Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 52(2); 2018 > Article
-
Original Article
The Clinicopathological and Prognostic Significance of the Gross Classification of Hepatocellular Carcinoma - Yangkyu Lee, Hyunjin Park1, Hyejung Lee1, Jai Young Cho2, Yoo-Seok Yoon2, Young-Rok Choi2, Ho-Seong Han2, Eun Sun Jang3, Jin-Wook Kim3, Sook-Hyang Jeong3, Soomin Ahn, Haeryoung Kim,1
-
Journal of Pathology and Translational Medicine 2018;52(2):85-92.
DOI: https://doi.org/10.4132/jptm.2017.11.13
Published online: November 24, 2017
Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
1Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
2Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
3Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- Corresponding Author Haeryoung Kim, MD, PhD Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, 103 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-740-8322 Fax: +82-2-765-5600 E-mail: haeryoung.kim@snu.ac.kr
© 2018 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- We aimed to determine the clinicopathological significance of the gross classification of hepatocellular carcinoma (HCC) according to the Korean Liver Cancer Association (KLCA) guidelines.
-
Methods
- A retrospective analysis was performed on 242 cases of consecutively resected solitary primary HCC between 2003 and 2012 at Seoul National University Bundang Hospital. The gross classification (vaguely nodular [VN], expanding nodular [EN], multinodular confluent [MC], nodular with perinodular extension [NP], and infiltrative [INF]) was reviewed for all cases, and were correlated with various clinicopathological features and the expression status of “stemness”-related (cytokeratin 19 [CK19], epithelial cell adhesion molecule [EpCAM]), and epithelial-mesenchymal transition (EMT)–related (urokinase plasminogen activator receptor [uPAR] and Ezrin) markers.
-
Results
- Significant differences were seen in overall survival (p=.015) and disease-free survival (p = .034) according to the gross classification; INF type showed the worst prognosis while VN and EN types were more favorable. When the gross types were simplified into two groups, type 2 HCCs (MC/NP/INF) were more frequently larger and poorly differentiated, and showed more frequent microvascular and portal venous invasion, intratumoral fibrous stroma and higher pT stages compared to type 1 HCCs (EN/VN) (p<.05, all). CK19, EpCAM, uPAR, and ezrin expression was more frequently seen in type 2 HCCs (p<.05, all). Gross classification was an independent predictor of both overall and disease-free survival by multivariate analysis (overall survival: p=.030; hazard ratio, 4.118; 95% confidence interval, 1.142 to 14.844; disease-free survival: p=.016; hazard ratio, 1.617; 95% confidence interval, 1.092 to 2.394).
-
Conclusions
- The gross classification of HCC had significant prognostic value and type 2 HCCs were associated with clinicopathological features of aggressive behavior, increased expression of “stemness”- and EMT-related markers, and decreased survival.
- Patient selection and clinicopathological analysis
- Two hundred and ninety-eight consecutive cases of primary HCCs that were surgically resected between 2003 and 2012 at Seoul National University Bundang Hospital, Seongnam, Republic of Korea were evaluated in this study. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-1708-412-304), and patient consent was waived due to the retrospective nature of this study. Clinicopathological data were analyzed by reviewing electronic medical records, pathology reports and glass slides, and included patient sex, age at operation, tumor size, gross type, histologic differentiation (Edmondson-Steiner grade), serum α-fetoprotein (AFP) levels and the pathological T and N categories according to AJCC TNM staging system (7th edition). The presence of intratumoral fibrous stroma was also noted; we defined the presence of intratumoral fibrous stroma as fibrous stroma occupying more than 30% of the tumor area [14]. Cases with multiple HCCs were excluded from the study, leaving a total of 242 cases for further analysis. Follow-up data was retrieved from the electronic medical records, including the status at last follow up and occurrence of distant or intrahepatic metastasis or local recurrence.
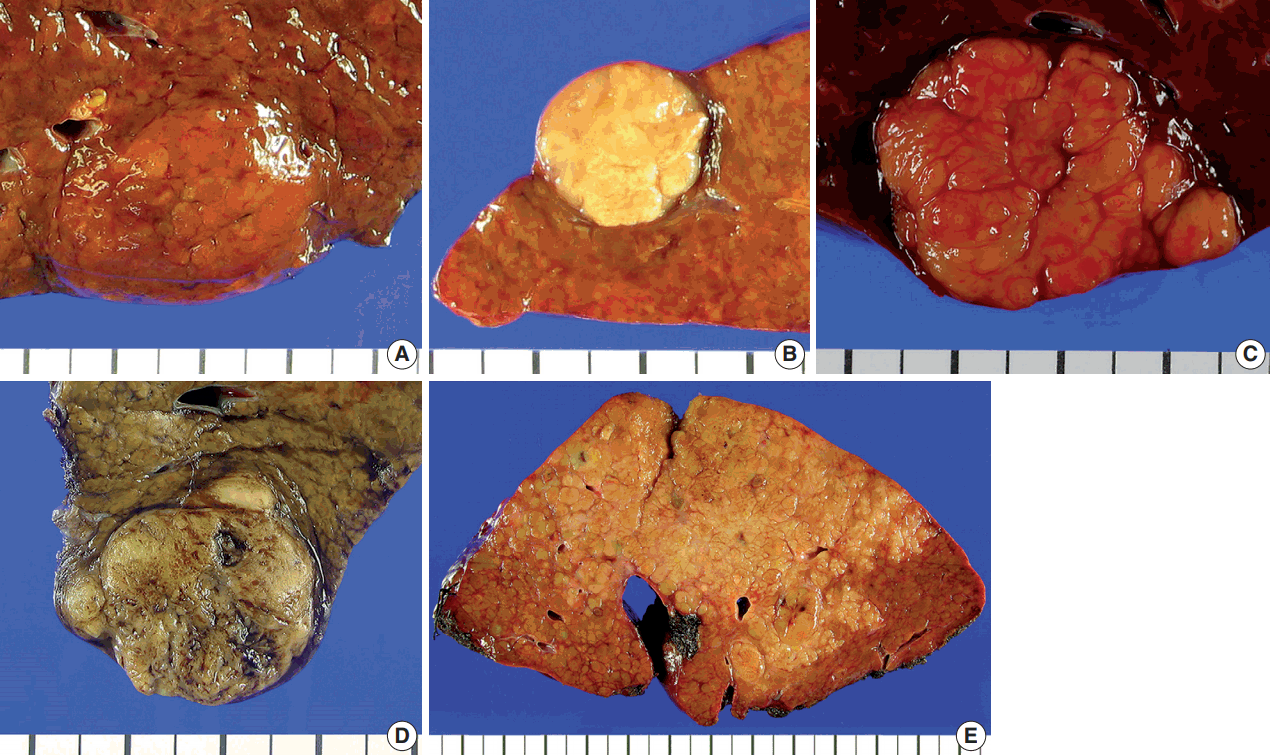
- The gross type was determined by examining the largest cross section of the tumor by two pathologists (Y.L. and H.K.), according to the General Rules for the Study of Primary Liver Cancer by the KLCA (Fig. 1) [13]. “Vaguely nodular” (VN) type was defined as a nodule with indistinct margins. While VN type morphology is an important characteristic of early HCC [15], we classified HCCs as VN type purely by gross appearance, regardless of the tumor size and histologic differentiation status. “Expanding nodular” (EN) type was defined by as a round expansile nodule with a distinct margin. “Multinodular confluent” (MC) type was defined as a cluster of small and confluent nodules. “Nodular with perinodular extension” (NP) type was defined as an expanding nodule similar to EN type HCCs that had extranodular growth in less than 50% of the tumor circumference. INF type HCCs showed extranodular growth in more than 50% of the tumor circumference. The gross types were correlated with the clinicopathological features of the HCCs.
- Tissue microarray construction and immunohistochemistry
- Two-millimeter-core tissue microarrays were constructed from the HCCs (Superbiochips Laboratories, Seoul, Korea), and 4 μm-thick tissue sections obtained from the tissue microarray blocks. Immunohistochemical staining was performed for “stemness”-related markers (cytokeratin 19 [CK19]; 1:100, mouse monoclonal, Dako, Glostrup, Denmark), epithelial cell adhesion molecule (EpCAM; 1:3,000, mouse monoclonal, Millipore, Billerica, MA, USA), and epithelial-mesenchymal transition (EMT)–related markers (urokinase plasminogen activator receptor [uPAR; 1:40, mouse monoclonal, Abcam, Cambridge, UK] and ezrin [1:100, mouse monoclonal, Abcam]). Briefly, after deparaffinization in xylene and rehydration in graded alcohol, antigen retrieval was performed on tissue sections using citrate buffer (pH 6.0) for CK19, EpCAM, and ezrin, and protease for uPAR. Incubation with primary antibodies was performed for 1 hour at room temperature, and with secondary antibody (EnVision kit, Dako) for 30 minutes. The presence of cytoplasmic expression in > 5% of the tumor cells was regarded as positive for CK19, ezrin, and uPAR expression. EpCAM was expressed in the tumor cell membranes.
- Statistical analysis
- All statistical analyses were performed using SPSS ver. 19.0 K (SPSS Korea, Seoul, Korea). Chi-square tests and Fisher exact tests were performed as deemed appropriate. Survival analyses for overall and disease-free survivals were performed by the Kaplan-Meier method and log-rank test. The Cox regression models were used for multivariate analysis. Statistical significance was defined as p < .05.
MATERIALS AND METHODS
- Clinicopathological characteristics according to HCC gross classification
- The clinicopathological characteristics of the 242 cases studied are summarized in Table 1. The most common etiologic factor was hepatitis B virus (HBV) infection (171/242, 70.7%), followed by alcohol (35/242, 14.5%), hepatitis C virus (HCV) infection (17/242, 7.0%) and combined HBV + HCV infection (1/242, 0.4%). The etiology was uncertain for the remainder of patients (18/298, 7.4%). The most common gross type of HCCs was the EN type (107/242, 44.2%), followed by the MC type (78/242, 32.2%), NP type (32/242, 13.2%), INF type (16/242, 6.6%), and the VN type (9/242, 3.7%).
- When the clinicopathological features were compared among the five different gross types, we found that INF type HCCs were associated with larger tumor size, poor histologic differentiation, more frequent microvascular and portal venous invasion and higher pathologic T stage compared to the other gross types (Table 2). Large tumor size, microvascular and portal venous invasion and high T stage were rare or absent in VN or EN type HCCs. The clinicopathological features of MC and NP type HCCs were similar. We regrouped the five gross types into type 1 and type 2, as previously described by Gong et al. [5]: type 1 HCCs consisted of VN and EN type, and type 2 HCCs consisted of MC, NP, and INF types. On comparing the clinicopathological findings between the two types, we found that type 2 HCCs were more frequently larger (p < .001) and poorly differentiated (p = .001), and showed more frequent microvascular invasion (p < .001), portal venous invasion (p < .001), higher pT stages (p < .001), and intratumoral fibrous stroma (p < .001) compared to type 1 HCCs.
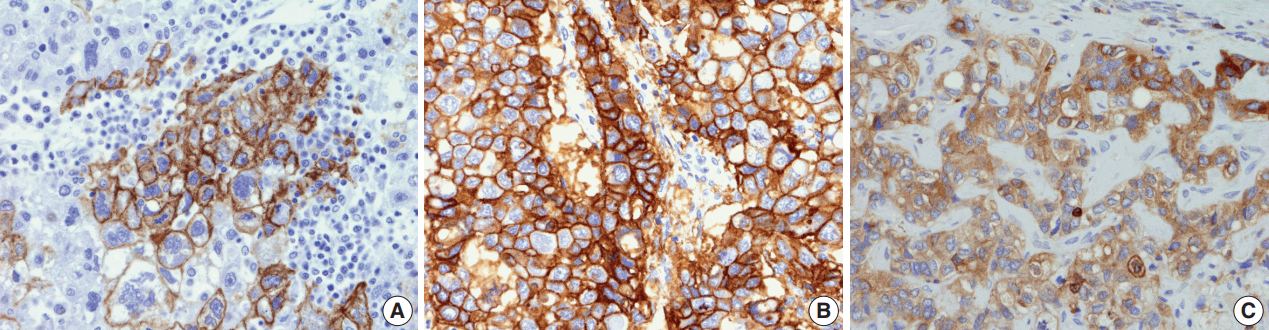
- Differences in expression status of “stemness”- and EMT-related markers in HCC according to gross classification
- The immunohistochemical stain results are summarized in Table 2 and Fig. 2. The expression of “stemness”-related markers, CK19 and EpCAM, was seen in 18.6% and 43.4% of HCCs, respectively. CK19 and EpCAM expression rates were significantly higher in INF type HCCs compared to EN type HCCs (CK19, 37.5% vs 9.3%; EpCAM, 75.0% vs 34.6%). Significant differences were seen in the frequencies of CK19 and EpCAM positivity between type 1 and type 2 HCCs (CK19, p = .002; EpCAM, p = .009). uPAR and ezrin, EMT-related markers, were more frequently expressed in type 2 HCCs compared to type 1 HCCs (uPAR, p < .001; ezrin, p = .036).
- Survival analysis results
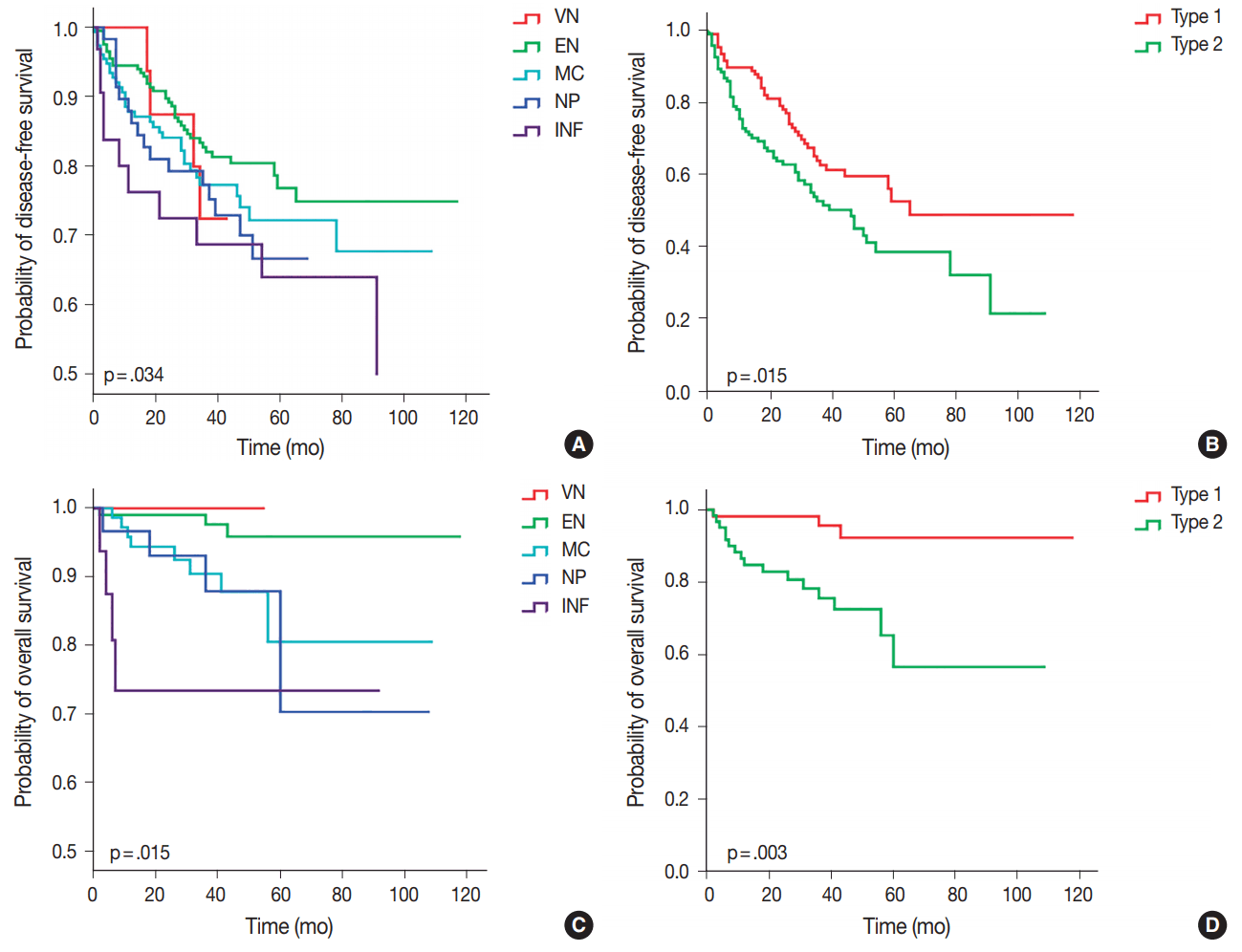
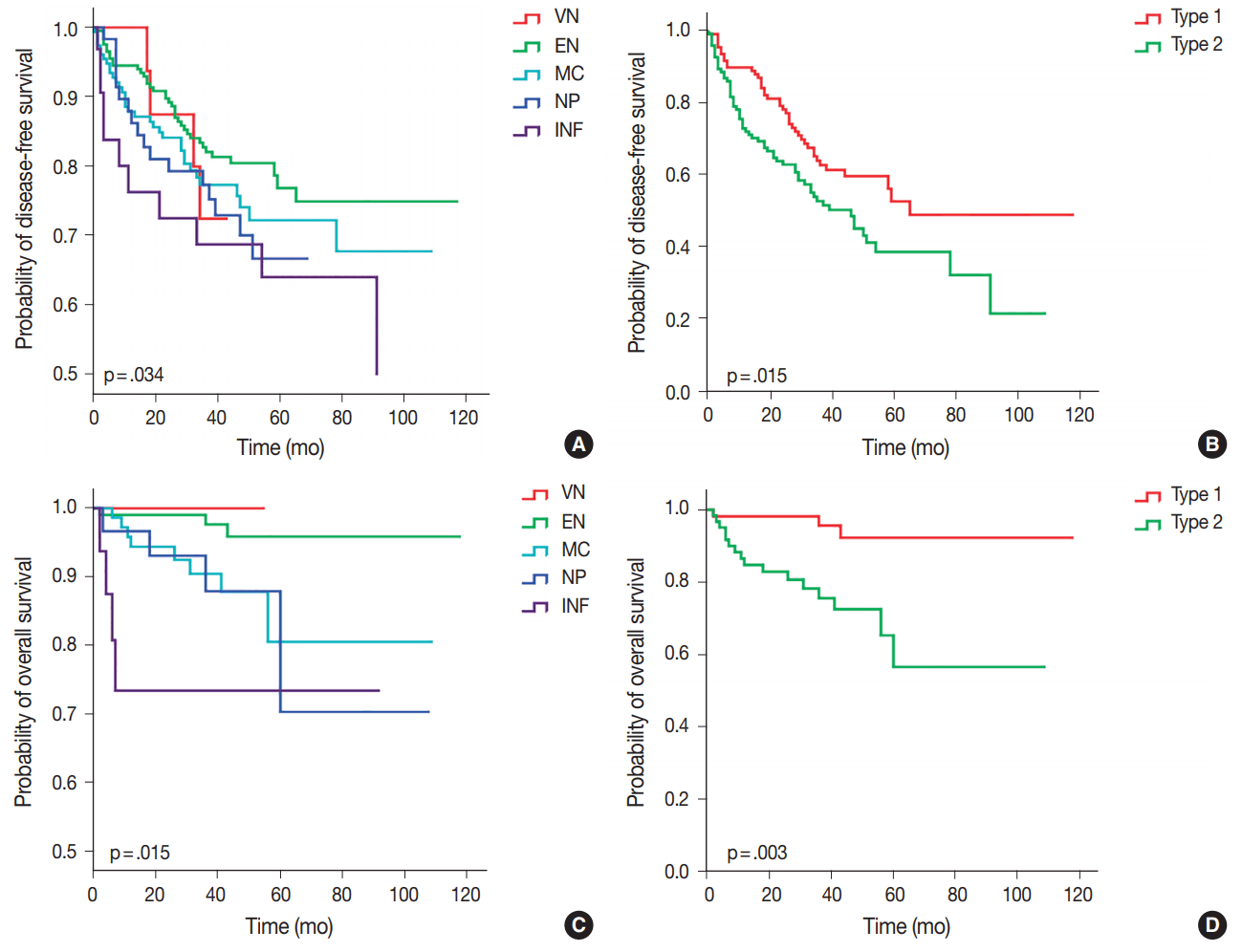
- Of the five different gross types, the INF type demonstrated the worst overall survival (p = .015) and disease-free survival (p=.034) compared to other types (Table 3, Fig. 3). The most favorable outcome was seen for EN and VN types, and the survival curves for MC and NP types were in between that of EN and INF type HCCs. When the gross types were simplified into two groups, type 2 HCCs showed significantly decreased disease-free (p = .015) and overall survival (p = .003) compared to type 1 HCCs. Of the other clinicopathological variables, tumor size of larger than 5 cm was associated with a decreased disease-free survival (p = .041), portal venous invasion (p < .001) and higher pT stage (p < .001) were associated with decreased overall survival, and microvascular invasion was marginally associated with decreased disease-free and overall survivals although not statistically significant.
- Multivariate analysis demonstrated that gross classification (type 1 vs 2) was a significant independent predictor of both overall and disease-free survival, after adjusting for patient sex and age. Type 2 HCCs showed significantly decreased overall survival (p = .030; hazard ratio, 4.118; 95% confidence interval, 1.142 to 14.844) and disease-free survival (p = .016; hazard ratio, 1.617; 95% confidence interval, 1.092 to 2.394). High pathologic T stage also remained a significant predictive factor for overall survival (p = .025; hazard ratio, 3.173; 95% confidence interval, 1.156 to 8.710).
RESULTS
- In this study, we found that INF type HCCs had the worst prognosis out of the five different gross types, and when the HCCs were further grouped into types 1 and 2, gross type 2 HCCs more frequently showed clinicopathological features of aggressive behavior and poor prognosis compared to type 1 HCCs. The gross classification had a strong impact on patient survival; type 2 gross morphology was a significant independent predictor of decreased overall and disease-free survivals on multivariate analysis.
- Our findings are similar to the results of a few previous studies. Increased overall and disease-free survival was noted for single nodular type HCCs compared to those with extranodular growth or MC growth patterns [6,9]. Interestingly, Shimada et al. [9] analyzed small HCCs (< 3 cm) separately and found similar associations between gross morphology and prognosis, and in another analysis [3] on huge HCCs (> 10 cm), single nodular type HCCs showed more favorable outcomes compared to non-single nodular HCCs. Taken together, it could be suggested that the gross classification may be an important predictor of prognosis regardless of tumor size. When tumor size was entered into our multivariate analysis model, we found that gross type 2 was a strong independent predictor of both overall survival and disease-free survival.
- The gross classification consists of five different morphological types of HCCs and clear definitions for the different types have been proposed in the guidelines of both the LCSGJ and KLCA. However, in practice, the distinction between NP, MC, and INF type HCCs is not always clear cut, and prone to interobserver variability. On the other hand, EN and VN types (known as “single nodular with distinct margin” and “single nodular with indistinct margin” types in the LCSGJ guidelines, respectively) are relatively easier to discriminate from the other types as they lack the multilobulated irregular contour. If the gross classification is an important prognostic factor for HCC on surgically resected specimens, this could also be implemented in the preoperative evaluation of HCC patients, as the gross features can be determined on preoperative imaging. Therefore, it may be sufficient and more practical to classify HCCs as single nodular types (type 1) and non-single nodular types (type 2) for guiding patient management strategies. Interestingly, Fu et al. [4] analyzed the survival of patients with small HCCs (< 5 cm) treated with radiofrequency ablation according to the gross type on imaging, and found that HCCs with single nodular HCCs without extranodular growth or irregular margins were associated with favorable survival.
- A subset of HCCs that have morphological features consistent with HCC have been demonstrated to express immunophenotypes associated with “stemness,” such as CK19, EpCAM, CD133, and c-kit positivity. These tumors have been associated with higher preoperative serum AFP levels, less frequent fibrous capsule formation, intratumoral fibrous stroma, frequent vascular invasion and poor prognosis compared to typical HCCs that do not express these markers [16,17]. As expected, we found in this study that larger tumor size, poor histological differentiation, microvascular and portal venous invasion, intratumoral fibrous stroma, “stemness” and EMT-related marker expression and higher T stages were significantly more frequent in type 2 HCCs compared to type 1 HCCs. Therefore, HCCs that have a solitary, well-circumscribed and expansile growth pattern were less likely to exhibit features associated with “stemness.” The higher prevalence of CK19 expression in type 2 HCCs has been recently demonstrated by another group [5].
- EMT refers to the process in which tumor epithelial cells lose their epithelial characteristics (e.g., loss of membranous E-cadherin expression) and acquire mesenchymal features, facilitating tumor invasion and distant metastasis [18]. This process has been described in HCCs, and HCCs expressing ezrin and uPAR have been associated with poor prognoses [19,20]. We found in this study that INF type HCCs showed frequent uPAR expression (75%) compared to other types, especially VN and EN types which were uPAR positive in 11% and 15%, respectively. MC and NP types showed uPAR expression frequency intermediate between INF types and EN/VN types. In a previous study from Japan, E-cadherin loss was more frequently seen in single nodular with extranodal growth type and confluent multinodular type HCCs of less than 6 cm in diameter (which can be translated to NP and MC type HCCs according to the KLCA classification) [7]. Taken together, it could be suggested that invasiveness and metastatic ability of HCCs could be reflected by the gross appearance.
- On examining the Kaplan-Meier curves for overall and disease-free survival according to the five different gross types of our cohort, significant differences in survival were noted between the EN types and INF types. The MC and NP types showed survivals intermediate between the EN and INF types, without significant differences between the two types. Interestingly, while the VN type showed the best overall survival (no HCC-related deaths), early recurrences were noted for two VN type HCCs (at 17 and 18 months) for the disease-free survival analysis. Although VN type morphology is a characteristic feature of early HCC [15], we included all cases that were macroscopically of VN type regardless of the histological differentiation or tumor size; indeed, poor histological differentiation was noted in 4/9 (44.4%) cases and 2/9 (22.2%) cases were larger than 3 cm. Therefore, the VN type in this study does not refer to early HCC, and we grouped VN and EN types together into type 1 (single nodular) HCCs for analytical purposes.
- Although this is not the first report on the clinicopathological significance of the gross classification of HCCs, this is a large-scale cohort study of 242 surgically resected solitary HCCs using the definitions in the guidelines of the KLCA, and we also demonstrate for the first time the associations between the different gross types and the expression status of “stemness”- and EMT-related markers. A limitation of this study is that we excluded multiple HCCs (including multicentricity and intrahepatic metastasis) from the study cohort in order to exclude cases showing multiple gross types in the same liver. This resulted in the lower percentage of cases with higher pT stage, and the exclusion of pT3a cases using the current AJCC staging system (seventh edition). Nevertheless, we demonstrate that the gross classification of HCCs according to the KLCA guidelines has prognostic value, and that gross type 2 HCCs with non-single nodular patterns are associated with clinicopathological features of aggressive behavior, increased expression of “stemness”- and EMT-related markers and decreased survival. Further validation would be required in independent cohorts and also radio-pathological correlation studies would be needed to validate the utility of the gross classification in HCC patient management.
DISCUSSION
Acknowledgments

| Variable |
Overall survival |
Disease-free survival |
||
|---|---|---|---|---|
| Hazard ratio (95% confidence interval) | p-value | Hazard ratio (95% confidence interval) | p-value | |
| Univariate analysis | ||||
| Gross type (type 1 vs type 2)a | 5.439 (1.583–18.683) | .007 | 1.617 (1.092–2.394) | .016 |
| Tumor size (> 5 cm) | 1.229 (0.407–3.712) | .714 | 1.590 (1.013–2.495) | .044 |
| High E-S grade (III or IV) | 0.636 (0.256–1.583) | .331 | 1.054 (0.694–1.599) | .805 |
| Microvascular invasion | 2.178 (0.881–5.383) | .092 | 1.445 (0.977–2.138) | .065 |
| Portal vein invasion | 5.311 (1.906–14.796) | < .001 | 1.702 (0.858–3.376) | .128 |
| High T category (pT3 or pT4) | 5.206 (1.974–13.728) | < .001 | 1.795 (0.982–3.284) | .057 |
| Intratumoral stromal fibrosis | 2.037 (0.801–5.179) | .135 | 0.973 (0.608–1.559) | .973 |
| Multivariate analysis | ||||
| Gross type (type 1 vs type 2) | 4.118 (1.142–14.844) | .030 | 1.617 (1.092–2.394) | .016 |
| Tumor size (> 5 cm) | 0.586 (0.166–2.060) | .404 | 1.303 (0.802–2.115) | .285 |
| Microvascular invasion | 1.953 (0.765–4.982) | .161 | 1.300 (0.866–1.952) | .205 |
| Portal vein invasion | 1.091 (0.150–7.931) | .931 | 0.954 (0.292–3.124) | .938 |
| High T category (pT3 or pT4) | 3.173 (1.156–8.710) | .025 | 1.462 (0.500–4.275) | .487 |
- 1. Liver Cancer Study Group of Japan. General rules for the clinical and pathological study of primary liver cancer. 2nd ed. Tokyo: Kanehara & Co, 2003.
- 2. Kanai T, Hirohashi S, Upton MP, et al. Pathology of small hepatocellular carcinoma: a proposal for a new gross classification. Cancer 1987; 60: 810-9. ArticlePubMed
- 3. Choi GH, Han DH, Kim DH, et al. Outcome after curative resection for a huge (>or=10 cm) hepatocellular carcinoma and prognostic significance of gross tumor classification. Am J Surg 2009; 198: 693-701. PubMed
- 4. Fu X, Mao L, Tang M, et al. Gross classification of solitary small hepatocellular carcinoma on preoperative computed tomography: prognostic significance after radiofrequency ablation. Hepatol Res 2016; 46: 298-305. ArticlePubMed
- 5. Gong SC, Cho MY, Lee SW, Kim SH, Kim MY, Baik SK. The meaning of gross tumor type in the aspects of cytokeratin 19 expression and resection margin in patients with hepatocellular carcinoma. J Gastroenterol Hepatol 2016; 31: 206-12. ArticlePubMedPDF
- 6. Hui AM, Takayama T, Sano K, et al. Predictive value of gross classification of hepatocellular carcinoma on recurrence and survival after hepatectomy. J Hepatol 2000; 33: 975-9. ArticlePubMed
- 7. Inayoshi J, Ichida T, Sugitani S, et al. Gross appearance of hepatocellular carcinoma reflects E-cadherin expression and risk of early recurrence after surgical treatment. J Gastroenterol Hepatol 2003; 18: 673-7. ArticlePubMed
- 8. Murakata A, Tanaka S, Mogushi K, et al. Gene expression signature of the gross morphology in hepatocellular carcinoma. Ann Surg 2011; 253: 94-100. ArticlePubMed
- 9. Shimada M, Rikimaru T, Hamatsu T, et al. The role of macroscopic classification in nodular-type hepatocellular carcinoma. Am J Surg 2001; 182: 177-82. ArticlePubMed
- 10. Trevisani F, Caraceni P, Bernardi M, et al. Gross pathologic types of hepatocellular carcinoma in Italian patients: relationship with demographic, environmental, and clinical factors. Cancer 1993; 72: 1557-63. ArticlePubMed
- 11. Tsujita E, Yamashita Y, Takeishi K, et al. The clinicopathological impact of gross classification on solitary small hepatocellular carcinoma. Hepatogastroenterology 2013; 60: 1726-30. PubMed
- 12. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998; 28: 751-5. PubMed
- 13. Jang JY, Lee JS, Kim HJ, et al. The general rules for the study of primary liver cancer. J Liver Cancer 2017; 17: 19-44.
- 14. Kurogi M, Nakashima O, Miyaaki H, Fujimoto M, Kojiro M. Clinicopathological study of scirrhous hepatocellular carcinoma. J Gastroenterol Hepatol 2006; 21: 1470-7. ArticlePubMed
- 15. International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology 2009; 49: 658-64. ArticlePubMed
- 16. Kim H, Choi GH, Na DC, et al. Human hepatocellular carcinomas with "stemness"-related marker expression: keratin 19 expression and a poor prognosis. Hepatology 2011; 54: 1707-17. ArticlePubMed
- 17. Kim H, Park YN. Hepatocellular carcinomas expressing ‘stemness’-related markers: clinicopathological characteristics. Dig Dis 2014; 32: 778-85. ArticlePubMedPDF
- 18. Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 2006; 172: 973-81. ArticlePubMedPMCPDF
- 19. Okamura D, Ohtsuka M, Kimura F, et al. Ezrin expression is associated with hepatocellular carcinoma possibly derived from progenitor cells and early recurrence after surgical resection. Mod Pathol 2008; 21: 847-55. ArticlePubMedPDF
- 20. Zhou L, Hayashi Y, Itoh T, Wang W, Rui J, Itoh H. Expression of urokinase-type plasminogen activator, urokinase-type plasminogen activator receptor, and plasminogen activator inhibitor-1 and -2 in hepatocellular carcinoma. Pathol Int 2000; 50: 392-7. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Long-Term Outcomes of Transarterial Chemoembolization plus Ablation versus Surgical Resection in Patients with Large BCLC Stage A/B HCC
Ying-Wen Hou, Tian-Qi Zhang, Li-Di Ma, Yi-Quan Jiang, Xue Han, Tian Di, Lu Tang, Rong-Ping Guo, Min-Shan Chen, Jin-Xin Zhang, Zhi-Mei Huang, Jin-Hua Huang
Academic Radiology.2025; 32(7): 3960. CrossRef - Membranous Overexpression of Fibronectin Predicts Microvascular Invasion and Poor Survival Outcomes in Patients with Hepatocellular Carcinoma
Yoon Jung Hwang, Hyejung Lee, Suk Kyun Hong, Su Jong Yu, Haeryoung Kim
Gut and Liver.2025; 19(2): 275. CrossRef - Association between tumor morphology and efficacy of atezolizumab plus bevacizumab for advanced hepatocellular carcinoma
Nobuaki Ishihara, Shohei Komatsu, Keitaro Sofue, Eisuke Ueshima, Yoshihiko Yano, Yoshimi Fujishima, Jun Ishida, Masahiro Kido, Hidetoshi Gon, Kenji Fukushima, Takeshi Urade, Hiroaki Yanagimoto, Hirochika Toyama, Yoshihide Ueda, Yuzo Kodama, Takamichi Mura
Hepatology Research.2024; 54(8): 773. CrossRef - Macroscopic Characterization of Hepatocellular Carcinoma: An Underexploited Source of Prognostic Factors
Stéphanie Gonvers, Sebastiao Martins-Filho, André Hirayama, Julien Calderaro, Rebecca Phillips, Emilie Uldry, Nicolas Demartines, Emmanuel Melloul, Young Nyun Park, Valérie Paradis, Swan Thung, Venancio Alves, Christine Sempoux, Ismail Labgaa
Journal of Hepatocellular Carcinoma.2024; Volume 11: 707. CrossRef - Serum Total Superoxide Dismutase Activity as a Predictive Factor in Patients with Hepatocellular Carcinoma
Yanqiu Xu, Bin Liu, Shiqing Cheng, Junguo Zhang, Xiue Cao, Yong Wang, Fang Luan
Hepatitis Monthly.2024;[Epub] CrossRef - Diagnostic value of expressions of cancer stem cell markers for adverse outcomes of hepatocellular carcinoma and their associations with prognosis: A Bayesian network meta‑analysis
Zhengrong Ou, Shoushuo Fu, Jian Yi, Jingxuan Huang, Weidong Zhu
Oncology Letters.2024;[Epub] CrossRef - Recurrence of hepatocellular carcinoma in noncirrhotic patients with nonalcoholic fatty liver disease versus hepatitis B infection
Jungnam Lee, Jong-In Chang, Young-Joo Jin, Jeong-Hoon Lee, Ju Yeon Kim, Dong Hyun Sinn, Soon Sun Kim, Hyun Woong Lee, Sun Hong Yoo, Jung Hwan Yu, Jin-Woo Lee
European Journal of Gastroenterology & Hepatology.2023; 35(4): 431. CrossRef - Comprehensive investigating of MMR gene in hepatocellular carcinoma with chronic hepatitis B virus infection in Han Chinese population
Ning Ma, Ao Jin, Yitong Sun, Yiyao Jin, Yucheng Sun, Qian Xiao, XuanYi Sha, Fengxue Yu, Lei Yang, Wenxuan Liu, Xia Gao, Xiaolin Zhang, Lu Li
Frontiers in Oncology.2023;[Epub] CrossRef - LI-RADS Morphological Type Predicts Prognosis of Patients with Hepatocellular Carcinoma After Radical Resection
Chunhui Zhang, Rui Yang, Xinxin Wang, Yuqing Tao, Shuli Tang, Zhennan Tian, Yang Zhou
Annals of Surgical Oncology.2023; 30(8): 4876. CrossRef - From clinical variables to multiomics analysis: a margin morphology-based gross classification system for hepatocellular carcinoma stratification
Zhongqi Fan, Meishan Jin, Lei Zhang, Nanya Wang, Mingyue Li, Chuanlei Wang, Feng Wei, Ping Zhang, Xiaohong Du, Xiaodong Sun, Wei Qiu, Meng Wang, Hongbin Wang, Xiaoju Shi, Junfeng Ye, Chao Jiang, Jianpeng Zhou, Wengang Chai, Jun Qi, Ting Li, Ruoyan Zhang,
Gut.2023; 72(11): 2149. CrossRef - A clinical and pathological update on hepatocellular carcinoma
Salvatore Lorenzo Renne, Luca Di Tommaso
Journal of Liver Cancer.2022; 22(1): 14. CrossRef - An approach to grossing of hepatectomy specimens
Archana Rastogi
Indian Journal of Pathology and Microbiology.2021; 64(5): 121. CrossRef - Hepatocellular carcinoma: a clinical and pathological overview
Salvatore Lorenzo Renne, Samantha Sarcognato, Diana Sacchi, Maria Guido, Massimo Roncalli, Luigi Terracciano, Luca Di Tommaso
Pathologica.2021; 113(3): 203. CrossRef - The Clinicopathological Significance of YAP/TAZ Expression in Hepatocellular Carcinoma with Relation to Hypoxia and Stemness
Hyunjin Park, Yangkyu Lee, Kiryang Lee, Hyejung Lee, Jeong Eun Yoo, Soomin Ahn, Young Nyun Park, Haeryoung Kim
Pathology and Oncology Research.2021;[Epub] CrossRef - Adjuvant versus Neoadjuvant Immunotherapy for Hepatocellular Carcinoma: Clinical and Immunologic Perspectives
Yung-Yeh Su, Chia-Chen Li, Yih-Jyh Lin, Chiun Hsu
Seminars in Liver Disease.2021; 41(03): 263. CrossRef - Prognostic significance of viable tumor size measurement in hepatocellular carcinomas after preoperative locoregional treatment
Yoon Jung Hwang, Youngeun Lee, Hyunjin Park, Yangkyu Lee, Kyoungbun Lee, Haeryoung Kim
Journal of Pathology and Translational Medicine.2021; 55(5): 338. CrossRef - Survival according to recurrence patterns after resection for transplantable hepatocellular carcinoma in HBV endemic area: Appraisal of liver transplantation strategy
Chung Gyo Seo, Sun Young Yim, Soon Ho Um, Yoo Ra Lee, Yoo Jin Lee, Tae Hyung Kim, Hyun Gil Goh, Young Sun Lee, Sang Jun Suh, Na Yeon Han, Hyuk Soon Choi, Eun Sun Kim, Bora Keum, Yeon Seok Seo, Hyung Joon Yim, Ji Hoon Kim, Dong Sik Kim, Yoon Tae Jeen, Hoon
Clinics and Research in Hepatology and Gastroenterology.2020; 44(4): 532. CrossRef - A radiomics-based biomarker for cytokeratin 19 status of hepatocellular carcinoma with gadoxetic acid–enhanced MRI
Wentao Wang, Dongsheng Gu, Jingwei Wei, Ying Ding, Li Yang, Kai Zhu, Rongkui Luo, Sheng-Xiang Rao, Jie Tian, Mengsu Zeng
European Radiology.2020; 30(5): 3004. CrossRef Paeonol Inhibits Cell Proliferation, Migration and Invasion and Induces Apoptosis in Hepatocellular Carcinoma by Regulating miR-21-5p/KLF6 Axis
Miaoguo Cai, Wei Shao, Huijun Yu, Ye Hong, Lili Shi
Cancer Management and Research.2020; Volume 12: 5931. CrossRef- Update on Hepatocellular Carcinoma: a Brief Review from Pathologist Standpoint
Nese Karadag Soylu
Journal of Gastrointestinal Cancer.2020; 51(4): 1176. CrossRef - Clinico-Radio-Pathological and Molecular Features of Hepatocellular Carcinomas with Keratin 19 Expression
Hyungjin Rhee, Haeryoung Kim, Young Nyun Park
Liver Cancer.2020; 9(6): 663. CrossRef - Histopathological characteristics of needle core biopsy and surgical specimens from patients with solitary hepatocellular carcinoma or intrahepatic cholangiocarcinoma
Ju-Shan Wu, Ji-Liang Feng, Rui-Dong Zhu, San-Guang Liu, Da-Wei Zhao, Ning Li
World Journal of Gastrointestinal Oncology.2019; 11(5): 404. CrossRef - The strengths and weaknesses of gross and histopathological evaluation in hepatocellular carcinoma: a brief review
Sebastião N. Martins-Filho, Venâncio Avancini Ferreira Alves
Surgical and Experimental Pathology.2019;[Epub] CrossRef - Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma
Archana Rastogi
World Journal of Gastroenterology.2018; 24(35): 4000. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1.
Fig. 2.
Fig. 3.
| Characteristic | No. (%) (n = 242) |
|---|---|
| Sex | |
| Male | 181 (74.8) |
| Female | 61 (25.2) |
| Age at operation, median (range, yr) | 59 (29-87) |
| Preoperative serum AFP level, median (range, ng/mL) | 14.35 (1–40,000) |
| Etiology | |
| Hepatitis B virus (HBV) | 171 (70.7) |
| Alcohol | 35 (14.5) |
| Hepatitis C virus (HCV) | 17 (7.0) |
| HBV + HCV | 1 (0.4) |
| Uncertain etiology | 18 (7.4) |
| Tumor size, median (range, cm) | 3.0 (0.9–17.0) |
| ≤ 2 | 52 (21.5) |
| > 2 and ≤ 5 | 145 (59.9) |
| > 5 | 45 (18.6) |
| Gross type | |
| Vaguely nodular | 9 (3.7) |
| Expanding nodular | 107 (44.2) |
| Multinodular confluent | 78 (32.2) |
| Nodular with perinodular extension | 32 (13.2) |
| Infiltrative | 16 (6.6) |
| Edmondson-Steiner grade | |
| Grade I | 2 (0.8) |
| Grade II | 74 (30.6) |
| Grade III | 142 (58.7) |
| Grade IV | 24 (9.9) |
| Microvascular invasion | |
| Absent | 154 (63.6) |
| Present | 88 (36.4) |
| Portal vein invasion | |
| Absent | 225 (93.0) |
| Present | 17 (7.0) |
| Cirrhosis in background liver | |
| Absent | 109 (45.0) |
| Present | 126 (52.1) |
| Intratumoral fibrous stroma (> 30%) | |
| Absent | 187 (77.3) |
| Present | 55 (22.7) |
| Pathologic T category (AJCC 7th edition) | |
| pT1 | 140 (57.9) |
| pT2 | 82 (33.9) |
| pT3 | 17 (7.0) |
| pT4 | 3 (1.2) |
| Pathologic N category (AJCC 7th edition) | |
| pN0 | 240 (99.2) |
| pN1 | 2 (0.8) |
| Recurrence on follow-up | |
| Absent | 138 (57.0) |
| Present | 104 (43.0) |
| Status at last follow-up | |
| Alive | 160 (66.1) |
| Deceased of disease | 19 (7.9) |
| Deceased of other cause | 5 (2.1) |
| Follow-up loss | 58 (24.0) |
| VN (n = 9) | EN (n = 107) | MC (n = 78) | NP (n = 32) | INF (n = 16) | Type 1 (VN, EN) (n = 116) | Type 2 (MC, NP, INF) (n = 126) | p-value (type 1 vs 2) | |
|---|---|---|---|---|---|---|---|---|
| Tumor size (> 5 cm) | 0 | 11 (10.3) | 21 (26.9) | 7 (21.9) | 6 (37.5) | 11 (9.5) | 34 (27.0) | < .001 |
| HBV etiology | 5 (55.6) | 73 (68.2) | 58 (74.4) | 21 (65.6) | 15 (93.8) | 78 (67.2) | 94 (74.6) | .207 |
| Edmondson-Steiner grade III/IV | 4 (44.4) | 64 (59.8) | 58 (74.4) | 25 (78.1) | 15 (93.8) | 68 (58.6) | 98 (77.8) | .001 |
| Microvascular invasion | 1 (11.1) | 29 (27.1) | 34 (43.6) | 14 (43.8) | 10 (62.5) | 30 (25.9) | 58 (46.0) | < .001 |
| Portal vein invasion | 0 | 0 | 4 (5.1) | 5 (15.6) | 8 (50.0) | 0 | 17 (13.55) | < .001 |
| High T category (pT3 or pT4) | 0 | 1 (0.9) | 7 (9.0) | 5 (15.6) | 7 (43.8) | 1 (0.9) | 19 (15.1) | < .001 |
| Cirrhosis in background liver | 6 (66.7) | 49 (46.7) | 42 (57.5) | 15 (46.9) | 14 (87.5) | 55 (48.2) | 71 (58.1) | .118 |
| Serum AFP level > 1,000 ng/mL | 0 | 12 (13.6) | 10 (14.7) | 6 (20.7) | 6 (37.5) | 12 (12.6) | 22 (19.5) | .194 |
| Fibrous stroma (> 30%) | 1 (11.1) | 14 (13.1) | 25 (32.1) | 9 (28.1) | 6 (37.5) | 15 (12.9) | 40 (31.7) | < .001 |
| CK19 positive | 2 (22.2) | 10 (9.3) | 20 (25.6) | 7 (21.9) | 6 (37.5) | 12 (10.3) | 33 (26.2) | .002 |
| EpCAM positive | 3 (33.3) | 37 (34.6) | 34 (43.6) | 19 (59.4) | 12 (75.0) | 40 (34.5) | 65 (51.6) | .009 |
| uPAR positive | 1 (11.1) | 16 (15.2) | 22 (28.2) | 14 (43.8) | 12 (75.0) | 17 (14.9) | 48 (38.1) | < .001 |
| Ezrin positive | 4 (44.4) | 34 (32.4) | 40 (51.3) | 12 (37.5) | 7 (43.8) | 38 (33.3) | 59 (46.8) | .036 |
| Variable | Overall survival |
Disease-free survival |
||
|---|---|---|---|---|
| Hazard ratio (95% confidence interval) | p-value | Hazard ratio (95% confidence interval) | p-value | |
| Univariate analysis | ||||
| Gross type (type 1 vs type 2) |
5.439 (1.583–18.683) | .007 | 1.617 (1.092–2.394) | .016 |
| Tumor size (> 5 cm) | 1.229 (0.407–3.712) | .714 | 1.590 (1.013–2.495) | .044 |
| High E-S grade (III or IV) | 0.636 (0.256–1.583) | .331 | 1.054 (0.694–1.599) | .805 |
| Microvascular invasion | 2.178 (0.881–5.383) | .092 | 1.445 (0.977–2.138) | .065 |
| Portal vein invasion | 5.311 (1.906–14.796) | < .001 | 1.702 (0.858–3.376) | .128 |
| High T category (pT3 or pT4) | 5.206 (1.974–13.728) | < .001 | 1.795 (0.982–3.284) | .057 |
| Intratumoral stromal fibrosis | 2.037 (0.801–5.179) | .135 | 0.973 (0.608–1.559) | .973 |
| Multivariate analysis | ||||
| Gross type (type 1 vs type 2) | 4.118 (1.142–14.844) | .030 | 1.617 (1.092–2.394) | .016 |
| Tumor size (> 5 cm) | 0.586 (0.166–2.060) | .404 | 1.303 (0.802–2.115) | .285 |
| Microvascular invasion | 1.953 (0.765–4.982) | .161 | 1.300 (0.866–1.952) | .205 |
| Portal vein invasion | 1.091 (0.150–7.931) | .931 | 0.954 (0.292–3.124) | .938 |
| High T category (pT3 or pT4) | 3.173 (1.156–8.710) | .025 | 1.462 (0.500–4.275) | .487 |
AFP, α-fetoprotein; AJCC, American Joint Committee on Cancer.
VN, vaguely nodular; EN, expanding nodular; MC, multinodular confluent; NP, nodular with perinodular extension; INF, infiltrative; HBV, hepatitis B virus; AFP, α-fetoprotein; CK19, cytokeratin 19; EpCAM, epithelial cell adhesion molecule; uPAR, urokinase plasminogen activator receptor.
E-S grade, Edmondson-Steiner grade. Type 1: vaguely nodular and expanding nodular types, type 2: multinodular confluent, nodular with perinodular extension and infiltrative types.

 E-submission
E-submission