Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 52(6); 2018 > Article
-
Case Study
Squamous Metaplasia in Pleomorphic Adenoma: A Diagnostic and Prognostic Enigma -
Swati Sharma
 , Monica Mehendiratta
, Monica Mehendiratta , Nivedita Chaudhary
, Nivedita Chaudhary , Vineet Gupta
, Vineet Gupta , Maulshree Kohli, Anjana Arora1
, Maulshree Kohli, Anjana Arora1 -
Journal of Pathology and Translational Medicine 2018;52(6):411-415.
DOI: https://doi.org/10.4132/jptm.2018.07.15
Published online: October 1, 2018
Department of Oral and Maxillofacial Pathology and Microbiology, I.T.S. Dental College, Hospital and Research Centre, Greater Noida, India
1Department of Oral Medicine and Radiology, I.T.S. Dental College, Hospital and Research Centre, Greater Noida, India
- Corresponding Author Swati Sharma, MDS Department of Oral and Maxillofacial Pathology and Microbiology, I.T.S. Dental College, Hospital and Research Centre, Greater Noida, Uttar Pradesh 201308, India Tel: +91-0120-2331089 Fax: +91-0120-2323702 E-mail: sharmaswatisharma86@gmail.com
• Received: April 27, 2018 • Revised: June 20, 2018 • Accepted: July 14, 2018
© 2018 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Pleomorphic adenoma (PA) is the most common benign salivary gland tumor. Histologically, squamous metaplasia has been reported in PA, but has rarely been documented as being extensive enough to cause significant misdiagnosis. Here, we present an unusual case of PA in a 50-year-old female patient presenting with swelling on the postero-lateral aspect of the palate for a week. Histopathologically, the tumor exhibited the features of conventional PA with extensive squamous metaplasia and giant keratotic lamellae in cyst-like areas. Such exuberant squamous metaplasia and keratin can be a diagnostic and prognostic pitfall and lead to overtreatment of the patient.
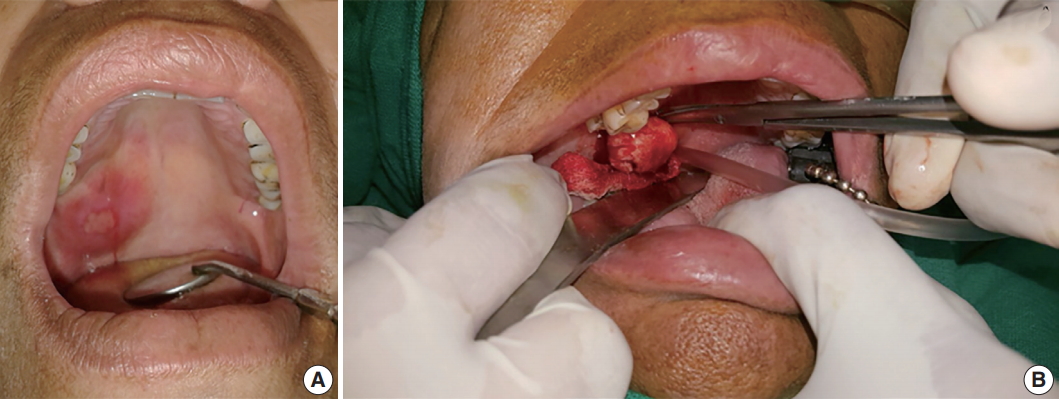
- A 50-year-old female patient reported with a chief complaint of swelling on the right postero-lateral aspect of the palate for the past 1 week. Intraorally, a swelling associated with central ulceration and a fibrino-purulent membrane covering was present on the right side of the hard palate, with an approximate size of 5.0 × 5.0 × 1.5 cm3 (Fig. 1). The swelling was firm in consistency, non-tender, slightly movable and had an erythematous oedematous periphery. There were no associated palpable lymph nodes.
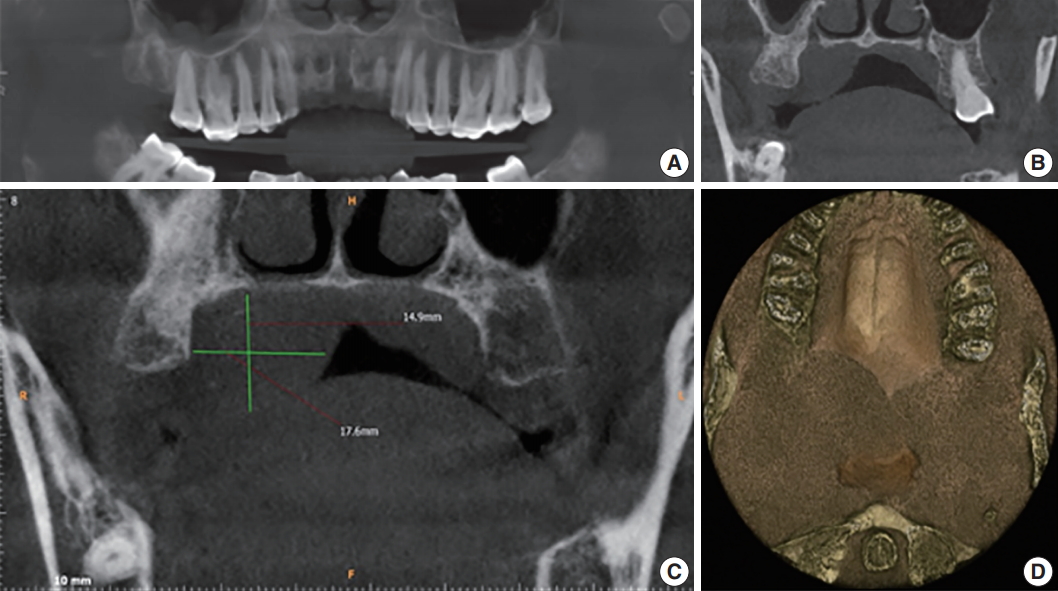
- Further investigations were carried out, including hematological examination, computed tomography, aspiration cytology and biopsy. Hematological examination included complete blood count, prothrombin time, erythrocyte sedimentation rate, and routine blood sugar without any significant alarming result. Aspiration of the swelling did not yield much and was not helpful for evaluating the diagnosis. A subsequent cone beam computed tomography (CBCT) scan captured coronal sectional images revealing a well-defined dome-shaped soft tissue shadow measuring 2.19 × 2.42 cm2, extending between the palatal aspect of 17 and 18, and mid-line of the palate medio-laterally. There was no evidence of bone resorption. The three dimensional image revealed an ill-defined posterior-most extent of this soft tissue swelling up to the oropharynx. No radiographic signs of malignancy such as invasive borders, irregular cortical boundary and aggressive bone destruction, root resorption, tooth displacement or periosteal reaction were evident (Fig. 2).
- Wide local excision was conducted and the specimen was removed in toto in an uneventful surgery. Grossly, the tumor appeared well-encapsulated (Fig. 1), grayish-white, firm in consistency, smooth in texture and measured 2.6 × 2.9 × 1.7 cm3. A provisional diagnosis of lymphadenopathy and palatal minor salivary gland tumor were made.
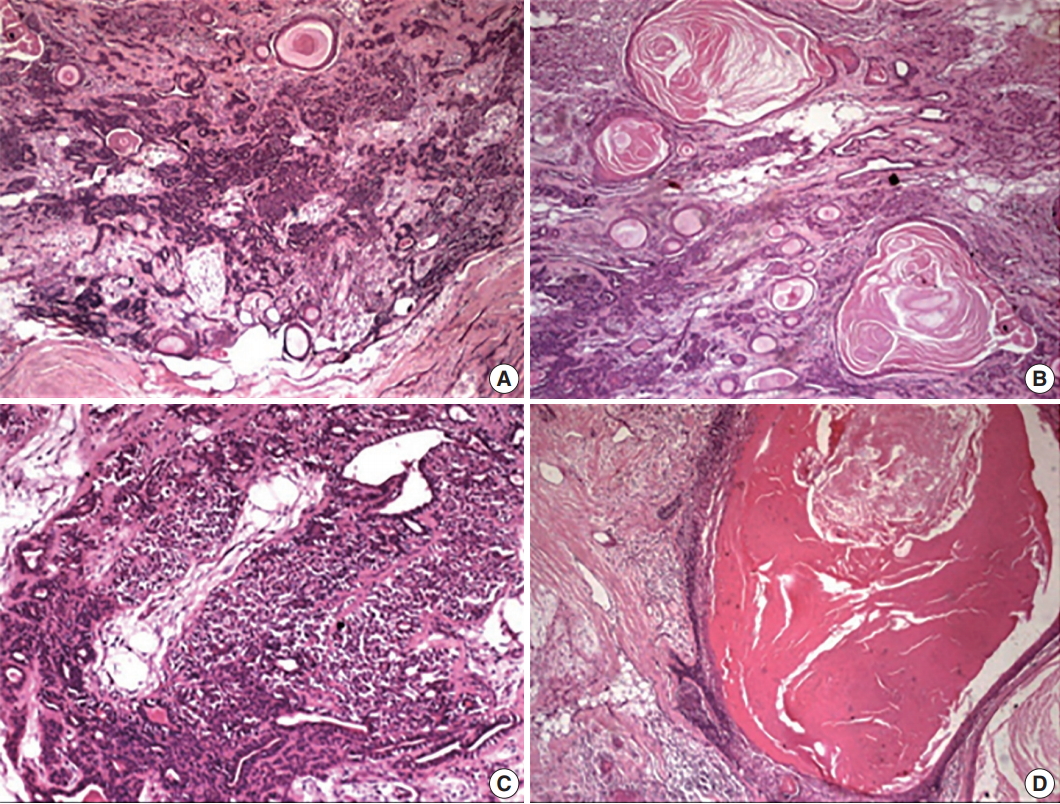
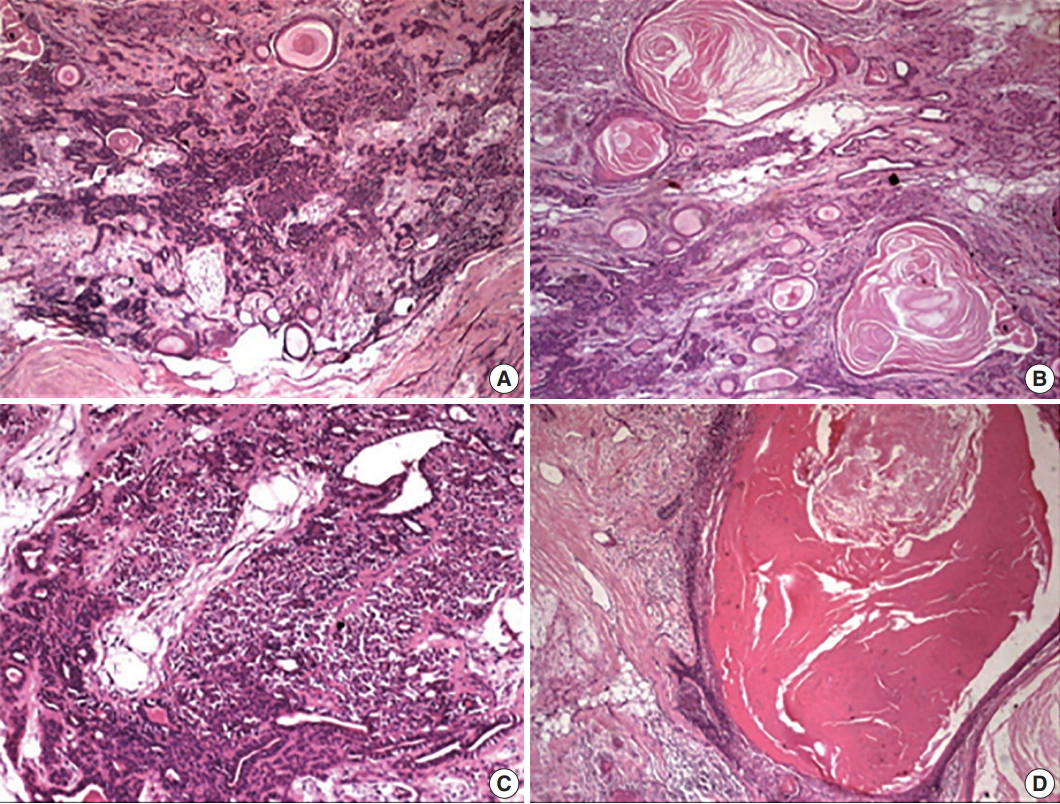
- Hematoxylin and eosin–stained sections revealed a well-delineated but partly encapsulated tumor mass flanked with abundant adipocytes. Under low magnification, the central pathology consisted of variably shaped, abundant ducts and massive squamous epithelium-lined cysts with extensive keratin in a myxoid and hyalinized stroma (Fig. 3). Under higher magnification, a classical bilayered ductal pattern with luminal cuboidal cells and variably-shaped, abluminal, myoepithelial cells was ascertained. The myoepithelial cell layers varied from a single layer to collar to an extensive collection, imparting the appearance of a swarm of bees. The other important feature of the lesion was florid squamous cells (50% of the tumor) arranged in nests, islands or in sheets with or without extensive cystic cavities containing massive keratotic lamellae and/or areas of degeneration. The cystic cavities were lined by 4–5 compressed layers of hyperchromatic, stratified squamous epithelium with hypergranulosis and sparse mitotic figures in the outer cells, but no evidence of atypia. The lining epithelium showed bud-shaped projections in the stromal tissue away from the cystic cavities (Fig. 3).
- The stroma also revealed histogenetic diversity with extensive myxoid, fibrous to hyalinized areas with evidence of dystrophic calcification. The features were clearly suggestive of PA with florid squamous metaplasia and keratin-filled cysts. Post-operative healing of the patient was uneventful without any recurrence till 1.5 years after excision.
- The presence of an extensive squamous component with keratin lamellae in the tumor background with ducts and variably shaped epithelial cells dispersed in a myxoid and hyalinized background created a multitude of differential diagnoses. The absence of frank mucous and intermediate cells as well as extensive mucin pooling helped distinguish from mucoepidermoid carcinoma. Conventional SCC was considered, as it may invade or entrap nontumorous salivary glands, but was ultimately ruled out due to the presence of extensive morphological, epithelial and stromal diversity with no evidence of dysplasia. Adenoid SCC and adenosquamous cell carcinoma owing to the presence of squamous and glandular components were also considered as differentials, although the former malignancy does not show any true glands/ducts or intracytoplasmic mucins. The latter and other malignancies such as in-situ (intracapsular) PA and carcinoma ex-PA were ruled out for several reasons. First, the received tissue was in toto and thoroughly examined for evidence of any atypical changes. Second, there was an absence of cellular and nuclear atypia, necrosis, capsular invasion, an aggressive growth pattern and nerve/surrounding tissue permeation.
- Clinically, because the tumor was located on the palate with a short history of presentation, a diagnosis of necrotizing sialometaplasia or chronic sialadenitis was also considered, but ultimately ruled out due to the absence of necrosis and presence of rich morphological diversity consistent with the diagnosis of PA.
- Another important feature of note in the present case was squamous metaplasia, which could have been the result of the fine needle aspiration cytology (FNAC) conducted prior to excision of the lesion. However, the exuberant amount of squamous metaplasia evident in the lesion did not appear to be correlated with a needle-induced change.
CASE REPORT
- The present case is a classical case of PA without cytological atypia, but demonstrating extensive squamous metaplasia, which can be of serious concern. Squamous metaplasia is an incidental microscopic finding in various benign and malignant (de-novo or induced) tumors in humans or animal models. The origin is unknown and has been associated erratically with the intra-tumoral/tissue environment, like trauma, infarction/ischemia and repair following infarction [3]. Squamous metaplasia has been experimentally induced by arterial ligation in rat salivary glands by Dardick et al. [4], and appears to have formed via the gradual dedifferentiation and hyperplasia of the acinar-intercalated duct system. Tonofilaments and desmosomes begin to appear in the luminal and abluminal myoepithelial cells, and thus keratinization of central cells materializes [4]. The varying degree of squamous metaplasia could be a consequence of the rapidity and ease of switch in the genetic programming of cytokeratin filaments induced by ischemia in the salivary glands, and so the most probable etiology for this change appears to be ischemia [3]. FNAC for diagnostic purposes has been shown to induce the same in tumors during histopathological evaluation [5]. FNAC was conducted in our case, but there was no evidence of necrosis/repair in the sections, and the amount of squamous metaplasia appeared to be correlated with the extent of injury induced by ischemia.
- Foci of squamous cells are an integral feature of PA, but extensive squamous metaplasia is uncommon and can be easily misinterpreted as SCC, especially in FNAC and incisional biopsies due to the limited and selective sampling. In addition, diagnosis becomes challenging in the absence of chondro-myxoid stroma, making it imperative to understand this diagnostic pitfall [6].
- The presence of squamous metaplasia is also a prognostic pitfall, as its transition into SCC has been further emphasized by Takegawa et al. [7] in the submandibular glands of rats by the application of potassium iodide. Takegawa et al. [7] observed the development of squamous metaplasia in proliferative ductules and interlobular ducts that apparently transited to SCC, and emphasized that this occurred via a non-genotoxic, proliferation-dependent mechanism [7].
- To summarize, similar case reports have been provided by various authors with or without application of immunohistochemistry (IHC) markers. One case described a 32-year-old patient with 45% of the tumor consisting of squamous cells, wherein IHC helped distinguish the squamous metaplastic cells from SCC [8]. The presence of low molecular weight cytokeratin and p63 in squamous cells helps rule out SCC or even reactive squamous hyperplasia in such PA cases [9]. Multiple IHC markers are used to ascertain differences between glandular cells or metaplasticallyformed squamous cells. Although no conclusive differences have been established using cytokeratin or even MIB-1 [8] (a proliferative marker), Ki-67 as used by Goulart et al. [10] had a higher proliferative index in the epithelial lining of a large keratin cyst.
- Diagnosis of PA requires physical examination, CBCT, cytology and histopathology. FNAC and incisional biopsy can help determine the proper management regimen, but must be thoroughly sampled to rule out any misdiagnosis, especially in cases of misleading short histories like our present case. Other supportive investigations like computed tomography scanning and magnetic resonance imaging can provide information on the location and size of the tumor and its extension into surrounding superficial and deep structures.
- The treatment for PA is surgical excision, and although radiotherapy is not indicated, correct diagnosis is essential to avoid overtreatment [2]. The present case, to the best of our knowledge, is among the first 20 cases reported in the English language literature and thus a rarity. The misleading short history of one week, enormous size of 5.0 × 5.0 × 1.5 cm3 and massive squamous islands could have led to an incorrect diagnosis of malignancy. Thus, the thorough examination of samples, particularly in FNAC and incisional cases, is important.
DISCUSSION
Fig. 1.(A) Swelling associated with central ulceration covered by a fibrino-purulent membrane and surrounded by palatal erythema can be seen on the right distal side of the hard palate, laterally. (B) A well-encapsulated tumor removed in toto from the palate.
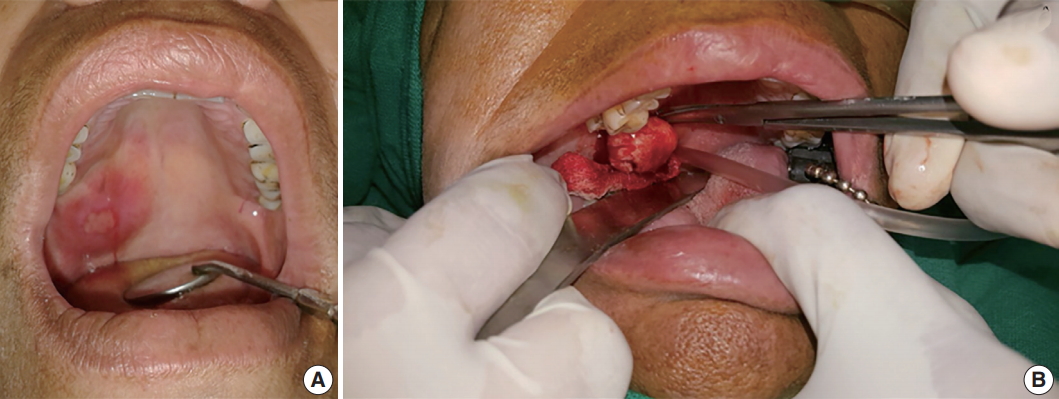

Fig. 2.(A) Reconstructed panoramic image shows the polyp at the level of the mid right maxillary sinus. (B) Axial section shows inflamed sinus lining involving the right maxillary sinus. (C) Cone beam computed tomography (CBCT) mid-coronal section of the right maxillary third molar (18) showing the soft tissue shadow palatally. (D) Two-three dimensional CBCT image showing the antero-posterior extent of the swelling.
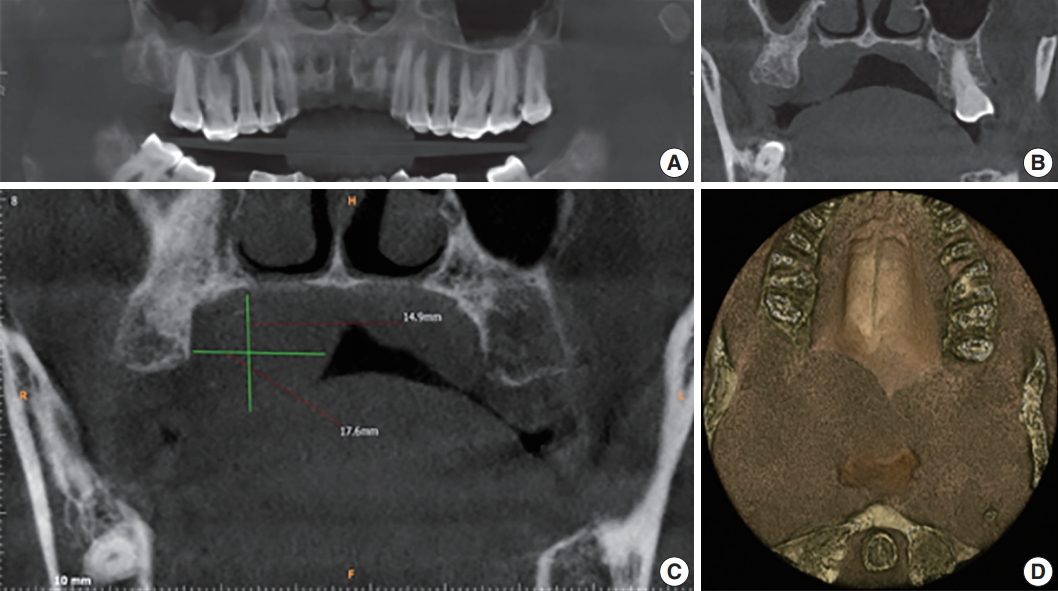

Fig. 3.(A) The periphery of the tumor with classical features of pleomorphic adenoma having abundant ducts dispersed in a myxoid and hyalinized background with multiple keratin-filled cystic areas. (B) Multitude of features, including variably shaped epithelial cells, ducts, and squames. Keratin-filled cysts dispersed in the myxoid stroma. (C) Abundant myoepithelial cells around the ducts giving the appearance of a swarm of bees. (D) Massive epithelial lined cysts with keratotic lamellae and the lining budding into the stroma.

- 1. Neville B, Damm DD, Allen C, Bouquot J. Oral and maxillofacial pathology. 3rd ed. St. Louis: Elsevier, 2009; 473-506.
- 2. Rajendran R, Sivapathasundharam B. Shafer’s text book of oral pathology. 7th ed. Noida: Elsevier, 2012; 223-57.
- 3. Kaveri H, Gopalkrishnana K, Venkatesh A. Cystic and florid squamous metaplasia in pleomorphic adenoma of palate: a diagnostic dilemma. Asian J Med Sci 2014; 5: 108-10. ArticlePDF
- 4. Dardick I, Jeans MT, Sinnott NM, Wittkuhn JF, Kahn HJ, Baumal R. Salivary gland components involved in the formation of squamous metaplasia. Am J Pathol 1985; 119: 33-43. PubMedPMC
- 5. Jaishankar HP, Hegde U, Nagpal B. Florid squamous metaplasia and keratin cyst formation in palatal minor salivary gland tumor: a diagnostic challenge. Int J Health Sci Res 2016; 6: 516-20.
- 6. Compagno J, Wong RT. Intranasal mixed tumors (pleomorphic adenomas): a clinicopathologic study of 40 cases. Am J Clin Pathol 1977; 68: 213-8. ArticlePubMedPDF
- 7. Takegawa K, Mitsumori K, Onodera H, et al. Induction of squamous cell carcinomas in the salivary glands of rats by potassium iodide. Jpn J Cancer Res 1998; 89: 105-9. ArticlePubMedPMC
- 8. Lam KY, Ng IO, Chan GS. Palatal pleomorphic adenoma with florid squamous metaplasia: a potential diagnostic pitfall. J Oral Pathol Med 1998; 27: 407-10. ArticlePubMed
- 9. Lim S, Cho I, Park JH, Lim SC. Pleomorphic adenoma with exuberant squamous metaplasia and keratin cysts mimicking squamous cell carcinoma in minor salivary gland. Open J Pathol 2013; 3: 113-6. ArticlePDF
- 10. Goulart MC, Freitas-Faria P, Goulart GR, et al. Pleomorphic adenoma with extensive squamous metaplasia and keratin cyst formations in minor salivary gland: a case report. J Appl Oral Sci 2011; 19: 182-8. ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Retrospective Clinicopathological Study of 33 Cases of Pleomorphic Salivary Adenoma Diagnosed in Benghazi
Siraj S. Najem, Elhoni Ashour, Rehab Elmaddani, Ali M. Elmurtadi
Libyan Journal of Dentistry .2025; 8(2): 29. CrossRef - Fine‐Needle Aspiration Cytology Diagnosis of Pleomorphic Adenoma With Spontaneous Infarction in the Salivary Gland: A Multicenter Retrospective Study
Jie‐Qiong Wang, Ge Li, Shao‐Hua Wang, Bo Yang, Yun Liu, Yu Wan, Cong‐Gai Huang, Fan Li
Cytopathology.2025; 36(5): 484. CrossRef - Keratocystoma: Molecular insights and diagnostic challenges in a rare salivary gland tumor
Yoshitaka Utsumi, Masato Nakaguro, Justin A. Bishop, Toshitaka Nagao
Seminars in Diagnostic Pathology.2025; 42(5): 150940. CrossRef - Bronchial pleomorphic adenoma successfully diagnosed and resected with left lower sleeve lobectomy; a case report and literature review
Katsuhiro Itogawa, Tomohiro Oba, Mitsuru Maki, Masako Amano, Akiko Adachi, Hidekazu Matsushima
Respiratory Medicine Case Reports.2025; 57: 102253. CrossRef - Effective Management of a Giant Deforming Pleomorphic Adenoma With Airway Displacement in a 93-Year-Old Patient: A Case Report
Julio A Palomino-Payan, Jessica Guillen-Valles, Daniel A Meza-Martinez, Fernanda Urias, Luis D Montes de Oca-Gordoa
Cureus.2024;[Epub] CrossRef - ECTOPIC PLEOMORPHIC ADENOMA OF BUCCAL SPACE: CASE REPORT WITH REVIEW OF LITERATURE
SANCHIT BAJPAI
UP STATE JOURNAL OF OTOLARYNGOLOGY AND HEAD AND NECK SURGERY.2024; VOLUME 12(ISSUE 1): 55. CrossRef - Pleomorphic adenoma with extensive squamous metaplasia and keratinizing cysts: Diagnostic and clinical pitfalls – A report of two cases and review of literature
Mahadevi B. Hosur, Rudrayya S. Puranik, Satyajit G. Dandagi, Vivekanand M. Patil
Journal of Oral and Maxillofacial Pathology.2024; 28(4): 689. CrossRef - Pleomorphic adenoma of the upper lip: A rare site for a common tumor- Case report
Prasath Sathiah, Sujaya Mazumder, Santosh Tummidi, Vijay Kannaujiya
SN Comprehensive Clinical Medicine.2023;[Epub] CrossRef - Variable metaplastic entities in pleomorphic adenoma a review of a rare case report with a note on its significance
N. Mahapatra, L. Bhuyan, Dash Chandra, P. Mishra
Archive of Oncology.2023; 29(2): 18. CrossRef - Pleomorphic adenoma with extensive oncocytic papillary cystic areas and trichilemmal keratinisation – A unique presentation
CV Aiswarya, Raghunath Vandana, Kamal Firoz, Meda Samatha
Journal of Oral and Maxillofacial Pathology.2023; 27(3): 562. CrossRef - Pleomorphic Adenoma with Extensive Squamous and Adipocytic Metaplasia Mimicking as Low Grade Mucoepidermoid Carcinoma on FNAC
Anu Singh, Ravi Hari Phulware, Arvind Ahuja, Ankur Gupta, Manju Kaushal
Indian Journal of Otolaryngology and Head & Neck Surgery.2022; 74(S2): 2132. CrossRef - Aspiration cytology of pleomorphic adenoma with squamous metaplasia: A case series and literature review illustrating diagnostic challenges
Joshua J. X. Li, Joanna K. M. Ng, Eric H. L. Lau, Amy B. W. Chan
Diagnostic Cytopathology.2022; 50(2): 64. CrossRef - Pleomorphic adenoma with extensive squamous metaplasia: The first well-documented case involving the submandibular gland
David A. Gaskin, Alain Reid, Pamela S. Gaskin
Human Pathology Reports.2022; 27: 300600. CrossRef - Salivary Gland Pleomorphic Adenomas Presenting With Extremely Varied Clinical Courses. A Single Institution Case-Control Study†
Krzysztof Piwowarczyk, Ewelina Bartkowiak, Paweł Kosikowski, Jadzia Tin-Tsen Chou, Małgorzata Wierzbicka
Frontiers in Oncology.2021;[Epub] CrossRef - A case report of pleomorphic adenoma squamous metaplasia resembling metastatic oral squamous cell carcinoma
E. Donohoe, R. Courtney, S. Phelan, P.J. McCann
Advances in Oral and Maxillofacial Surgery.2021; 2: 100074. CrossRef - Extensive squamous metaplasia in minor salivary gland neoplasm mimicking squamous cell carcinoma: Diagnostic dilemma in aspiration cytology
Renu Sukumaran, Nileena Nayak, RariP Mony
Clinical Cancer Investigation Journal.2021; 10(5): 257. CrossRef - Navigating small biopsies of salivary gland tumors: a pattern-based approach
J. Stephen Nix, Lisa M. Rooper
Journal of the American Society of Cytopathology.2020; 9(5): 369. CrossRef - Giant Parotid Pleomorphic Adenoma with Atypical Histological Presentation and Long‐Term Recurrence‐Free Follow‐Up after Surgery: A Case Report and Review of the Literature
Mohammed AlKindi, Sundar Ramalingam, Lujain Abdulmajeed Hakeem, Manal A. AlSheddi, Pravinkumar G. Patil
Case Reports in Dentistry.2020;[Epub] CrossRef - Pleomorphic adenoma of soft palate with extensive squamous metaplasia – A diagnostic enigma
Rashmi Patnayak, Sandip Mohanty, Anjan Kumar Sahoo, Adya Kinkara Panda, Amitabh Jena
Journal of Dr. NTR University of Health Sciences.2019; 8(4): 268. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Squamous Metaplasia in Pleomorphic Adenoma: A Diagnostic and Prognostic Enigma



Fig. 1. (A) Swelling associated with central ulceration covered by a fibrino-purulent membrane and surrounded by palatal erythema can be seen on the right distal side of the hard palate, laterally. (B) A well-encapsulated tumor removed in toto from the palate.
Fig. 2. (A) Reconstructed panoramic image shows the polyp at the level of the mid right maxillary sinus. (B) Axial section shows inflamed sinus lining involving the right maxillary sinus. (C) Cone beam computed tomography (CBCT) mid-coronal section of the right maxillary third molar (18) showing the soft tissue shadow palatally. (D) Two-three dimensional CBCT image showing the antero-posterior extent of the swelling.
Fig. 3. (A) The periphery of the tumor with classical features of pleomorphic adenoma having abundant ducts dispersed in a myxoid and hyalinized background with multiple keratin-filled cystic areas. (B) Multitude of features, including variably shaped epithelial cells, ducts, and squames. Keratin-filled cysts dispersed in the myxoid stroma. (C) Abundant myoepithelial cells around the ducts giving the appearance of a swarm of bees. (D) Massive epithelial lined cysts with keratotic lamellae and the lining budding into the stroma.
Fig. 1.
Fig. 2.
Fig. 3.
Squamous Metaplasia in Pleomorphic Adenoma: A Diagnostic and Prognostic Enigma

 E-submission
E-submission