Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 52(6); 2018 > Article
-
Original Article
The Prognostic Impact of Synchronous Ipsilateral Multiple Breast Cancer: Survival Outcomes according to the Eighth American Joint Committee on Cancer Staging and Molecular Subtype -
Jinah Chu
 , Hyunsik Bae
, Hyunsik Bae , Youjeong Seo
, Youjeong Seo , Soo Youn Cho
, Soo Youn Cho , Seok-Hyung Kim
, Seok-Hyung Kim , Eun Yoon Cho
, Eun Yoon Cho
-
Journal of Pathology and Translational Medicine 2018;52(6):396-403.
DOI: https://doi.org/10.4132/jptm.2018.10.03
Published online: October 23, 2018
Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
-
Corresponding Author Eun Yoon Cho, MD, PhD Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea Tel: +82-2-3410-2796 Fax: +82-2-3410-0025 E-mail: eunyoon.cho@samsung.com
Seok-Hyung Kim, PhD Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea Tel: +82-2-3410-2898 Fax: +82-2-3410-0025 E-mail: parmenides.kim@samsung.com
© 2018 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- In the current American Joint Committee on Cancer staging system of breast cancer, only tumor size determines T-category regardless of whether the tumor is single or multiple. This study evaluated if tumor multiplicity has prognostic value and can be used to subclassify breast cancer.
-
Methods
- We included 5,758 patients with invasive breast cancer who underwent surgery at Samsung Medical Center, Seoul, Korea, from 1995 to 2012.
-
Results
- Patients were divided into two groups according to multiplicity (single, n = 4,744; multiple, n = 1,014). Statistically significant differences in lymph node involvement and lymphatic invasion were found between the two groups (p < .001). Patients with multiple masses tended to have luminal A molecular subtype (p < .001). On Kaplan-Meier survival analysis, patients with multiple masses had significantly poorer disease-free survival (DFS) (p = .016). The prognostic significance of multiplicity was seen in patients with anatomic staging group I and prognostic staging group IA (p = .019 and p = .032, respectively). When targeting patients with T1-2 N0 M0, hormone receptor–positive, and human epidermal growth factor receptor 2 (HER2)–negative cancer, Kaplan-Meier survival analysis also revealed significantly reduced DFS with multiple cancer (p = .031). The multivariate analysis indicated that multiplicity was independently correlated with worse DFS (hazard ratio, 1.23; 95% confidence interval, 1.03 to 1.47; p = .025). The results of this study indicate that tumor multiplicity is frequently found in luminal A subtype, is associated with frequent lymph node metastasis, and is correlated with worse DFS.
-
Conclusions
- Tumor multiplicity has prognostic value and could be used to subclassify invasive breast cancer at early stages. Adjuvant chemotherapy would be necessary for multiple masses of T1–2 N0 M0, hormone-receptor-positive, and HER2-negative cancer.
- Study population
- We identified 5,758 patients with invasive breast cancer who underwent conserving breast surgery or total mastectomy at Samsung Medical Center in Seoul, Korea, from 1995 to 2012. For inclusion in the study, patients needed to meet the following criteria: no distant metastasis at the time of diagnosis, no neoadjuvant therapy prior to surgery, and a follow-up period longer than 36 months. The mean age of the patients was 47 years (age range, 21 to 86 years), and the median follow-up period was 64 months. This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB No. 2018-06-098-001). Formal written informed consent was not required due to a waiver by the appropriate IRB.
- Clinicopathological evaluation
- Clinicopathological information, including multiplicity, age, tumor size, axillary nodal status, and histological grade, was obtained from electronic medical records or surgical pathology reports. According to the eighth edition of the American Joint Committee on Cancer (AJCC) staging, a patient with multiple breast cancer was defined if two or more separate masses were grossly or microscopically identified in a resection specimen no matter whether they were present in the same or different quadrants. In some cases, through assistance of careful gross examination and correlation with imaging findings, we can determine multiple breast cancer. Pathological tumor stage was assessed according to the eighth AJCC TNM classification [16]. If an invasive carcinoma has been transected by vacuum-assisted biopsy or excisional biopsy, then the sizes in each fragment were not added together, and correlation with the size on breast imaging was helpful to determine the best size for classification. If there had been a prior core needle biopsy or incisional biopsy showing a larger area of invasion than in the excisional specimen, the largest dimension of the invasive carcinoma in the prior specimen should be used for T classification. Histological grade was evaluated according to the Scarff-Bloom-Richardson classification modified by Elston and Ellis [16]. The expression status of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2) were evaluated by immunohistochemistry based on the surgical specimen. For ER and PR, only nuclear (not cytoplasmic) staining was scored. A positive test was defined as positive staining greater than or equal to 1% of tumor cells. A negative test was defined as staining of less than 1% of tumor cells. HER2 was scored as 0, 1+, 2+, or 3+. Only membrane staining intensity and pattern were evaluated using the recommendations of the American Society of Clinical Oncology/College of American Pathologists [17,18]. A positive test was defined as a staining score of 3+. Tumors with a 2+ score were submitted for silver in situ hybridization. The tumor was considered positive for HER2 amplification if the HER2/chromosome 17 probe signal ratio was greater than 2.0 and/or the average HER2 copy number was greater than 6.0 signals per cell. Molecular subtypes of breast cancer were classified into luminal A, luminal B1, luminal B2, HER2, and triple-negative subtypes based on histological grade and the results of ER, PR, and HER2 immunochemistry as follows: luminal A (ER-positive and/or PR-positive, HER2-negative, and low histological grade [grade 1 or 2]); luminal B1 (ER-positive and/or PR-positive, and HER2-positive); luminal B2 (ER-positive and/or PR-positive, HER2-negative, and high histological grade [grade 3]), HER2-positive (ER-negative, PRnegative, and HER2-positive); and triple-negative (ER-negative, PR-negative, and HER2-negative) [19].
- Statistical analysis
- The primary outcome was disease-free survival (DFS), defined as the time interval from the date of surgery to the date of first recurrence, including local or distant. Survival curves were estimated using the Kaplan-Meier method, and survival differences were analyzed by log-rank test. The clinicopathological variables were analyzed in univariate and multivariate analyses of DFS with Cox proportional hazards model. Statistical analysis was performed using the R v3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
MATERIALS AND METHODS
- Patient characteristics
- Patients were divided into two groups according to multiplicity. We found breast cancers involving a single mass in 4,744 cases (82.4%) and breast cancers involving multiple masses in 1,014 cases (17.6%). Table 1 shows the results of the comparison between patients with a single mass and patients with multifocal or multicentric masses.
- Patients with multiple cancers were more likely to be young and have undergone total mastectomy. Statistically significant differences in lymph node positivity (single 38.0% vs multiple 47.3%, p < .001) and lymphatic invasion (single 24.7% vs multiple 32.6%, p < .001) were found between the two groups. In addition, multiplicity was associated with non–high histological grade (p < .001), ER positivity (p < .001), PR positivity (p < .001), and HER2 negativity (p = .003) of tumor. Therefore, breast cancers with multiple masses were more likely to have luminal A molecular subtype and less likely to be triple-negative subtype compared to those with a single mass (p < .001).
- Disease-free survival
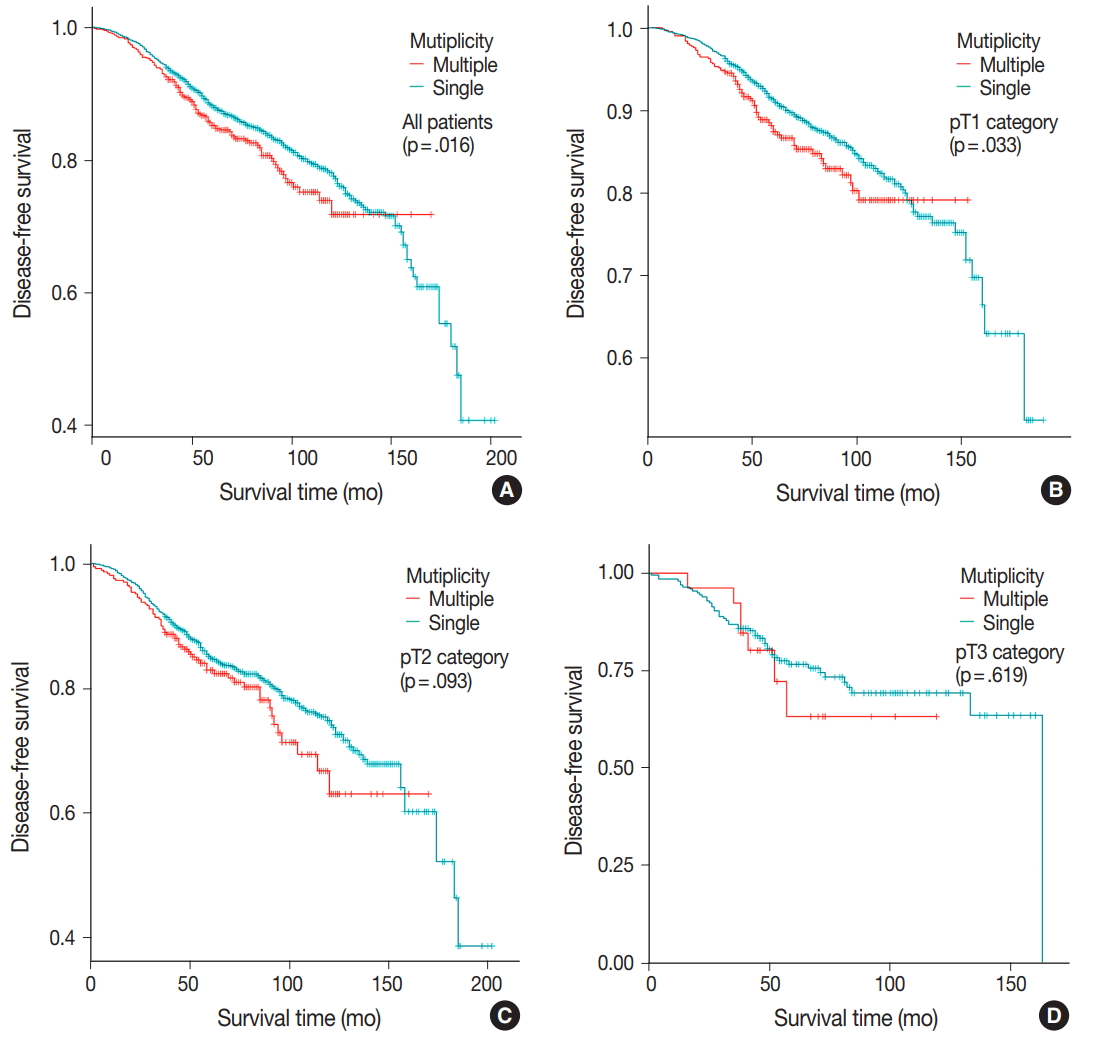
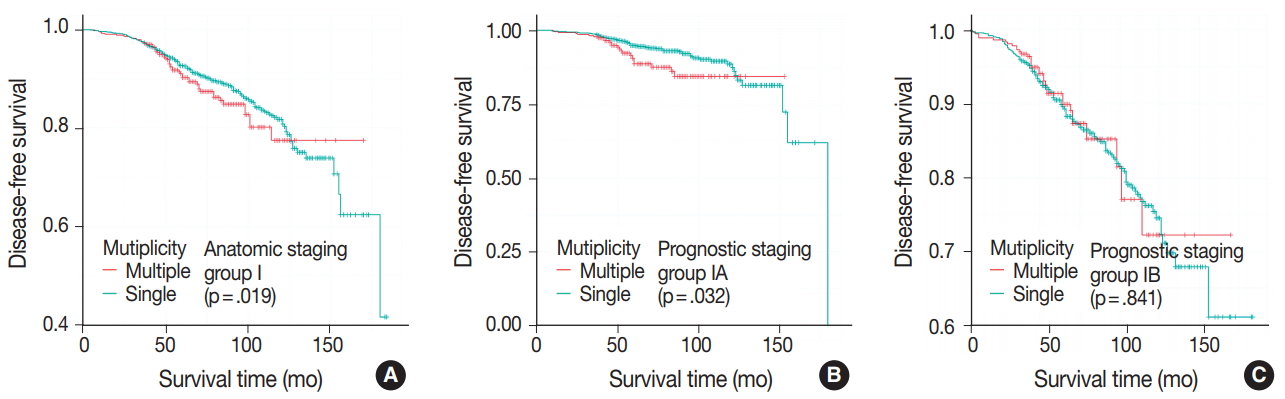
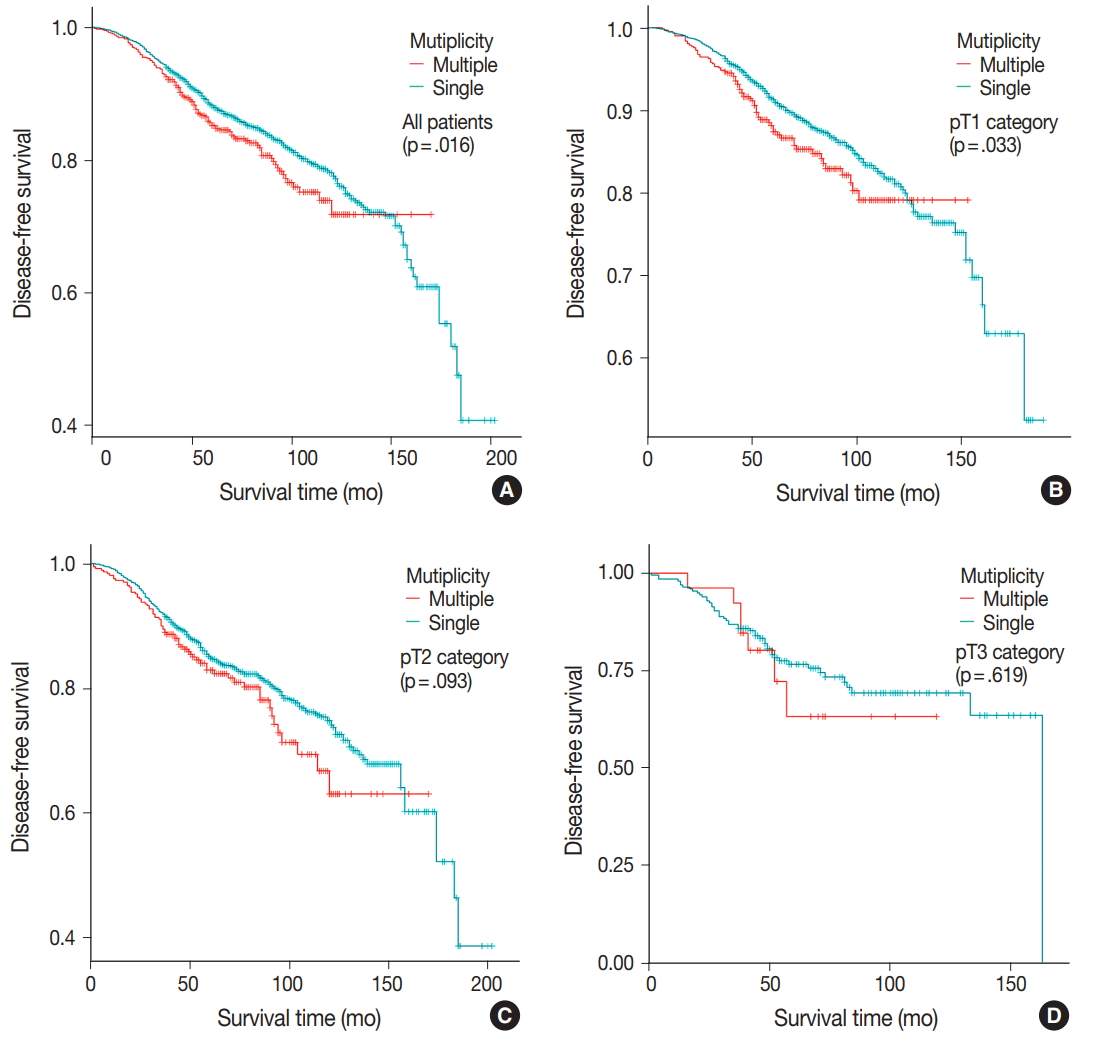
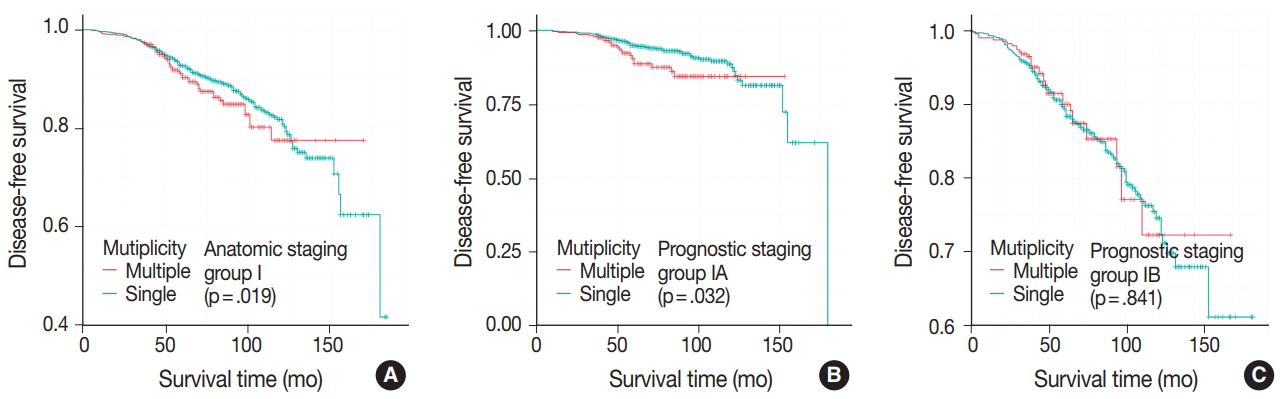
- DFS was evaluated in patients with single breast mass and multiple masses. Kaplan-Meier survival analysis indicated that patients with multiple masses had significantly poorer DFS than did those with a single mass (5-year rate, 88.2% vs 85.2%; p = .016) (Fig. 1A). When patients were subclassified according to T-category, Kaplan-Meier survival analysis in the T1 category group revealed significantly worse DFS for multiple breast cancer (5-year rate, 91.3% vs 87.4%; p = .033) (Fig. 1B). There was no significant prognostic difference in T2 and T3 category groups (p = .093 and p = .619, respectively) (Fig. 1C, D). Using the anatomic stage group table in the AJCC eighth edition for tumor staging, breast cancer with multiplicity had poor prognosis in stage I (5-year rate, 92.7% vs 90.3%; p = .019) (Fig. 2A). When using the prognostic stage group table in the AJCC eighth edition, multiple breast masses were found to have significantly shorter DFS than single breast masses in stage group IA (5-year rate, 94.9% vs 88.7%; p = .032) (Fig. 2B). However, no significant difference was found between single and multiple tumors in the other stage groups (i.e., anatomic staging group II or III and prognostic staging group IB, II, or III) (Fig. 2C).
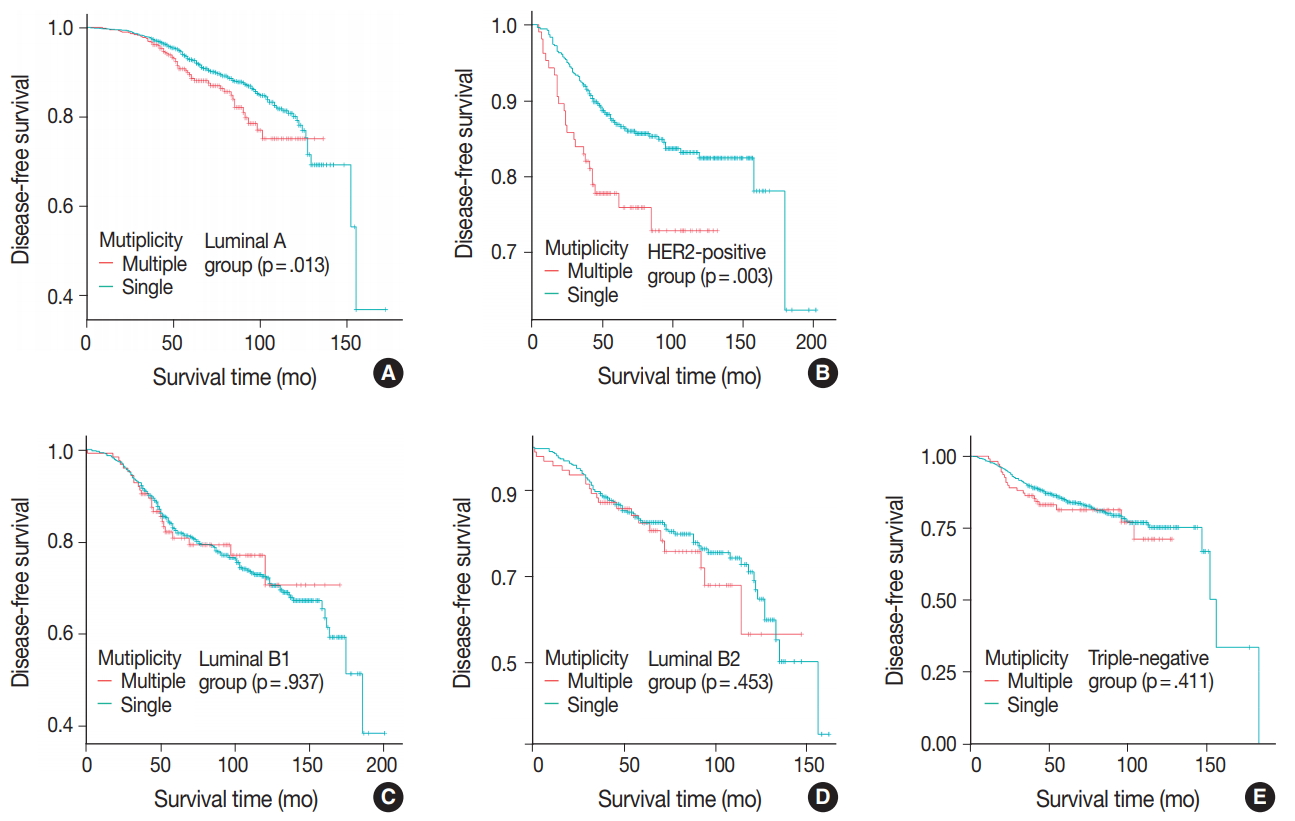
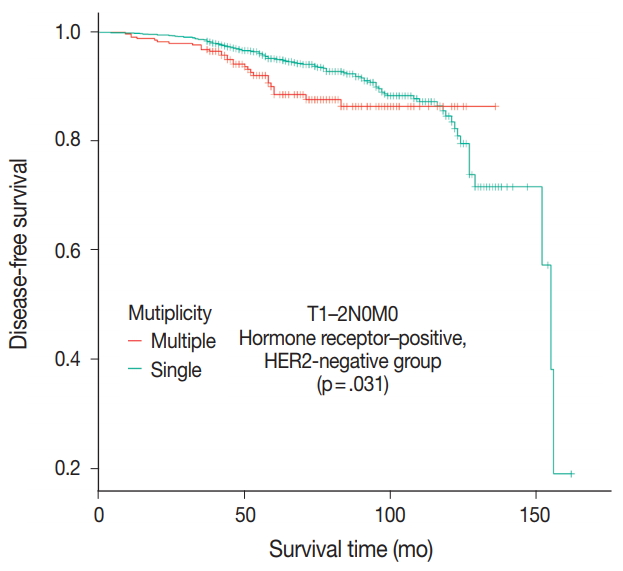
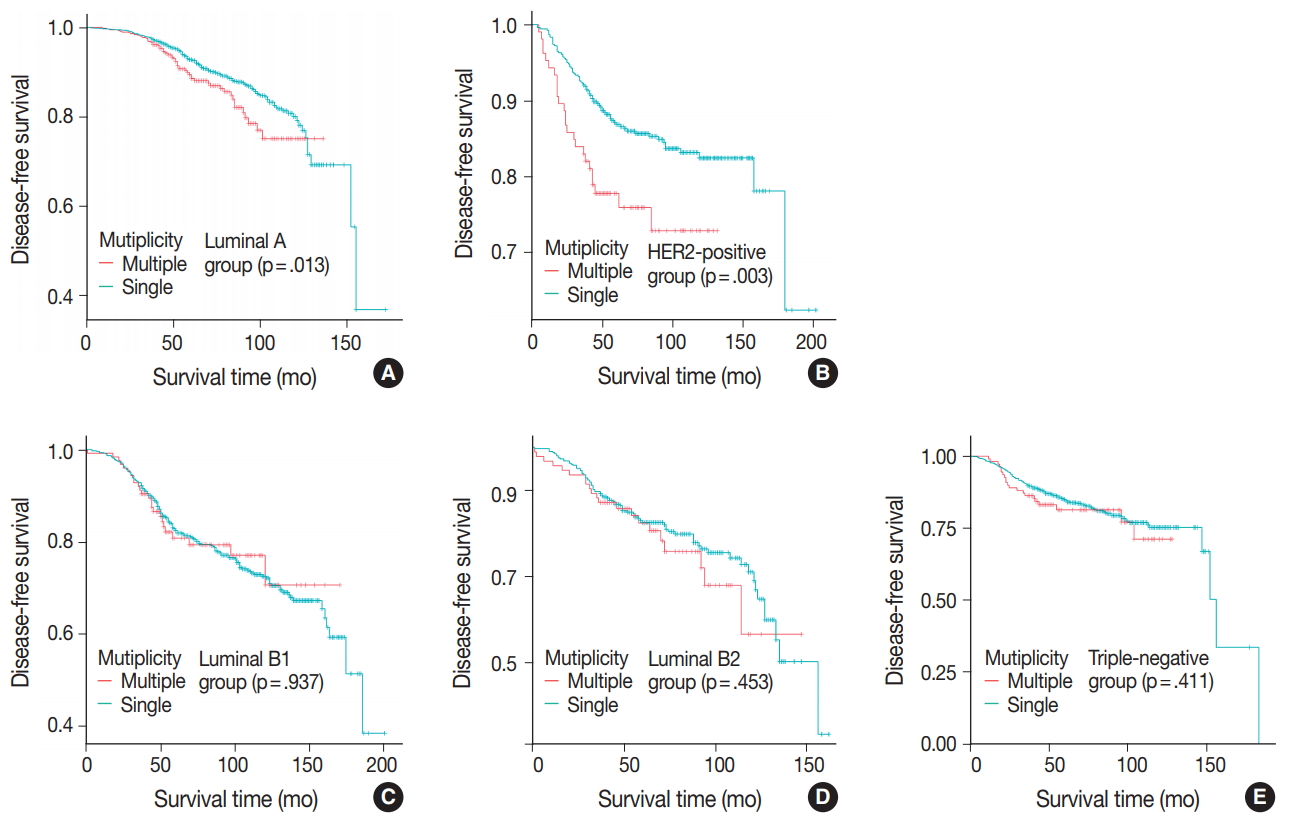
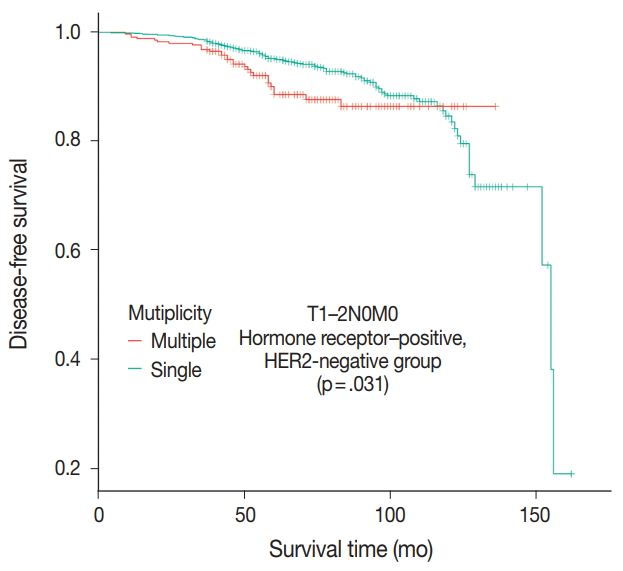
- Patients were divided into five molecular subtypes (i.e., luminal A, B1, and B2; HER2-positive; and triple-negative). The prognostic significance of multiplicity was only seen in patients with luminal A and HER2-positive groups in terms of DFS (5-year rate, 92.8% vs. 88.6%; p = .013 and 5-year rate, 86.9% vs 77.8%; p = .003, respectively) (Fig. 3A, B). There was no significant difference among the luminal B1 and B2 and triple-negative subtypes (p = .937, p = .453, and p = .411, respectively) (Fig. 3C–E). In addition, when targeting patients with T1–2 N0 M0, hormone-receptor-positive, and HER2-negative cancer, Kaplan-Meier survival analysis revealed a significantly reduced DFS of multiple breast cancer (5-year rate, 95.2% vs 88.6%; p = .031) (Fig. 4).
- Univariate analysis using Cox proportional hazard model indicated that high tumor stage (T3) (hazard ratio [HR], 2.44; 95% confidence interval [CI], 1.84 to 3.23; p < .001), positive lymph node metastasis (HR, 2.06; 95% CI, 1.8 to 2.36; p < .001), high anatomic staging group (i.e., stage III) (HR, 3.47; 95% CI, 2.89 to 4.18; p < .001), positive lymphatic emboli (HR, 2.16; 95% CI, 1.88 to 2.49; p < .001), high histological grade (i.e., grade 3) (HR, 1.52; 95% CI, 1.33 to 1.74; p < .001), negative ER status (HR, 1.23; 95% CI, 1.07 to 1.42; p < .001), positive HER2 status (HR, 1.21; 95% CI, 1.05 to 1.40; p = .004), and the presence of multiplicity (HR, 1.24; 95% CI, 1.04 to 1.48; p = .016) are significant variables associated with lower DFS (Table 2).
- These significant factors in the univariate model were included in multivariate analysis, which demonstrated that tumor multiplicity correlated independently with worse DFS (adjusted HR, 1.23; 95% CI, 1.05 to 1.47; p = .021). Other independent factors were high tumor stage (T3) (adjusted HR, 1.81; 95% CI, 1.35 to 2.41; p < .001), positive lymph node metastasis (adjusted HR, 1.84; 95% CI, 1.60 to 2.13; p < .001), and high histological grade (i.e., grade 3) (adjusted HR, 1.33; 95% CI, 1.14 to 1.55; p < .001) (Table 3).
RESULTS
- In the present study, the 17.6% incidence of surgically removed breast cancer with multiplicity is in line with prior data series [9,11,20-23]. In previous studies, the incidence of multiple breast cancer had a wide range due to different definitions and inclusion criteria for multiple masses. Here, we used the term multiplicity if the cancer showed either multicentricity or multifocality. Many researchers have studied the characteristics of multicentric or multifocal breast cancer. In the literature, lymphovascular invasion and axillary nodal involvement were more frequent in multicentric or multifocal breast cancers [3,5-15]. The higher frequency of lymph node metastases could be due to the greater volume and surface area of multiple breast cancer or different biological behavior [8]. In agreement with reported series, patients in this study with multiple masses had a higher incidence of lymph node involvement than patients with single mass. In addition, multiplicity was associated with frequent lymphovascular invasion.
- Theoretically, as breast cancers with multiplicity are more likely to have lymph node involvement and lymphovascular invasion, it could be inferred that prognosis would be worse than that of single mass breast cancers. Of course, many researchers have studied multiplicity as a prognostic factor in breast cancer. However, the biological and clinical significances of multiplicity are still debated [3,7,9,11,12,14,20,21,24-26]. Vlastos et al. [11] studied 284 patients with early-stage breast cancer and found that locoregional recurrence, distant metastasis, and disease-specific survival and DFS were not different between multicentric versus unicentric tumors. On the other hand, Yerushalmi et al. [3] analyzed 1,554 patients and found multicentric/multifocal tumors to be associated with worse breast cancer–specific survival. Additionally, Neri et al. [22] reported on 191 cases of breast cancer and found multifocal/multicentric breast cancer to be related to significantly worse prognosis with breast cancer–specific survival.
- The results of our study suggest that multicentric and multifocal breast cancers may have different biological behaviors. Multiple masses were more likely to have non-high histology grade, ER positivity, PR positivity, and HER2 negativity compared with single mass cases. Interestingly, we found that breast cancers with multiplicity were associated with luminal A molecular subtype and non-high histology grade, which are known to have good prognosis. Additionally, multiple breast masses of the luminal A group were found to have a significantly shorter DFS than single breast masses in Kaplan-Meier survival analysis (p = .013). As with luminal A, multiplicity had prognostic significance in the HER2-positive group. According to our results, close observation during follow-up is needed, especially in patients of the luminal A and HER2-positive groups with multiple breast cancer. There have been conflicting reports about hormonal receptor status [22,27]. As in our study, Moon et al. [27] identified frequent ER positivity and HER2 negativity of multiple breast cancers in a series of 2,882 patients. Conversely, however, Neri et al. [22] reviewed 1,158 patients and found an association between multiplicity and ER-negative and HER2-positive status. On the other hand, Moon et al. [27] reported that the difference in overall survival was significant only in patients with the triple-negative subtype.
- Our results show that breast cancer with multiplicity has a negative effect on DFS, especially in early-stage cancer. The results of multivariate analysis confirmed the independent prognostic value of multiplicity, and Kaplan-Meier survival curve showed significantly reduced DFS for patients with multiple masses in the T1 stage group (p = .033). The AJCC eighth edition presents the Prognostic Stage Group table in addition to the anatomic stage group table using the T, N, and M categories. The Prognostic Stage Group table includes the anatomical T, N, and M categories; tumor grade; and the status of ER, PR, and HER2 biomarkers. The prognostic significance of multiplicity in terms of DFS was only seen in patients with anatomic staging group I and prognostic staging group IA by Kaplan-Meier survival analysis (p = .019 and p = .032, respectively). Therefore, the negative prognostic impact of multiplicity could be considered for subclassification in at least early breast cancer patients.
- The Oncotype Dx genomic test is now performed for consideration of adjuvant chemotherapy in patients with T1–2 N0 M0, hormone receptor–positive, and HER2-negative cancer [28]. In this patient population in our study, multiple breast masses were found to have a significantly shorter DFS than single breast mass (p = .031). Based on the difference of prognosis, adjuvant chemotherapy would be necessary for multiple breast masses even without the Oncotype Dx test.
- Our study has several limitations. First, this retrospective study had a relatively short-term follow-up period (median duration, 64 months). Second, molecular subtype was evaluated only using the largest among multiple masses. Because intertumoral heterogeneity could be a factor affecting survival, a further study should be conducted to investigate the relationship between intertumoral heterogeneity and survival in multiple breast cancer. Finally, patients with neoadjuvant therapy were not included. Therefore, the evaluation of advanced stage breast cancer was relatively limited.
- In conclusion, the results of this study indicate that tumor multiplicity is frequently found in luminal A breast cancer, is associated with frequent lymph node metastasis, and is correlated with worse DFS. Tumor multiplicity has prognostic value and could be used to subclassify invasive breast cancer in the early stage. Adjuvant chemotherapy would be necessary for multiple breast masses of the T1–2 N0 M0, hormone-receptorpositive, and HER2-negative cancer groups.
DISCUSSION
- 1. Qualheim RE, Gall EA. Breast carcinoma with multiple sites of origin. Cancer 1957; 10: 460-8. ArticlePubMed
- 2. Bendifallah S, Werkoff G, Borie-Moutafoff C, et al. Multiple synchronous (multifocal and multicentric) breast cancer: clinical implications. Surg Oncol 2010; 19: e115-23. ArticlePubMed
- 3. Yerushalmi R, Kennecke H, Woods R, Olivotto IA, Speers C, Gelmon KA. Does multicentric/multifocal breast cancer differ from unifocal breast cancer? An analysis of survival and contralateral breast cancer incidence. Breast Cancer Res Treat 2009; 117: 365-70. ArticlePubMedPDF
- 4. Egan RL. Multicentric breast carcinomas: clinical-radiographicpathologic whole organ studies and 10-year survival. Cancer 1982; 49: 1123-30. ArticlePubMed
- 5. Tot T. Axillary lymph node status in unifocal, multifocal, and diffuse breast carcinomas: differences are related to macrometastatic disease. Ann Surg Oncol 2012; 19: 3395-401. ArticlePubMedPDF
- 6. Duraker N, Caynak ZC. Axillary lymph node status and prognosis in multifocal and multicentric breast carcinoma. Breast J 2014; 20: 61-8. ArticlePubMed
- 7. Cabioglu N, Ozmen V, Kaya H, et al. Increased lymph node positivity in multifocal and multicentric breast cancer. J Am Coll Surg 2009; 208: 67-74. ArticlePubMed
- 8. Andea AA, Bouwman D, Wallis T, Visscher DW. Correlation of tumor volume and surface area with lymph node status in patients with multifocal/multicentric breast carcinoma. Cancer 2004; 100: 20-7. ArticlePubMed
- 9. Joergensen LE, Gunnarsdottir KA, Lanng C, Moeller S, Rasmussen BB. Multifocality as a prognostic factor in breast cancer patients registered in Danish Breast Cancer Cooperative Group (DBCG) 1996-2001. Breast 2008; 17: 587-91. ArticlePubMed
- 10. Coombs NJ, Boyages J. Multifocal and multicentric breast cancer: does each focus matter? J Clin Oncol 2005; 23: 7497-502. ArticlePubMed
- 11. Vlastos G, Rubio IT, Mirza NQ, et al. Impact of multicentricity on clinical outcome in patients with T1-2, N0-1, M0 breast cancer. Ann Surg Oncol 2000; 7: 581-7. ArticlePubMedPDF
- 12. Rezo A, Dahlstrom J, Shadbolt B, et al. Tumor size and survival in multicentric and multifocal breast cancer. Breast 2011; 20: 259-63. ArticlePubMed
- 13. Lynch SP, Lei X, Chavez-MacGregor M, et al. Multifocality and multicentricity in breast cancer and survival outcomes. Ann Oncol 2012; 23: 3063-9. ArticlePubMedPMCPDF
- 14. Pedersen L, Gunnarsdottir KA, Rasmussen BB, Moeller S, Lanng C. The prognostic influence of multifocality in breast cancer patients. Breast 2004; 13: 188-93. ArticlePubMed
- 15. Tot T, Gere M, Pekár G, et al. Breast cancer multifocality, disease extent, and survival. Hum Pathol 2011; 42: 1761-9. ArticlePubMed
- 16. Giuliano AE, Edge SB, Hortobagyi GN. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol 2018; 25: 1783-5. ArticlePubMedPDF
- 17. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010; 28: 2784-95. PubMedPMC
- 18. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med 2014; 138: 241-56. PubMed
- 19. Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011; 22: 1736-47. ArticlePubMedPMCPDF
- 20. Litton JK, Eralp Y, Gonzalez-Angulo AM, et al. Multifocal breast cancer in women < or =35 years old. Cancer 2007; 110: 1445-50. ArticlePubMed
- 21. Boyages J, Jayasinghe UW, Coombs N. Multifocal breast cancer and survival: each focus does matter particularly for larger tumours. Eur J Cancer 2010; 46: 1990-6. ArticlePubMed
- 22. Neri A, Marrelli D, Megha T, et al. “Clinical significance of multifocal and multicentric breast cancers and choice of surgical treatment: a retrospective study on a series of 1158 cases”. BMC Surg 2015; 15: 1.ArticlePubMedPMCPDF
- 23. O’Daly BJ, Sweeney KJ, Ridgway PF, et al. The accuracy of combined versus largest diameter in staging multifocal breast cancer. J Am Coll Surg 2007; 204: 282-5. ArticlePubMed
- 24. Weissenbacher TM, Zschage M, Janni W, et al. Multicentric and multifocal versus unifocal breast cancer: is the tumor-node-metastasis classification justified? Breast Cancer Res Treat 2010; 122: 27-34. ArticlePubMedPDF
- 25. Ustaalioglu BO, Bilici A, Kefeli U, et al. The importance of multifocal/multicentric tumor on the disease-free survival of breast cancer patients: single center experience. Am J Clin Oncol 2012; 35: 580-6. PubMed
- 26. Fish EB, Chapman JA, Link MA. Assessment of tumor size for multifocal primary breast cancer. Ann Surg Oncol 1998; 5: 442-6. ArticlePubMedPDF
- 27. Moon HG, Han W, Kim JY, et al. Effect of multiple invasive foci on breast cancer outcomes according to the molecular subtypes: a report from the Korean Breast Cancer Society. Ann Oncol 2013; 24: 2298-304. ArticlePubMedPDF
- 28. Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 2015; 373: 2005-14. PubMedPMC
REFERENCES
Figure & Data
References
Citations

- The Role of Serum Beta-Human Chorionic Gonadotropin (β-hCG) in Differentiating Benign and Malignant Breast Lesions at a Tertiary Care Center in Jharkhand
Neyaz Ahmad, Khushboo Rani, Zenith Kerketta, Krishna Murari, Anish Baxla, Ujala Murmu, Amit Nishant, Shreya .
Cureus.2025;[Epub] CrossRef - Role of Large Format Histology in Diagnosis of Breast Carcinoma
Hari Shankar Pandey, Sanya Bhasin, Suman Kumari Pandey
NMO Journal.2025; 19(2): 189. CrossRef - Prognostic Impact of Multiple Synchronous T1 Breast Cancer
Hongki Gwak, Sung Hoo Jung, Young Jin Suh, Seok Jin Nam, Jai Hong Han, Se Jeong Oh, Eun Hwa Park, Seong Hwan Kim
Cancers.2024; 16(23): 4019. CrossRef - Deep learning-based system for automatic prediction of triple-negative breast cancer from ultrasound images
Alexandre Boulenger, Yanwen Luo, Chenhui Zhang, Chenyang Zhao, Yuanjing Gao, Mengsu Xiao, Qingli Zhu, Jie Tang
Medical & Biological Engineering & Computing.2023; 61(2): 567. CrossRef - Multicentre prospective cohort study of unmet supportive care needs among patients with breast cancer throughout their cancer treatment trajectory in Penang: a PenBCNeeds Study protocol
Noorsuzana Mohd Shariff, Nizuwan Azman, Rohayu Hami, Noor Mastura Mohd Mujar, Mohammad Farris Iman Leong Bin Abdullah
BMJ Open.2021; 11(3): e044746. CrossRef - The subgross morphology of breast carcinomas: a single-institution series of 2033 consecutive cases documented in large-format histology slides
Tibor Tot, Maria Gere, Syster Hofmeyer, Annette Bauer, Ulrika Pellas
Virchows Archiv.2020; 476(3): 373. CrossRef - Editorial for “Synchronous Breast Cancer: Phenotypic Similarities on MRI”
Uma Sharma
Journal of Magnetic Resonance Imaging.2020; 52(1): 309. CrossRef - Synchronous Multiple Breast Cancers—Do We Need to Reshape Staging?
Minodora Onisâi, Adrian Dumitru, Iuliana Iordan, Cătălin Aliuș, Oana Teodor, Adrian Alexandru, Daniela Gheorghiță, Iulian Antoniac, Adriana Nica, Alexandra-Ana Mihăilescu, Sebastian Grădinaru
Medicina.2020; 56(5): 230. CrossRef - Molecular mechanism of triple‑negative breast cancer‑associated BRCA1 and the identification of signaling pathways
Feng Qi, Wen‑Xing Qin, Yuan‑Sheng Zang
Oncology Letters.2019;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure




Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
| Characteristic | Single (n = 4,744) | Multiple (n = 1,014) | p-value |
|---|---|---|---|
| Age (yr) | .001 | ||
| < 47 | 2,459 (51.8) | 591 (58.3) | |
| ≥ 47 | 2,285 (48.2) | 423 (41.7) | |
| Operation | < .001 | ||
| Partial | 3,089 (65.1) | 470 (46.4) | |
| Total | 1,655 (34.9) | 544 (53.6) | |
| Chemotherapy | .115 | ||
| Not done | 1,113 (23.5) | 214 (21.1) | |
| Done | 3,631 (76.5) | 800 (78.9) | |
| Hormonal therapy | < .001 | ||
| Not done | 1,412 (29.8) | 214 (21.1) | |
| Done | 3,332 (70.2) | 800 (78.9) | |
| Radiotherapy | < .001 | ||
| Not done | 1,241 (26.2) | 426 (42.0) | |
| Done | 3,503 (73.8) | 588 (58.0) | |
| pT | .016 | ||
| T1 | 2,720 (57.3) | 618 (60.9) | |
| T2 | 1,827 (38.5) | 370 (36.5) | |
| T3 | 197 (4.2) | 26 (2.6) | |
| Lymph node | < .001 | ||
| Negative | 2,941 (62.0) | 534 (52.7) | |
| Positive | 1,803 (38.0) | 480 (47.3) | |
| Anatomic stage group | .064 | ||
| Stage I | 2,014 (42.5) | 390 (38.5) | |
| Stage II | 2,089 (44.0) | 479 (47.2) | |
| Stage III | 641 (13.5) | 145 (14.3) | |
| Lymphatic invasion | < .001 | ||
| Negative | 3,572 (75.3) | 683 (67.4) | |
| Positive | 1,172 (24.7) | 331 (32.6) | |
| Histology grade | < .001 | ||
| Grade 1, 2 | 3,089 (65.1) | 737 (72.7) | |
| Grade 3 | 1,655 (34.9) | 277 (27.3) | |
| ER status | < .001 | ||
| Negative | 1,430 (30.1) | 232 (22.9) | |
| Positive | 3,314 (69.9) | 782 (77.1) | |
| PR status | < .001 | ||
| Negative | 1,789 (37.7) | 278 (27.4) | |
| Positive | 2,955 (62.3) | 736 (72.6) | |
| HER2 status | .003 | ||
| Negative | 3,448 (72.7) | 783 (77.2) | |
| Positive | 1,296 (27.3) | 231 (22.8) | |
| Molecular subtype | < .001 | ||
| Luminal A | 2,228 (47.0) | 580 (57.2) | |
| Luminal B1 | 753 (15.9) | 125 (12.3) | |
| Luminal B2 | 410 (8.6) | 94 (9.3) | |
| HER2 positive | 543 (11.4) | 106 (10.5) | |
| Triple negative | 810 (17.1) | 109 (10.7) |
| Hazard ratio | 95% CI | p-value | |
|---|---|---|---|
| Multiplicity | |||
| Single | 1 | ||
| Multiple | 1.24 | 1.04–1.48 | .016 |
| Age (yr) | |||
| ≥ 47 | 1 | ||
| < 47 | 1.08 | 0.94–1.24 | .258 |
| pT | |||
| T1 | 1 | ||
| T2 | 1.57 | 1.37–1.81 | < .001 |
| T3 | 2.44 | 1.84–3.23 | < .001 |
| Lymph node | |||
| Negative | 1 | ||
| Positive | 2.06 | 1.80–2.36 | < .001 |
| Anatomic stage | |||
| Stage I | 1 | ||
| Stage II | 1.65 | 1.40–1.95 | < .001 |
| Stage III | 3.47 | 2.89–4.18 | < .001 |
| Lymphatic invasion | |||
| Negative | 1 | ||
| Positive | 2.16 | 1.88–2.49 | < .001 |
| Histology grade | |||
| Grade 1, 2 | 1 | ||
| Grade 3 | 1.52 | 1.33–1.74 | < .001 |
| ER status | |||
| Positive | 1 | ||
| Negative | 1.23 | 1.07–1.42 | .004 |
| HER2 status | |||
| Negative | 1 | ||
| Positive | 1.21 | 1.05–1.40 | .009 |
| Molecular Subtype | |||
| Luminal A | 1 | ||
| Luminal B1 | 1.8 | 1.49–2.18 | < .001 |
| Luminal B2 | 2.17 | 1.73–2.71 | < .001 |
| HER2 positive | 1.37 | 1.09–1.73 | .007 |
| Triple negative | 1.83 | 1.51–2.23 | < .001 |
| Hazard ratio | 95% CI | p-value | |
|---|---|---|---|
| Multiplicity | |||
| Single | 1 | ||
| Multiple | 1.23 | 1.05–1.47 | .021 |
| pT | |||
| T1 | 1 | ||
| T2 | 1.27 | 1.09–1.47 | .002 |
| T3 | 1.81 | 1.35–2.41 | < .001 |
| Lymph node | |||
| Negative | 1 | ||
| Positive | 1.84 | 1.60–2.13 | < .001 |
| Histology grade | |||
| Grade 1, 2 | 1 | ||
| Grade 3 | 1.33 | 1.14–1.55 | < .001 |
| ER status | |||
| Positive | 1 | ||
| Negative | 1.09 | 0.93–1.28 | .255 |
| HER2 status | |||
| Negative | 1 | ||
| Positive | 1.1 | 0.95–1.28 | .206 |
Values are presented as number (%).
CI, confidence interval; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
CI, confidence interval; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.

 E-submission
E-submission