Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 52(3); 2018 > Article
-
Brief Case Report
Expression of CD34 and β-Catenin in Malignant Rhabdoid Tumor of the Liver Mimicking Proximal-Type Epithelioid Sarcoma -
Woo Cheal Cho
 , Fabiola Balarezo
, Fabiola Balarezo -
Journal of Pathology and Translational Medicine 2018;52(3):195-197.
DOI: https://doi.org/10.4132/jptm.2017.05.15
Published online: July 7, 2017
Department of Pathology and Laboratory Medicine, Hartford Hospital, Hartford, CT, USA
- Corresponding Author Woo Cheal Cho, MD Department of Pathology and Laboratory Medicine, Hartford Hospital, 80 Seymour Street, Hartford, CT 06102-5037, USA Tel: +1-860-972-2488, Fax: +1-860-545-2204 E-mail: woocheal.cho@hhchealth.org
• Received: March 12, 2017 • Revised: April 5, 2017 • Accepted: May 15, 2017
© 2018 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
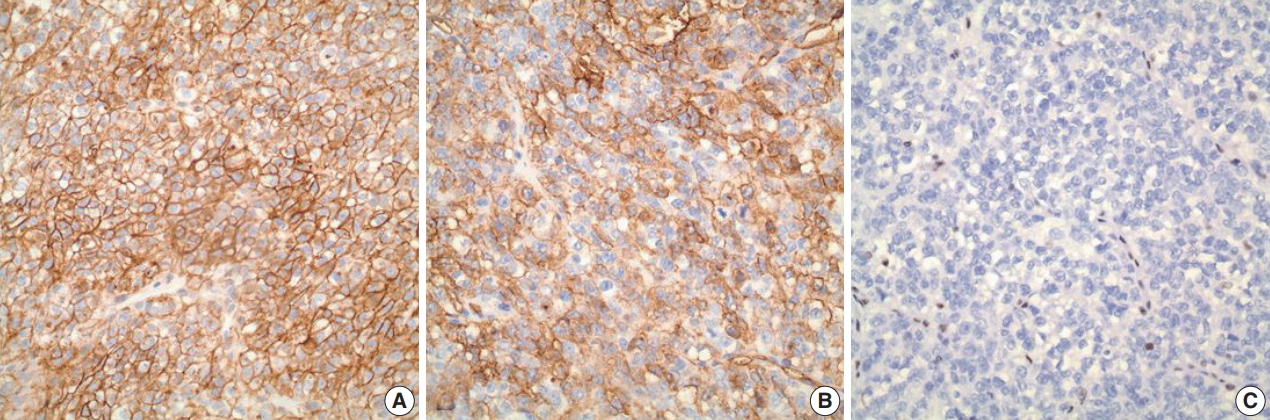
- A 1-year-old male infant presented with a right-sided, nontender abdominal mass. Imaging studies revealed a heterogeneously enhancing mass within the liver, measuring 10.0 × 10.0 × 7.9 cm, suspicious of hepatoblastoma. Liver biopsy showed a proliferation of malignant epithelioid cells with clear to amphophilic cytoplasm and small nucleoli (Fig. 1A–C). The tumor focally exhibited an organoid/trabecular growth pattern, and a rim of compressed non-neoplastic liver parenchyma was seen at the periphery of the tumor (Fig. 1A). Necrosis was present (Fig. 1B) and numerous mitotic figures (7 mitoses/10 high-power field) (Fig. 1C) were identified. No definitive rhabdoid cells were seen. Immunohistochemical analysis revealed diffuse immunoreactivity to cytokeratin (CK) 19, CK MNF116, vimentin, and β-catenin (membranous) (Fig. 2A). The tumor was also focally positive for epithelial membrane antigen, glypican-3, and CD34 (Fig. 2B). Immunostains for CK7, CK20, hepatocyte paraffin (Hep Par) 1, arginase-1, α-fetoprotein, desmin, myogenin, human melanoma black 45, Melan-A, octamer-binding transcription factor 3/4, carcinoembryonic antigen, chromogranin, synaptophysin, calponin, smooth muscle actin, S100, and SRY-related HMG-box 10 (SOX10) were negative. Loss of nuclear INI1 expression (Fig. 2C) was seen. The diagnosis of INI1-negative neoplasm was entertained with differential diagnoses including extrarenal MRT, proximal-type ES, and small cell undifferentiated (SCUD) hepatoblastoma. Despite the lack of classic rhabdoid morphology and the presence of positive expression of CD34 and β-catenin, extrarenal MRT was favored over proximal-type ES given the patient’s age and location of the tumor. In addition, the tumor lacked classic morphologic features of small round blue cell tumors, thereby making SCUD hepatoblastoma a less favored differential.
- Formal written informed consent was not required with a waiver by the appropriate institutional review board of Hartford Hospital and/or national research ethics committee.
CASE REPORT
- MRT is a rare, highly aggressive and lethal malignant neoplasm with poor prognosis. Histologically, MRT is classically characterized by sheets of large eosinophilic cells with eccentric vesicular nuclei, prominent nucleoli, and occasional intracytoplasmic inclusions of hyaline globules, reminiscent of rhabdomyoblasts. Loss of nuclear INI1 expression associated with deletions or mutations of the SMARCB1/INI1 gene at 22q11 [2]. is the characteristic hallmark of MRT [3,4]. This genetic alteration, however, is also seen in other rare neoplasms, including proximaltype ES [5] and SCUD hepatoblastoma [6]. In particular, distinction between extrarenal MRT and proximal-type ES can be problematic due to morphologic similarities between the two entities; proximal-type ES also shows large epithelioid cells, vesicular nuclei with prominent nucleoli, and rhabdoid cytoplasmic inclusions. Recently, CD34 and β-catenin have been suggested as potentially useful immunohistochemical markers for distinguishing extrarenal MRT from proximal-type ES. Proximal-type ES often exhibits positive expression of CD34 and β-catenin, while extrarenal MRT typically lacks immunoreactivity to these markers. In the present case, however, the tumor was diffusely and strongly positive for β-catenin while showing patchy positivity with CD34 in the absence of nuclear INI1 staining, mimicking proximal-type ES. Nonetheless, extrarenal MRT was still favored over proximal-type ES in our case given the fact that proximal-type ES is predominantly seen in middle-aged or older adults, frequently occurring in axial or proximal regions, such as the pelvis, perineum, and genitalia [7]. In contrast, MRT of the liver mainly occurs in infants [4]. SCUD hepatoblastoma is a rare variant of hepatoblastoma known to show loss of INI expression, similar to MRT [6]. In fact, some authors [6] have recently postulated that SCUD hepatoblastoma may actually not be a hepatoblastoma but rather a form of MRT arising in the liver, although further study is needed. The tumor in our case, however, lacked small cell morphology compatible with SCUD hepatoblastoma, thus making it a less favored differential.
- In conclusion, we report a case of MRT of the liver with unusual immunophenotypic features mimicking proximal-type ES and SCUD hepatoblastoma. To the best of our knowledge, this may be the first reported case of extrarenal MRT of the liver with CD34 and β-catenin expression.
DISCUSSION
Fig. 1.Microscopic findings on liver biopsy. (A) The tumor displays malignant epithelioid cells with clear to amphophilic cytoplasm and small nucleoli with a focal organoid growth pattern. A rim of compressed non-neoplastic liver parenchyma is seen at the periphery of the tumor. (B, C) Areas of necrosis (B) and frequent mitoses (C, circle) within the tumor are also seen.
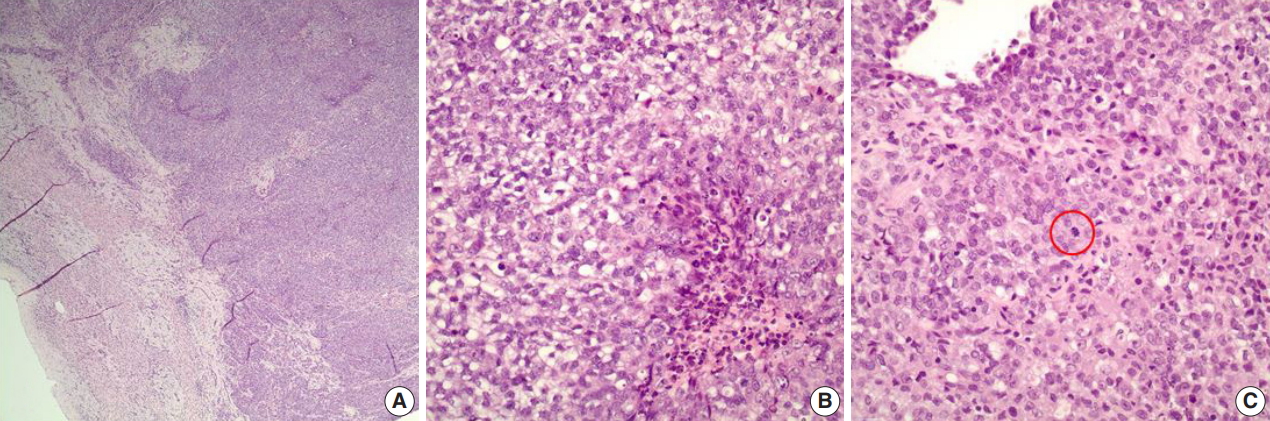

Fig. 2.Immunohistochemical analysis. (A) A diffuse and strong immunoreactivity (membranous) with β-catenin is seen in the tumor. (B) The tumor is also positive (patchy) for CD34. (C) Loss of nuclear INI1 expression is seen within the tumor.

- 1. Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumors: results from the First National Wilms’ Tumor Study. Cancer 1978; 41: 1937-48. ArticlePubMed
- 2. Rorke LB, Packer RJ, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg 1996; 85: 56-65. ArticlePubMed
- 3. Oita S, Terui K, Komatsu S, et al. Malignant rhabdoid tumor of the liver: a case report and literature review. Pediatr Rep 2015; 7: 5578.ArticlePubMedPMCPDF
- 4. Oda Y, Tsuneyoshi M. Extrarenal rhabdoid tumors of soft tissue: clinicopathological and molecular genetic review and distinction from other soft-tissue sarcomas with rhabdoid features. Pathol Int 2006; 56: 287-95. ArticlePubMed
- 5. Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol 2009; 33: 542-50. ArticlePubMed
- 6. Vokuhl C, Oyen F, Haberle B, von Schweinitz D, Schneppenheim R, Leuschner I. Small cell undifferentiated (SCUD) hepatoblastomas: All malignant rhabdoid tumors? Genes Chromosomes Cancer 2016; 55: 925-31. ArticlePubMed
- 7. Armah HB, Parwani AV. Epithelioid sarcoma. Arch Pathol Lab Med 2009; 133: 814-9. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- An Unusual Case of Metastasis of an Epithelioid Sarcoma to the Pleura and Bronchus: A Case Report and Literature Review
炳群 吴
Advances in Clinical Medicine.2018; 08(07): 615. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Expression of CD34 and β-Catenin in Malignant Rhabdoid Tumor of the Liver Mimicking Proximal-Type Epithelioid Sarcoma


Fig. 1. Microscopic findings on liver biopsy. (A) The tumor displays malignant epithelioid cells with clear to amphophilic cytoplasm and small nucleoli with a focal organoid growth pattern. A rim of compressed non-neoplastic liver parenchyma is seen at the periphery of the tumor. (B, C) Areas of necrosis (B) and frequent mitoses (C, circle) within the tumor are also seen.
Fig. 2. Immunohistochemical analysis. (A) A diffuse and strong immunoreactivity (membranous) with β-catenin is seen in the tumor. (B) The tumor is also positive (patchy) for CD34. (C) Loss of nuclear INI1 expression is seen within the tumor.
Fig. 1.
Fig. 2.
Expression of CD34 and β-Catenin in Malignant Rhabdoid Tumor of the Liver Mimicking Proximal-Type Epithelioid Sarcoma

 E-submission
E-submission




