxia Ma
Medicine.2017; 96(40): e8200. CrossRef - Wnt7a Deficiency Could Predict Worse Disease-Free and Overall Survival in Estrogen Receptor-Positive Breast Cancer
Kijong Yi, Kyueng-Whan Min, Young Chan Wi, Yeseul Kim, Su-Jin Shin, Min Sung Chung, Kiseok Jang, Seung Sam Paik
Journal of Breast Cancer.2017; 20(4): 361. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


Fig. 1.
Fig. 2.
| Variable | No. (%) | ypT0/is |
ypT0 |
ypT0/is ypN0 |
ypT0 ypN0 |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | p-value | No. (%) | p-value | No. (%) | p-value | No. (%) | p-value | ||
| All patients | 353 (100) | 86 (24.4) | 50 (14.2) | 62 (17.6) | 40 (11.3) | ||||
| Age (yr) | .192 | .284 | .152 | .308 | |||||
| Median | 44.0 | 45.5 | 45.5 | 46.5 | 45.5 | ||||
| Range | 22–68 | 22–64 | 22–64 | 22–64 | 22–64 | ||||
| Menopause | .246 | .372 | .328 | .432 | |||||
| Pre | 268 (75.9) | 61 (22.8) | 35 (13.1) | 44 (16.4) | 28 (10.4) | ||||
| Post | 85 (24.1) | 25 (29.4) | 15 (17.6) | 18 (21.2) | 12 (14.1) | ||||
| Tumor size before NAC (cm) | .001 | .001 | .036 | .058 | |||||
| Median | 4.6 | 3.75 | 3.45 | 4.0 | 3.9 | ||||
| Range | 0.7–11.0 | 1.1–11.0 | 1.1–11.0 | 0.7–10.0 | 1.1–11.0 | ||||
| Clinical N stage | .190 | .739 | .193 | .533 | |||||
| cN1 | 10 (2.8) | 5 (50.0) | 2 (20.0) | 4 (40.0) | 2 (20.0) | ||||
| cN2 | 61 (17.3) | 16 (26.2) | 11 (18.0) | 13 (21.3) | 9 (14.8) | ||||
| cN3 | 178 (50.4) | 38 (21.3) | 23 (12.9) | 29 (16.3) | 20 (11.2) | ||||
| cN4 | 104 (29.5) | 27 (26.0) | 14 (13.5) | 16 (15.4) | 9 (8.7) | ||||
| Lymph node metastasis after NAC | < .001 | < .001 | < .001 | < .001 | |||||
| No | 135 (38.2) | 62 (45.9) | 40 (29.6) | 62 (45.9) | 40 (29.6) | ||||
| Yes | 218 (61.8) | 24 (11.0) | 10 (4.6) | 0 | 0 | ||||
| Histologic type | .017 | .143 | .056 | .233 | |||||
| Ductal | 323 (91.5) | 85 (26.3) | 49 (15.2) | 61 (18.9) | 39 (12.1) | ||||
| Lobular | 9 (2.5) | 1 (11.1) | 1 (11.1) | 1 (11.1) | 1 (11.1) | ||||
| Others | 21 (5.9) | 0 | 0 | 0 | 0 | ||||
| Histologic grade of pre-NAC tumors | < .001 | < .001 | < .001 | < .001 | |||||
| 1 | 27 (7.6) | 1 (3.7) | 0 | 0 | 0 | ||||
| 2 | 148 (41.9) | 18 (12.2) | 8 (5.4) | 12 (8.1) | 6 (4.1) | ||||
| 3 | 178 (50.4) | 67 (37.6) | 42 (23.6) | 50 (28.1) | 34 (19.1) | ||||
| Lymphovascular invasion | < .001 | < .001 | < .001 | < .001 | |||||
| No | 223 (63.2) | 83 (37.2) | 47 (21.1) | 62 (27.8) | 40 (17.9) | ||||
| Yes | 130 (36.8) | 3 (2.3) | 3 (2.3) | 0 | 0 | ||||
| ER status | < .001 | < .001 | < .001 | < .001 | |||||
| Negative | 167 (47.3) | 68 (40.7) | 43 (25.7) | 48 (28.7) | 34 (20.4) | ||||
| Positive | 186 (52.7) | 18 (9.7) | 7 (3.8) | 14 (7.5) | 6 (3.2) | ||||
| PR status | < .001 | < .001 | < .001 | < .001 | |||||
| Negative | 214 (60.6) | 72 (33.6) | 44 (20.6) | 53 (24.8) | 35 (16.4) | ||||
| Positive | 139 (39.4) | 14 (10.1) | 6 (4.3) | 9 (6.5) | 5 (3.6) | ||||
| HER2 status | .027 | .395 | .019 | .714 | |||||
| Negative | 255 (72.2) | 54 (21.2) | 39 (15.3) | 37 (14.5) | 30 (11.8) | ||||
| Positive | 98 (27.8) | 32 (32.7) | 11 (11.2) | 25 (25.5) | 10 (10.2) | ||||
| Subgroups | < .001 | < .001 | < .001 | < .001 | |||||
| Luminal A–like | 120 (34.0) | 6 (5.0) | 3 (2.5) | 5 (4.2) | 2 (1.7) | ||||
| Luminal B–like | 71 (20.1) | 14 (19.7) | 5 (7.0) | 10 (14.1) | 5 (7.0) | ||||
| HER2-positive | 55 (15.6) | 23 (41.8) | 8 (14.5) | 18 (32.7) | 7 (12.7) | ||||
| Triple-negative | 107 (30.3) | 43 (40.2) | 34 (31.8) | 29 (27.1) | 26 (24.3) | ||||
| Definitions of pCR | Total, n (%) | Distant metastasis, n (%) | DDFS |
Died of disease, n (%) | OS |
||
|---|---|---|---|---|---|---|---|
| HR |
p-value |
HR |
p-value |
||||
| Breast only | |||||||
| ypT0/is | 86 (24.4) | 13 (15.1) | 2.472 (1.380–4.426) | .002 | 5 (5.9) | 2.497 (0.980–6.364) | .055 |
| ypT0 | 50 (14.2) | 6 (12.0) | 2.900 (1.270–6.618) | .011 | 2 (4.0) | 3.373 (0.814–13.969) | .094 |
| Breast and lymph nodes | |||||||
| ypT0/is ypN0 | 62 (17.6) | 6 (9.7) | 3.954 (1.732–9.026) | .001 | 2 (3.2) | 4.498 (1.086–18.638) | .038 |
| ypT0 ypN0 | 40 (11.3) | 3 (7.5) | 4.741 (1.502–14.958) | .008 | 1 (2.5) | 5.277 (0.725–38.398) | .100 |
pCR, pathologic complete response; NAC, neoadjuvant chemotherapy; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; Luminal A-like, ER/PR+HER2– tumors with histologic grade 1 or 2; Luminal B-like, ER/PR+HER2– tumors with histologic grade 3 or ER/PR+HER2+ tumors; HER2-positive, ER/PR–HER2+ tumors; Triple-negative, ER/PR/HER2– tumors.
pCR, pathologic complete response; DDFS, distant disease-free survival; OS, overall survival; HR, hazard ratio; CI, confidende interval. HRs and p-values are calculated from the comparison of survival in patients with or without pCR.

 E-submission
E-submission