Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 53(1); 2019 > Article
-
Case Study
Primary Peripheral Gamma Delta T-Cell Lymphoma of the Central Nervous System: Report of a Case Involving the Intramedullary Spinal Cord and Presenting with Myelopathy -
Jeemin Yim
 , Seung Geun Song
, Seung Geun Song , Sehui Kim
, Sehui Kim , Jae Won Choi1
, Jae Won Choi1 , Kyu-Chong Lee2
, Kyu-Chong Lee2 , Jeong Mo Bae
, Jeong Mo Bae , Yoon Kyung Jeon
, Yoon Kyung Jeon
-
Journal of Pathology and Translational Medicine 2019;53(1):57-61.
DOI: https://doi.org/10.4132/jptm.2018.08.21
Published online: October 1, 2018
Department of Pathology, Seoul National University Hospital, Seoul, Korea
1Department of Radiology, Seoul National University Hospital, Seoul, Korea
2Department of Radiology, Korea University Guro Hospital, Seoul, Korea
- Corresponding Author Yoon Kyung Jeon, MD, PhD Department of Pathology, Seoul National University College of Medicine, 103 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-740-8323 Fax: +82-2-743-5530 E-mail: ykjeon@snu.ac.kr
• Received: July 23, 2018 • Revised: August 20, 2018 • Accepted: August 21, 2018
© 2019 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Primary central nervous system lymphoma of T-cell origin (T-PCNSL) is rare, and its clinicopathological features remain unclear. Peripheral T-cell lymphoma of γδ T-cell origin is an aggressive lymphoma mainly involving extranodal sites. Here, we report a case of γδ T-PCNSL involving the intramedullary spinal cord and presenting with paraplegia. A 75-year-old Korean woman visited the hospital complaining of back pain and lower extremity weakness. Magnetic resonance imaging revealed multifocal enhancing intramedullary nodular lesions in the thoracic and lumbar spinal cord. An enhancing nodular lesion was observed in the periventricular white matter of the lateral ventricle in the brain. There were no other abnormalities in systemic organs or skin. Laminectomy and tumor removal were performed. The tumor consisted of monomorphic, medium-to-large atypical lymphocytes with pale-to-eosinophilic cytoplasm. Immunohistochemically, the tumor cells were CD3(+), TCRβF1(-), TCRγ(+), CD30(-), CD4(-), CD8(-), CD56(+), TIA1(+), granzyme B(+), and CD103(+). Epstein-Barr virus in situ was negative. This case represents a unique T-PCNSL of γδ T-cell origin involving the spinal cord.
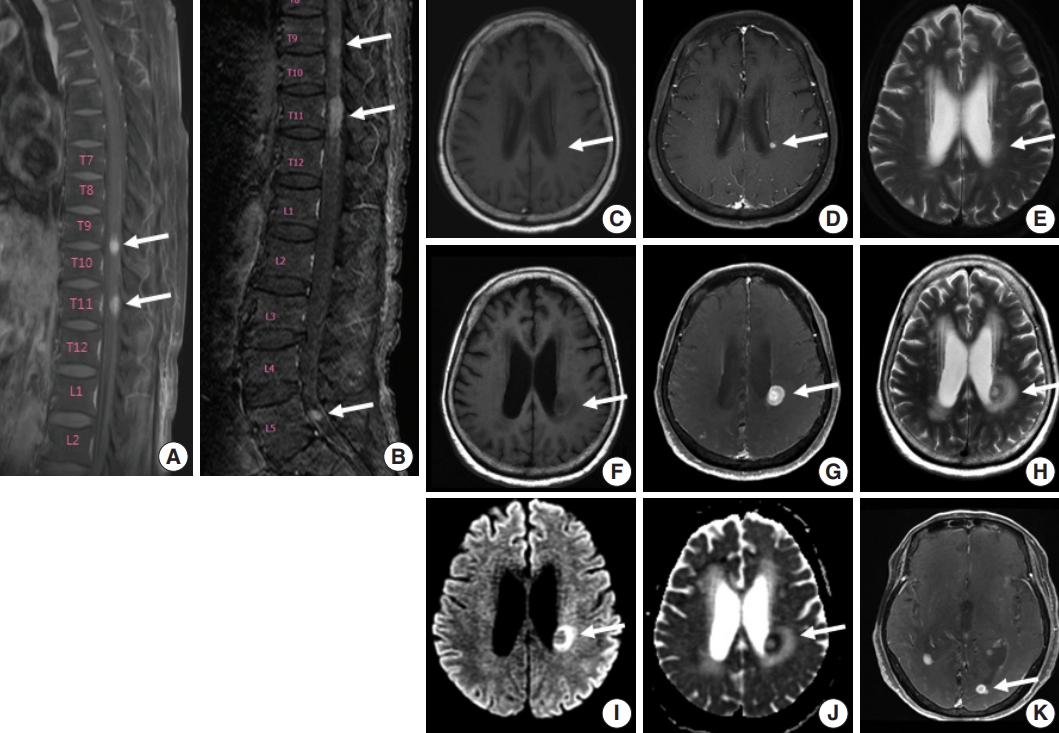
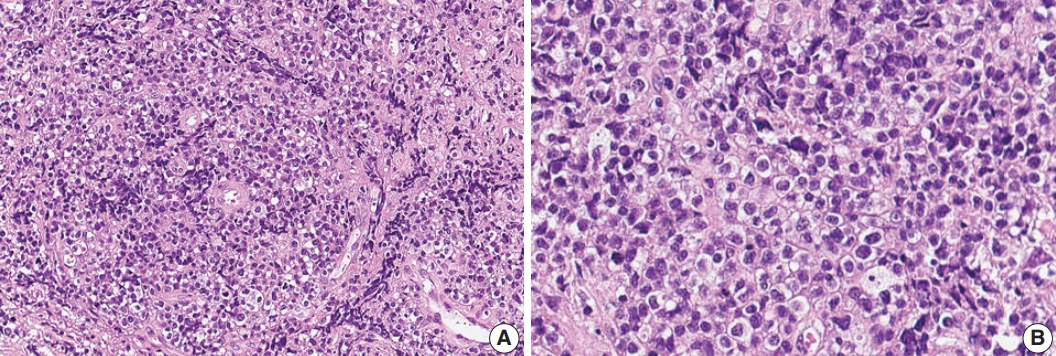
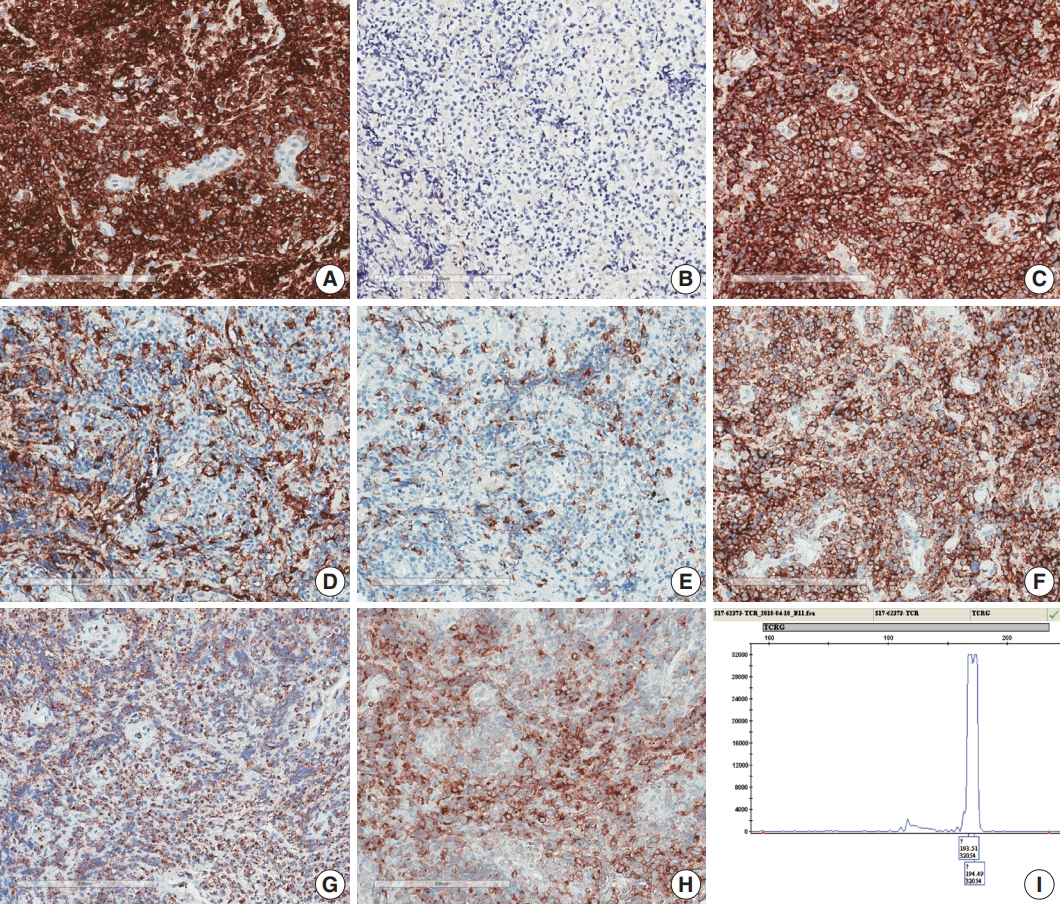
- A 75-year-old Korean woman presented with back pain and lower extremity weakness for 3.5 months. Lower extremity weakness causing difficulty in ambulation was temporarily relieved after steroid therapy. She had underlying hypertension, hyperlipidemia, and type 2 diabetes and had no history of immunodeficiency. Spine magnetic resonance imaging (MRI) revealed multiple enhancing intramedullary nodular lesions in the spinal cord at T9/10, T11, and L5 levels (Fig. 1A). Brain MRI revealed a small enhancing nodular lesion in the periventricular white matter of the left lateral ventricle (Fig. 1C–E). Clinicoradiological diagnoses included tumorous conditions such as lymphoma, glioma, and metastasis or nontumorous myelitis. Spinal and brain lesions had increased in size on MRI taken 1.5 months after initial presentation (Fig. 1B, F–J). Multiple newly developed enhancing nodules were observed in the lateral subependymal lining, left frontal lobe, and right temporal lobe (Fig. 1K). Positron emission tomography scan showed mild hypermetabolism in spinal cord lesions. No other abnormal findings were identified in the systemic organs and skin. The patient underwent T11 laminectomy and tumor removal. Microscopic examination of tumor revealed diffuse infiltration of monotonous, medium-to-large atypical lymphocytes with round nuclei, condensed chromatin, pale-to-eosinophilic cytoplasm, and small inconspicuous nucleoli (Fig. 2). Vasculature with high endothelial cells was noted throughout the tumor, and perivascular infiltration of tumor cells was occasionally observed along with diffuse infiltration of tumor cells in glial tissue (Fig. 2). Immunohistochemically, the atypical cells were CD3(+), CD20(–), TCRβF1(–), TCRγ(+), CD30(–), CD4(–), CD8(–), CD10(–), BCL6(–), MUM1(–), CD56(+), TIA-1(+), granzyme B(focal +), and CD103(+) (Fig. 3A–H). The Ki-67 index was about 80%, and Epstein-Barr virus in situ hybridization showed no positive cells. T-cell monoclonality was detected by TCRG gene rearrangement study using IdentiClone TCRG Gene Clonality Assay (Invivoscribe Technologies Inc., San Diego, CA, USA) (Fig. 3I). This case represents a unique PCNSL of γδ T-cell origin involving the spinal cord that presented with paraplegia.
- The Institutional Review Board (IRB) of Seoul National University Hospital (SNUH) approved this study (No. H-1807-070-958) and waived the need for informed consent from patients.
CASE REPORT
- The detailed pathological features of T-PCNSL remain unclear. In the largest series of T-PCNSL (n = 45) published 2005 by Shenkier et al. [2], tumor cells were “small or small-to-medium sized” in 12 cases and “pleomorphic or medium-to-large” in 13 cases. Of the nine Korean patients with T-PCNSL reported by Lim et al. [3], seven were diagnosed with PTCL, while two were diagnosed with T-lineage lymphoma with no further specification. Menon et al.’s series [4] (n = 18) of T-PCNSL comprised 15 cases of PTCL not otherwise specified (NOS) with small (n = 2), small-medium (n = 6), medium (n = 3), and medium-large or large (n = 4) tumor cells; one case was anaplastic lymphoma kinase (ALK) (+) ALCL and two cases were ALK(–) ALCLs. Of note, two of the 15 PTCL NOS cases expressed TCRγ, suggestive of γδ T-cell derivation. One of the patients with γδ T-PCNSL was a 31-year-old male with bilateral temporal lobe involvement. The other was a 56-year-old female with a solitary frontal mass. The tumors of both patients were composed of small-medium cells with CD4(–)CD8(+) phenotype [4]. Recently, Mooney et al. [10] reported another case of γδ T-PCNSL involving the cerebellum in a 26-year-old Korean female. To the best of our knowledge, our patient is the fourth case of γδ T-PCNSL and the first case of γδ T-PCNSL involving the spinal cord presenting with myelopathy.
- The clinical features and outcomes of patients with T-PCNSL remain unclear. Based on previous reports, T-PCNSL predominantly involves older patients, but with a wide age range from 3 to 84 years, and the male to female ratio is 1.8:1. In prior reports, involvement of deep brain structures and presentation with multifocal lesions were observed in about 34% and 43% patients, respectively [2-4]. The disease-specific survival of patients with T-PCNSL was 25 months (95% confidence interval, 11 to 38 months) [2]. Although the prognosis of T-PCNSL is controversial, Shenkier et al. [2] and Lim et al. [3] demonstrated that the clinical outcome of patients with T-PCNSL was comparable to that of patients with B-PCNSL, and performance status and high-dose methotrexate-based therapy were associated with patient prognosis. Of the four reported cases with γδ T-PCNSL including our case, detailed treatment modality and outcome are available in only one patient [10]. A 26-year-old female with cerebellar γδ T-PCNSL underwent subtotal mass resection followed by high-dose methotrexate and cytarabine therapy, and she remained alive at 3 months [10]. Although our patient was lost to follow-up after surgery, lesions involving the spinal cord and brain had rapidly progressed before surgery.
- The current revised 2016 World Health Organization (WHO) classification recognizes three entities of γδ T-cell lymphoma (γδ TCL) including hepatosplenic γδ TCL, primary cutaneous γδ TCL, and monomorphic epitheliotropic intestinal TCL [1]. However, γδ TCLs involving other extranodal sites were reported including the lung, orbit, and tongue [11-13]. Morphologic features of γδ TCL cells vary, but these cells often share the following immunophenotype: CD2(+), CD3(+), CD4(-), CD5(-), CD7(+/-), CD8(-/+), CD56(+/-), TIA1(+), granzyme B(+/-), perforin(+/-), TCRβF1(-), and TCRγ(+) [9]. In general, γδ TCL aggravates rapidly and responds poorly to standard chemotherapy [9]. Recently, recurrent genetic alterations involving the JAK/STAT pathway and epigenetic pathway were demonstrated in γδ TCLs [14,15]. Thus, it is necessary to gather clinical data of γδ TCLs and discover new therapeutics.
- In summary, we report a unique case of γδ T-PCNSL involving the intramedullary spinal cord that presented with myelopathy. This case will intrigue and stimulate clinicians and pathologists to engage in the study of γδ TCL and T-PCNSL to discover effective therapeutic strategies and new targets for therapy.
DISCUSSION
Acknowledgments
Fig. 1.Spine and brain magnetic resonance imaging (MRI) features at presentation (A, C–E) and 6 weeks later (B, F–K). (A) Spine MRI sagittal view revealed enhancing intramedullary nodular lesions at T9/10 and T11 levels (arrows). (B) Six weeks later, enhancing intramedullary nodular lesions (arrows) at T7/8, T9/10, and T11 levels were enlarged, and an enhancing nodule appeared at L5 level with leptomeningeal enhancement. (C–E) A nodular lesion (arrows) was observed in the periventricular white matter of the left lateral ventricle, which showed enhancement in T1 enhanced image (D) compared to T1 weighted image (C) and heterogeneous high signal intensity in T2 weighted images (E). (F–H) After 6 weeks, the lesion (arrows) increased in size with surrounding edema in T1 weighted (F), T1 enhanced (G), and T2 weighted images (H). (I, J ) Diffusion weighted images revealed diffusion restriction within the tumor with high signal intensity (arrow) (I) and corresponding low signal intensity (arrow) on the apparent diffuse coefficient map (J). (K) There were also multiple newly developed enhancing nodules in the lateral subependymal lining, left frontal lobe, and right temporal lobe (arrow).


Fig. 2.Histologic features of γδ T-cell lymphoma involving the spinal cord. (A) Monomorphic medium-to-large atypical lymphoid cells diffusely infiltrating the spinal cord parenchyma with occasional perivascular arrangement. (B) Atypical lymphoid cells showed clear to eosinophilic cytoplasm with distinct cell borders and hyperchromatic nuclei with small indistinct nucleoli.


Fig. 3.Immunohistochemical and genetic features of γδ T-cell lymphoma involving the spinal cord. Tumor cells were CD3(+) (A), TCRβF1(–) (B), TCRγ(+) (C), CD4(–) (D), CD8(–) (E), CD56(+) (F), TIA1(+) (G), and CD103(+) (H). (I) Monoclonal peak was observed in TCRγ gene rearrangement study.


- 1. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haemotopoietic and lymphoid tissues. Revised 4th ed. Lyon: International Agency for Research on Cancer, 2017.
- 2. Shenkier TN, Blay JY, O'Neill BP, et al. Primary CNS lymphoma of T-cell origin: a descriptive analysis from the international primary CNS lymphoma collaborative group. J Clin Oncol 2005; 23: 2233-9. ArticlePubMed
- 3. Lim T, Kim SJ, Kim K, et al. Primary CNS lymphoma other than DLBCL: a descriptive analysis of clinical features and treatment outcomes. Ann Hematol 2011; 90: 1391-8. ArticlePubMedPMC
- 4. Menon MP, Nicolae A, Meeker H, et al. Primary CNS T-cell Lymphomas: a clinical, morphologic, immunophenotypic, and molecular analysis. Am J Surg Pathol 2015; 39: 1719-29. PubMedPMC
- 5. Nael A, Walavalkar V, Wu W, et al. CD4-positive T-cell primary central nervous system lymphoma in an HIV positive patient. Am J Clin Pathol 2016; 145: 258-65. ArticlePubMedPDF
- 6. Guzzetta M, Drexler S, Buonocore B, Donovan V. Primary CNS T-cell lymphoma of the spinal cord: case report and literature review. Lab Med 2015; 46: 159-63. ArticlePubMedPDF
- 7. Flanagan EP, O'Neill BP, Porter AB, Lanzino G, Haberman TM, Keegan BM. Primary intramedullary spinal cord lymphoma. Neurology 2011; 77: 784-91. ArticlePubMed
- 8. Yang W, Garzon-Muvdi T, Braileanu M, et al. Primary intramedullary spinal cord lymphoma: a population-based study. Neuro Oncol 2017; 19: 414-21. ArticlePubMedPDF
- 9. Tripodo C, Iannitto E, Florena AM, et al. Gamma-delta T-cell lymphomas. Nat Rev Clin Oncol 2009; 6: 707-17. ArticlePubMedPDF
- 10. Mooney KL, Choy W, Woodard J, et al. Primary central nervous system gamma delta cytotoxic T-cell lymphoma. J Clin Neurosci 2016; 26: 138-40. ArticlePubMed
- 11. Arnulf B, Copie-Bergman C, Delfau-Larue MH, et al. Nonhepatosplenic gammadelta T-cell lymphoma: a subset of cytotoxic lymphomas with mucosal or skin localization. Blood 1998; 91: 1723-31. PubMed
- 12. Garcia-Herrera A, Song JY, Chuang SS, et al. Nonhepatosplenic gammadelta T-cell lymphomas represent a spectrum of aggressive cytotoxic T-cell lymphomas with a mainly extranodal presentation. Am J Surg Pathol 2011; 35: 1214-25. PubMedPMC
- 13. Choe JY, Bisig B, de Leval L, Jeon YK. Primary gammadelta T cell lymphoma of the lung: report of a case with features suggesting derivation from intraepithelial gammadelta T lymphocytes. Virchows Arch 2014; 465: 731-6. ArticlePubMedPDF
- 14. Küçük C, Jiang B, Hu X, et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from gammadelta-T or NK cells. Nat Commun 2015; 6: 6025.PubMed
- 15. Roberti A, Dobay MP, Bisig B, et al. Type II enteropathy-associated T-cell lymphoma features a unique genomic profile with highly recurrent SETD2 alterations. Nat Commun 2016; 7: 12602.ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- B-Cell Lymphoma Intramedullary Tumor: Case Report and Systematic Review
Daniel Gregório Gonsalves, Paulo Eduardo Albuquerque Zito Raffa, Gabriela Gerenutti de Sousa, Melissa Esposito Gomes Rigueiral, Iracema Araújo Estevão, Cesar Cozar Pacheco, Roger Thomaz Rotta Medeiros, Paulo Roberto Franceschini, Paulo Henrique Pires de A
Asian Journal of Neurosurgery.2023; 18(02): 231. CrossRef - Primary intramedullary spinal cord lymphoma misdiagnosed as longitudinally extensive transverse myelitis: a case report and literature review
Huizhen Ge, Li Xu, Huajie Gao, Suqiong Ji
BMC Neurology.2023;[Epub] CrossRef - Clinicopathologic and Genetic Features of Primary T-cell Lymphomas of the Central Nervous System
Jeemin Yim, Jiwon Koh, Sehui Kim, Seung Geun Song, Jeong Mo Bae, Hongseok Yun, Ji-Youn Sung, Tae Min Kim, Sung-Hye Park, Yoon Kyung Jeon
American Journal of Surgical Pathology.2022; 46(4): 486. CrossRef - Peripheral T-Cell Lymphomas Involving the Central Nervous System: A Report From the Czech Lymphoma Study Group Registry
Heidi Mocikova, Robert Pytlík, Katerina Benesova, Andrea Janikova, Juraj Duras, Alice Sykorova, Katerina Steinerova, Vit Prochazka, Vit Campr, David Belada, Marek Trneny
Frontiers in Oncology.2022;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Primary Peripheral Gamma Delta T-Cell Lymphoma of the Central Nervous System: Report of a Case Involving the Intramedullary Spinal Cord and Presenting with Myelopathy



Fig. 1. Spine and brain magnetic resonance imaging (MRI) features at presentation (A, C–E) and 6 weeks later (B, F–K). (A) Spine MRI sagittal view revealed enhancing intramedullary nodular lesions at T9/10 and T11 levels (arrows). (B) Six weeks later, enhancing intramedullary nodular lesions (arrows) at T7/8, T9/10, and T11 levels were enlarged, and an enhancing nodule appeared at L5 level with leptomeningeal enhancement. (C–E) A nodular lesion (arrows) was observed in the periventricular white matter of the left lateral ventricle, which showed enhancement in T1 enhanced image (D) compared to T1 weighted image (C) and heterogeneous high signal intensity in T2 weighted images (E). (F–H) After 6 weeks, the lesion (arrows) increased in size with surrounding edema in T1 weighted (F), T1 enhanced (G), and T2 weighted images (H). (I, J ) Diffusion weighted images revealed diffusion restriction within the tumor with high signal intensity (arrow) (I) and corresponding low signal intensity (arrow) on the apparent diffuse coefficient map (J). (K) There were also multiple newly developed enhancing nodules in the lateral subependymal lining, left frontal lobe, and right temporal lobe (arrow).
Fig. 2. Histologic features of γδ T-cell lymphoma involving the spinal cord. (A) Monomorphic medium-to-large atypical lymphoid cells diffusely infiltrating the spinal cord parenchyma with occasional perivascular arrangement. (B) Atypical lymphoid cells showed clear to eosinophilic cytoplasm with distinct cell borders and hyperchromatic nuclei with small indistinct nucleoli.
Fig. 3. Immunohistochemical and genetic features of γδ T-cell lymphoma involving the spinal cord. Tumor cells were CD3(+) (A), TCRβF1(–) (B), TCRγ(+) (C), CD4(–) (D), CD8(–) (E), CD56(+) (F), TIA1(+) (G), and CD103(+) (H). (I) Monoclonal peak was observed in TCRγ gene rearrangement study.
Fig. 1.
Fig. 2.
Fig. 3.
Primary Peripheral Gamma Delta T-Cell Lymphoma of the Central Nervous System: Report of a Case Involving the Intramedullary Spinal Cord and Presenting with Myelopathy

 E-submission
E-submission