Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 55(2); 2021 > Article
-
Brief Case Report
Multiple hepatocyte nuclear factor 1A (HNF1A)-inactivated hepatocellular adenomas arising in a background of congenital hepatic fibrosis -
Yangkyu Lee1
 , Hyunjin Park2
, Hyunjin Park2 , Kyoungbun Lee3
, Kyoungbun Lee3 , Youngeun Lee3
, Youngeun Lee3 , Kiryang Lee3
, Kiryang Lee3 , Haeryoung Kim3
, Haeryoung Kim3
-
Journal of Pathology and Translational Medicine 2021;55(2):154-158.
DOI: https://doi.org/10.4132/jptm.2020.11.12
Published online: December 23, 2020
1Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
2Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
3Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- Corresponding Author: Haeryoung Kim, MD, PhD, Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, 103 Daehak-ro, Jongno-gu, Seoul 03080, Korea, Tel: +82-2-740-8322, Fax: +82-2-765-5600, E-mail: haeryoung.kim@snu.ac.kr
© 2021 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
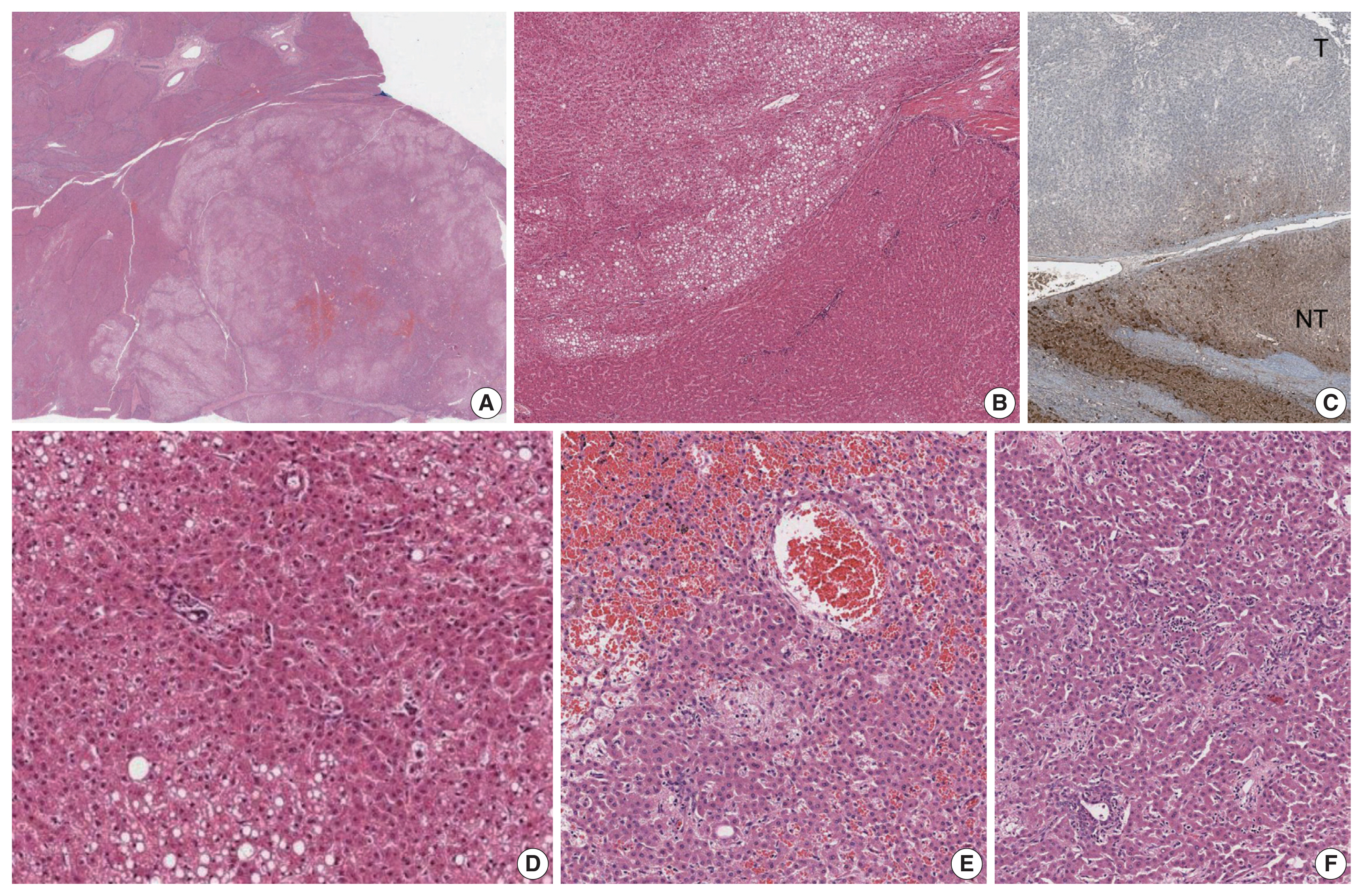
- A 25-year-old man was admitted to the hospital for liver transplantation for multiple hepatic masses that were incidentally discovered by imaging. He had previously been diagnosed with CHF on liver biopsy 14 years ago, and had received transjugular intrahepatic portosystemic shunt for portal hypertension at that time. He had no history of metabolic syndrome, alcohol intake or prior intake of androgens and his body mass index was 25.2 kg/m2. There was no evidence of other abnormalities associated with CHF, and there was no remarkable family history. Preoperative liver function tests were as follows: aspartate aminotransferase 35 U/L, alanine aminotransferase 29 U/L, gamma-glutamyl transferase 19 U/L, alkaline phosphatase 79 U/L, total bilirubin 4.2 mg/dL, and direct bilirubin 1.3 mg/dL. Serological tests for hepatitis B and C virus were negative. On computed tomography, there were multiple fat-containing nodules in both hepatic lobes.
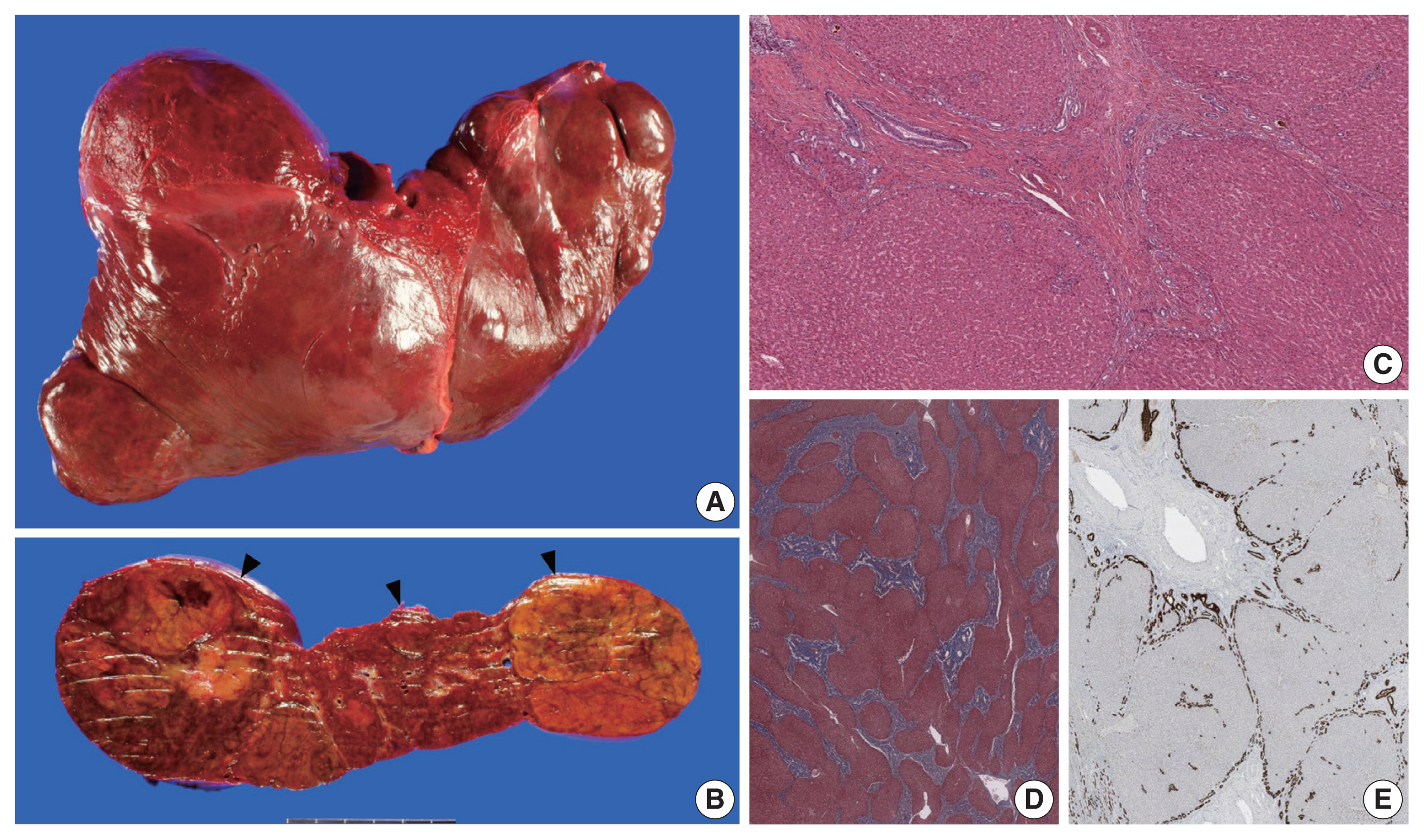
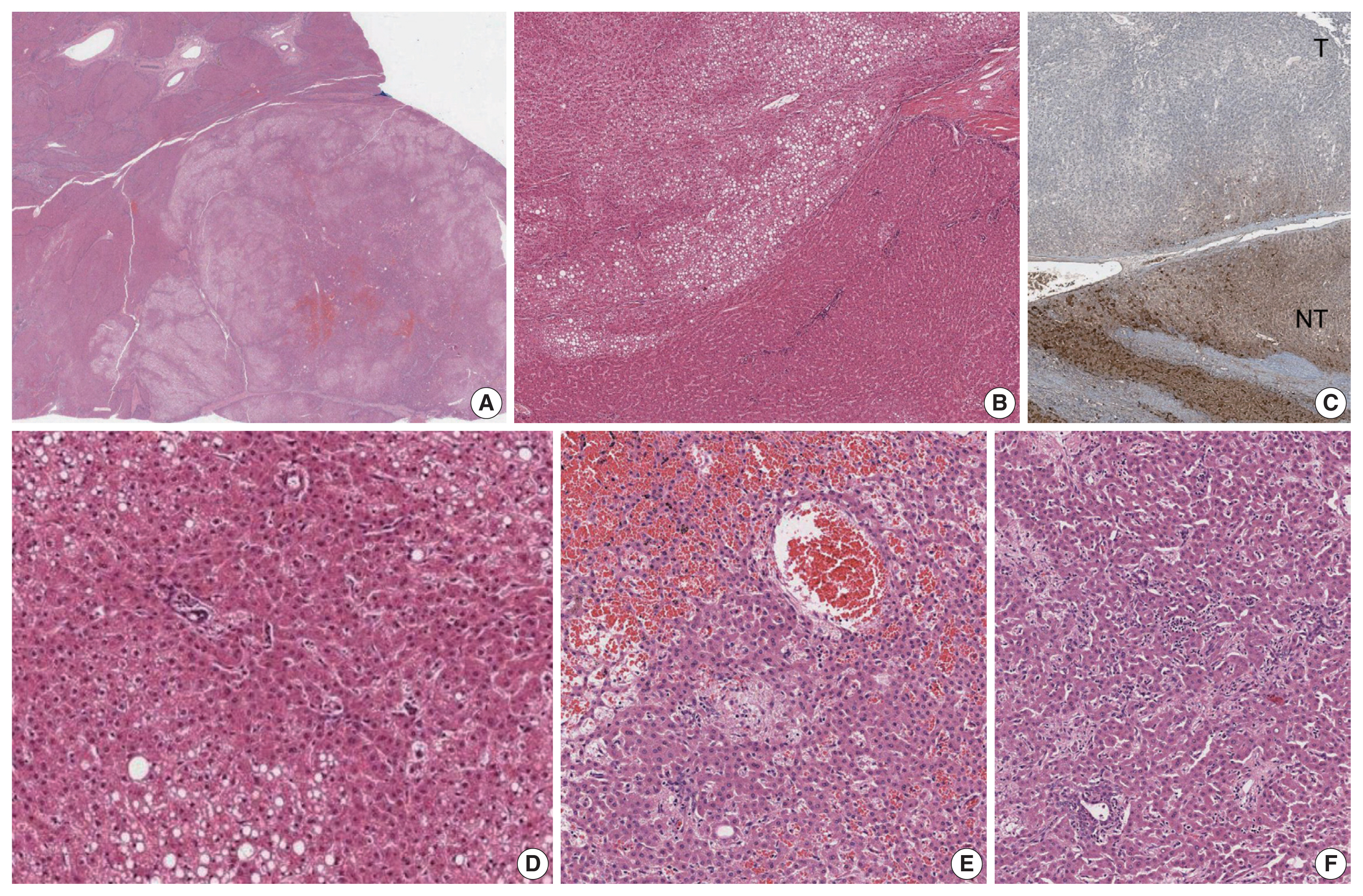
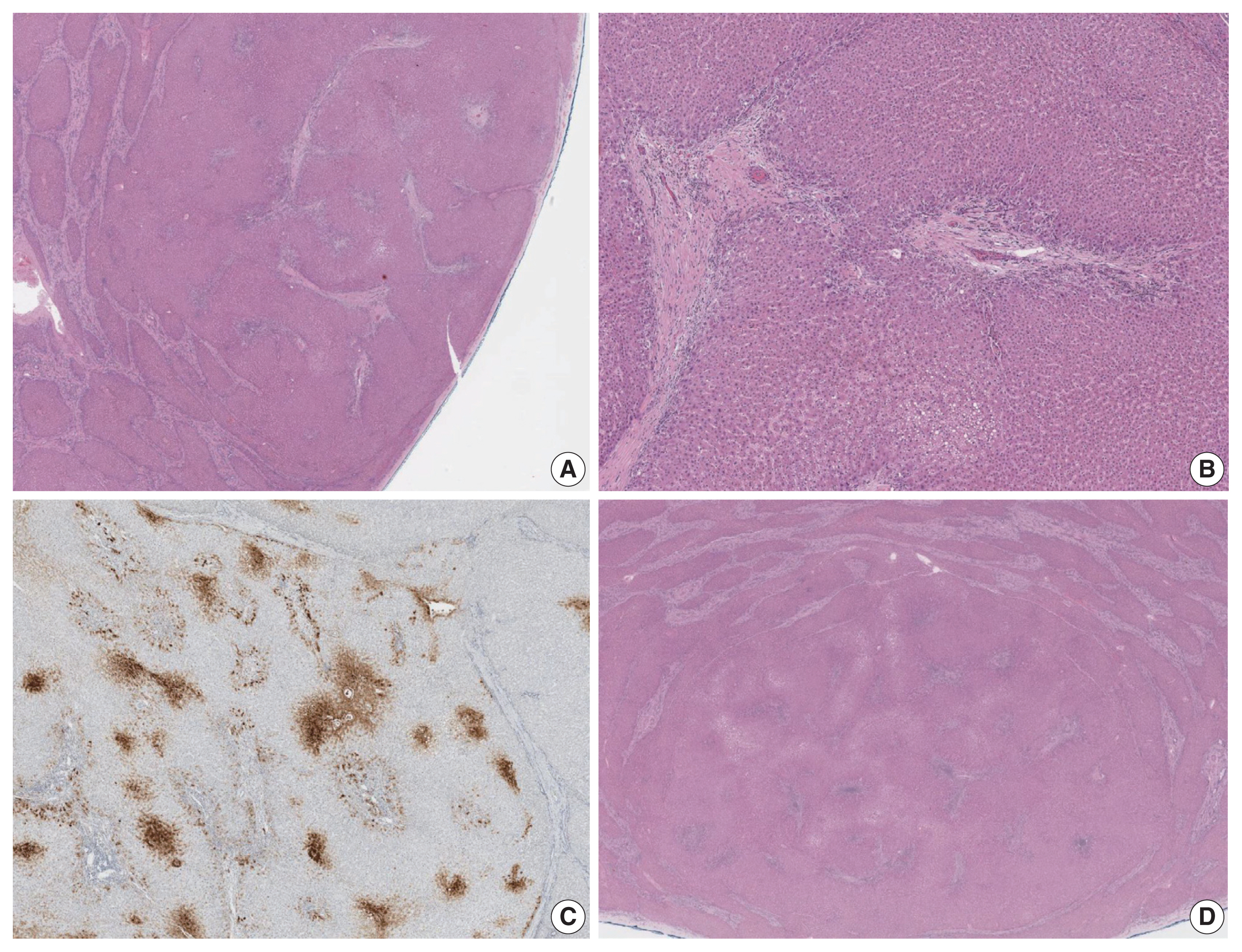
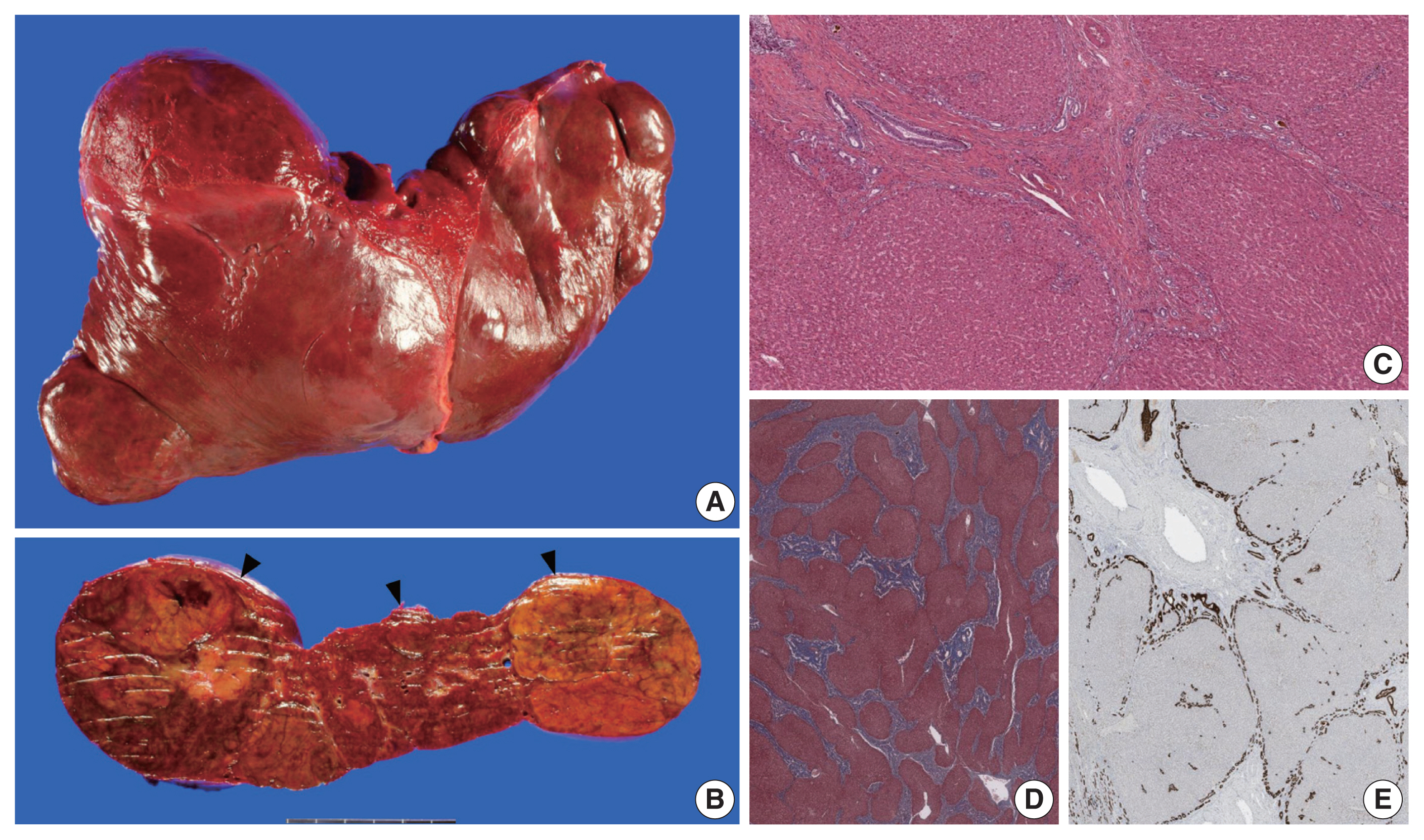
- The liver weighed 1,242 g, and sections revealed nine discrete tan-to-yellow nodules in both lobes, measuring 9.5 × 6.5 × 7.5 cm in the largest one. The background liver was firm with a reticular appearance (Fig. 1). On microscopic examination, the background liver showed the typical histological features of CHF: broad fibrous bands rimmed by small bile ductular structures with ductal plate malformation patterns (Fig. 1). The pathological findings of the nine nodules are summarized in Table 1 and Figs. 2 and 3. Six nodules were diagnosed as H-HCAs and the three smaller nodules as large regenerative nodule (LRN) or focal nodular hyperplasia (FNH)-like nodules, based on the histo-pathological and immunohistochemical characteristics. Notably, although LFABP loss was seen in all six H-HCAs, nodules #1, #2, and #9 showed only patchy mild steatosis, and nodules #1 and #2 also demonstrated focal peliosis and mild ductular reaction. The remaining 4 H-HCAs demonstrated diffuse steatosis. On follow-up, the patient is currently well without evidence of recurrence.
CASE REPORT
- To our knowledge, this is the second reported case of H-HCA arising in a background of CHF. Hepatocellular nodules, including FNH and FNH-like nodules, HCAs and even hepatocellular carcinomas (HCCs), have been demonstrated to occur in the setting of hepatic vascular abnormalities, such as Budd-Chiari syndrome and congenital extrahepatic portosystemic shunts [4–8]. While CHF belongs to a group of fibropolycystic diseases of the liver that affects the biliary system, portal hypertension dominates the clinical presentation of this disease, as the number of portal vein branches is often reduced [9]. Treatment of CHF with portosystemic shunt surgery may also add to the abnormal heterogeneous vascularity in the parenchyme [10]. As a result, the uneven hepatic perfusion may give rise to the formation of hepatocellular nodules. However, although this vascular concept explains the pathogenesis of reactive hyperplastic hepatocellular nodules such as FNH and LRN/FNH-like nodules, the relationship between this vascular abnormality and the occurrence of neoplastic hepatocellular nodules is still not well understood. Indeed, the neoplastic nature of smaller hepatocellular nodules resembling HCAs (previously referred to as “adenoma-like” nodules or adenomatous hyperplasia) arising in vascular disorders have been questioned, due to the morphological overlap with LRNs and FNH-like nodules and their frequent co-existence within the same liver [4]. However, as the molecular classification of HCAs has been better characterized over the past decade, immunohistochemical stains have facilitated the diagnosis of HCAs [7]. Interestingly, Sempoux et al. [7] described three cases of H-HCAs arising in backgrounds of hepatic vascular disorders which were either associated with HCCs or contained atypical foci. This is a notable finding as it implies that H-HCAs associated with vascular disorders may be at increased risk of malignant transformation, in contrast to those arising in “conventional” settings (e.g., females, oral contraceptives, MODY3, etc) [2]. Another interesting finding is that H-HCAs in the context of hepatic vascular disorders often demonstrated a lack of intratumoral steatosis, which differs from the characteristic histology (diffuse steatosis) of typical H-HCAs [7]. Indeed, in our case, three H-HCAs demonstrated only focal mild steatosis, and the diagnosis was rendered based on the loss of LFABP expression.
- Although further validation would be required, the identification of nodular lesions on imaging in the liver of patients with CHF or hepatic vascular disorders may warrant histological examination to rule out the possibility of HCCs or HCAs. In addition, H-HCAs arising in the context of hepatic vascular disorders may not be associated with the typical clinicopathological features, such as steatosis and low risk of malignant transformation.
DISCUSSION
Ethics Statement
This case study was approved by the Institutional Review Board of Seoul National University Hospital (#H-1809-141-975) and informed consent was waived.
Author Contributions
Conceptualization: YL (Yangkyu Lee), HK. Funding acquisition: HK. Investigation: YL (Yangkyu Lee), HK. Supervision: HK. Writing—original draft: YL (Yangkyu Lee). Writing—review & editing: YL (Yangkyu Lee), HP, KL, YL (Youngeun Lee), KL, HK. Approval of final manuscript: all authors.
Conflicts of Interest
H.K., a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
No funding to declare.

- 1. Bioulac-Sage P, Sempoux C, Balabaud C. Hepatocellular adenoma: classification, variants and clinical relevance. Semin Diagn Pathol 2017; 34: 112-25. ArticlePubMed
- 2. Nault JC, Paradis V, Cherqui D, Vilgrain V, Zucman-Rossi J. Molecular classification of hepatocellular adenoma in clinical practice. J Hepatol 2017; 67: 1074-83. ArticlePubMed
- 3. Rebouissou S, Imbeaud S, Balabaud C, et al. HNF1alpha inactivation promotes lipogenesis in human hepatocellular adenoma independently of SREBP-1 and carbohydrate-response element-binding protein (ChREBP) activation. J Biol Chem 2007; 282: 14437-46. PubMed
- 4. Ibarrola C, Castellano VM, Colina F. Focal hyperplastic hepatocellular nodules in hepatic venous outflow obstruction: a clinicopathological study of four patients and 24 nodules. Histopathology 2004; 44: 172-9. ArticlePubMed
- 5. Kondo F. Benign nodular hepatocellular lesions caused by abnormal hepatic circulation: etiological analysis and introduction of a new concept. J Gastroenterol Hepatol 2001; 16: 1319-28. PubMed
- 6. Sempoux C, Balabaud C, Paradis V, Bioulac-Sage P. Hepatocellular nodules in vascular liver diseases. Virchows Arch 2018; 473: 33-44. ArticlePubMedPDF
- 7. Sempoux C, Paradis V, Komuta M, et al. Hepatocellular nodules expressing markers of hepatocellular adenomas in Budd-Chiari syndrome and other rare hepatic vascular disorders. J Hepatol 2015; 63: 1173-80. ArticlePubMed
- 8. Sanada Y, Mizuta K, Niki T, et al. Hepatocellular nodules resulting from congenital extrahepatic portosystemic shunts can differentiate into potentially malignant hepatocellular adenomas. J Hepatobiliary Pancreat Sci 2015; 22: 746-56. PubMed
- 9. Bertheau P, Degott C, Belghiti J, et al. Adenomatous hyperplasia of the liver in a patient with congenital hepatic fibrosis. J Hepatol 1994; 20: 213-7. ArticlePubMed
- 10. Fukushima N, Kuromatsu R, Uchiyama D, et al. Hyperplastic nodular hepatic lesions following end-to-side portacaval shunting in childhood. Intern Med 2007; 46: 1203-8. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Molecular profiling in paediatric hepatocellular adenomas: phenotypic correlations and clinical significance
Yan Zhou, Antonio R Perez‐Atayde, Xuchen Zhang, Allison F O'Neill, Alanna J Church, Juan Putra
Histopathology.2025;[Epub] CrossRef - HNF1A-inactived Hepatocellular Adenomas in Fibropolycystic Kidney and Liver Disease Are Associated With Germline HNF1B Variant
Catherine E. Hagen, Dipti M. Karamchandani, Luisa Ricaurte, Nagaswaroop Nagaraj, Vivekananda Sarangi, Christopher P. Hartley, Chirag Patel, Marcela Salomao, Rish Pai, Rondell P. Graham
Modern Pathology.2025; 38(11): 100880. CrossRef - Hepatocellular adenoma: what we know, what we do not know, and why it matters
Paulette Bioulac‐Sage, Annette S H Gouw, Charles Balabaud, Christine Sempoux
Histopathology.2022; 80(6): 878. CrossRef - Hepatocellular adenomas: recent updates
Haeryoung Kim, Young Nyun Park
Journal of Pathology and Translational Medicine.2021; 55(3): 171. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1
Fig. 2
Fig. 3
| No. | Location (segment) | Size (cm) | Diagnosis | Histopathological feature | Immunohistochemical stain result |
|---|---|---|---|---|---|
| 1 | S7 | 9.5 | H-HCA | Patchy steatosis, focal peliosis, bile ductules | LFABP loss, SAA/CRP/GS negative, membranous β-catenin expression |
| 2 | S6 | 6.6 | H-HCA | Patchy steatosis, focal peliosis, bile ductules | LFABP loss, SAA/CRP/GS negative, membranous β-catenin expression |
| 3 | S1 | 3.5 | H-HCA | Diffuse steatosis | LFABP loss, SAA/CRP/GS negative, membranous β-catenin expression |
| 4 | S4 | 4.5 | H-HCA | Diffuse steatosis | LFABP loss, SAA/CRP/GS negative, membranous β-catenin expression |
| 5 | S4 | 1.0 | LRN | Fibrous septa with bile ductules | No LFABP loss, focal SAA/CRP expression, GS negative, membranous β-catenin |
| 6 | S4 | 1.0 | FNH-like nodule | Fibrous septa with bile ductules | No LFABP loss, focal SAA/CRP expression, patchy GS expression, membranous β-catenin |
| 7 | S2 | 9.5 | H-HCA | Diffuse steatosis | LFABP loss, SAA/CRP/GS negative, membranous β-catenin expression |
| 8 | S2 | 0.9 | FNH-like nodule | Fibrous septa with bile ductules | No LFABP loss, focal SAA/CRP expression, patchy GS expression, membranous β-catenin |
| 9 | S6 | 1.6 | H-HCA | Focal mild steatosis | LFABP loss, SAA/CRP/GS negative, membranous β-catenin expression |
H-HCA, Hepatocyte nuclear factor 1A–inactivated hepatocellular adenoma; LFABP, liver fatty acid-binding protein; SAA, serum amyloid A; CRP, C-reactive peptide; GS, glutamine synthetase; LRN, large regenerative nodule; FNH-like nodule, focal nodular hyperplasia-like nodule.

 E-submission
E-submission