Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 55(2); 2021 > Article
-
Case Study
A case of concomitant EGFR/ALK alteration against a mutated EGFR background in early-stage lung adenocarcinoma -
Ki-Chang Lee1
 , Jiwon Koh2
, Jiwon Koh2 , Doo Hyun Chung2
, Doo Hyun Chung2 , Yoon Kyung Jeon2,3
, Yoon Kyung Jeon2,3
-
Journal of Pathology and Translational Medicine 2021;55(2):139-144.
DOI: https://doi.org/10.4132/jptm.2020.12.16
Published online: January 22, 2021
1Seoul National University College of Medicine, Seoul, Korea
2Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
3Cancer Research Institute, Seoul National University, Seoul, Korea
- Corresponding Author: Yoon Kyung Jeon, MD, PhD, Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, 103 Daehak-ro, Jongno-gu, Seoul 03080, Korea, Tel: +82-2-740-8323, Fax: +82-2-743-5530, E-mail: ykjeon@snu.ac.kr
© 2021 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Rare cases of lung adenocarcinoma (LUAD) with concomitant epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) translocation have been reported. However, their clonal and evolutional relationship remains unclear. We report a case of early-stage EGFR-mutated LUAD with a focal concomitant EGFR/ALK alteration. A 63-year-old male underwent lobectomy to remove a 1.9-cm-sized lung nodule, which was diagnosed with EGFR-mutated LUAD. ALK immunohistochemistry (IHC) showed focal positivity within the part of the tumor characterized by lepidic pattern, also confirmed by fluorescence in-situ hybridization (FISH). Targeted next-generation sequencing was performed separately on the ALK IHC/FISH-positive and -negative areas. EGFR L833V/L858R mutations were detected in both areas, whereas EML4 (echinoderm microtubule-associated protein-like 4)-ALK translocations was confirmed only in the ALK IHC/FISH-positive area, suggesting the divergence of an EGFR/ALK co-altered subclone from the original EGFR-mutant clone. Our study suggests that concurrent alterations of EGFR and ALK can arise via divergent tumor evolution, even in the relatively early phases of tumorigenesis.
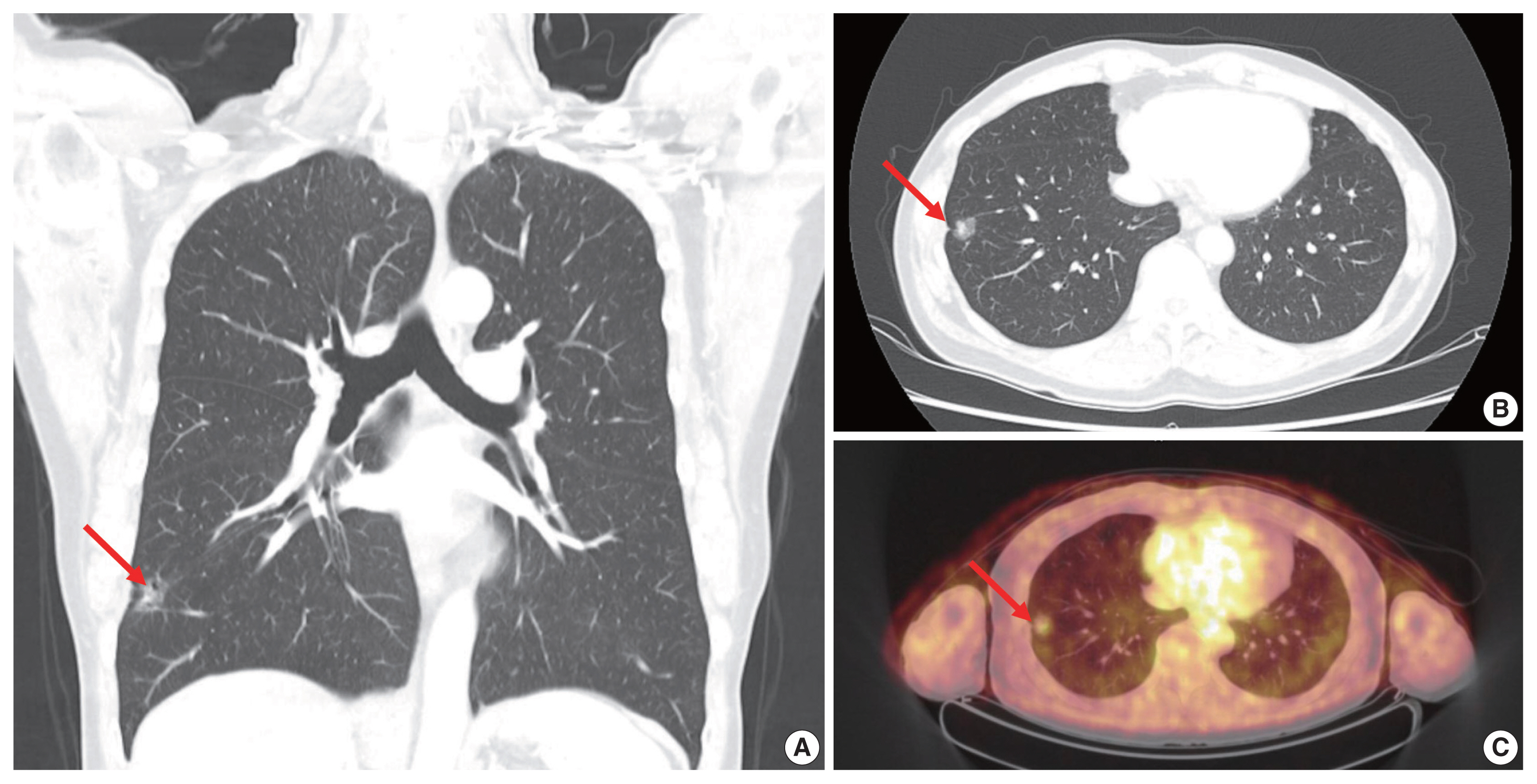
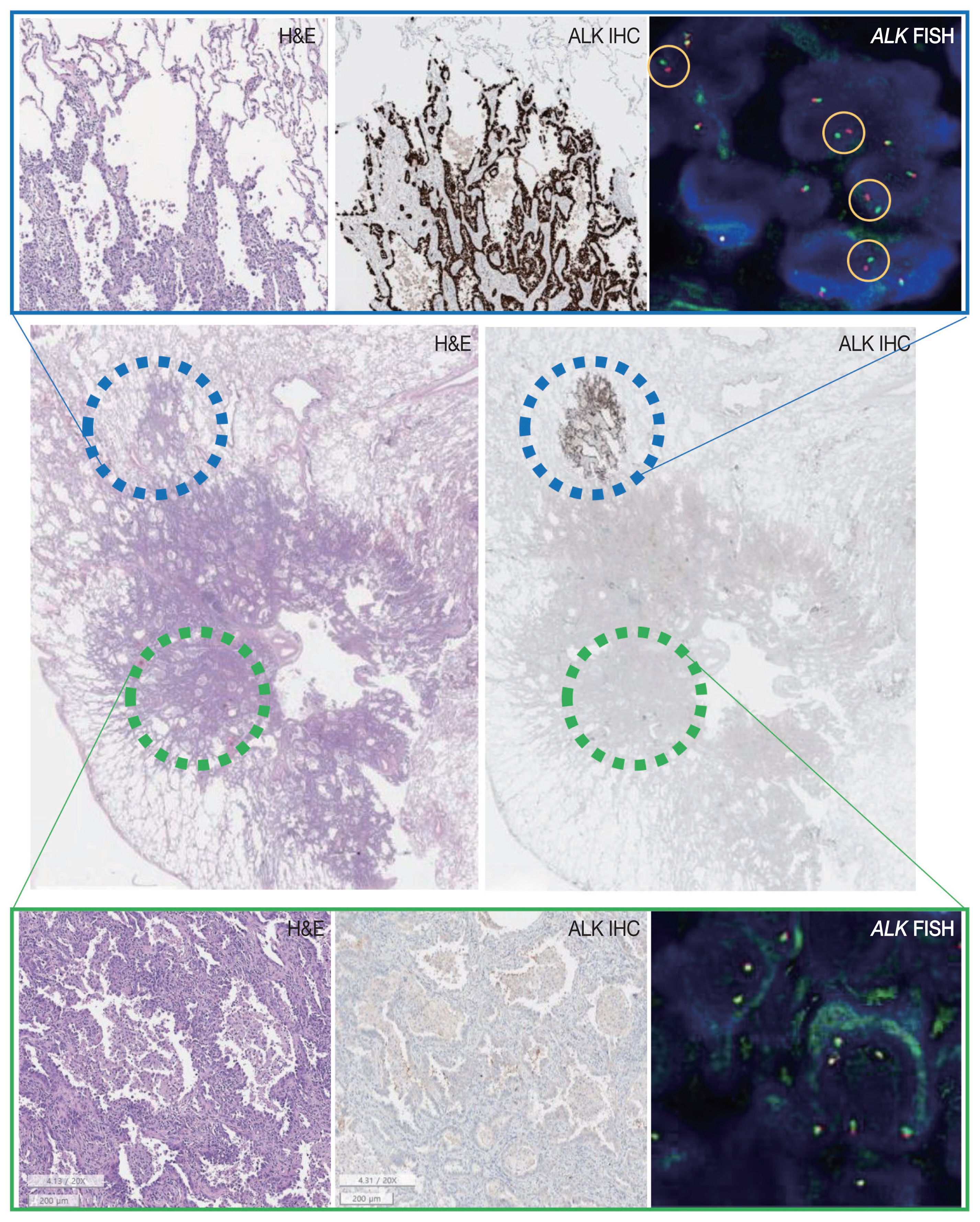
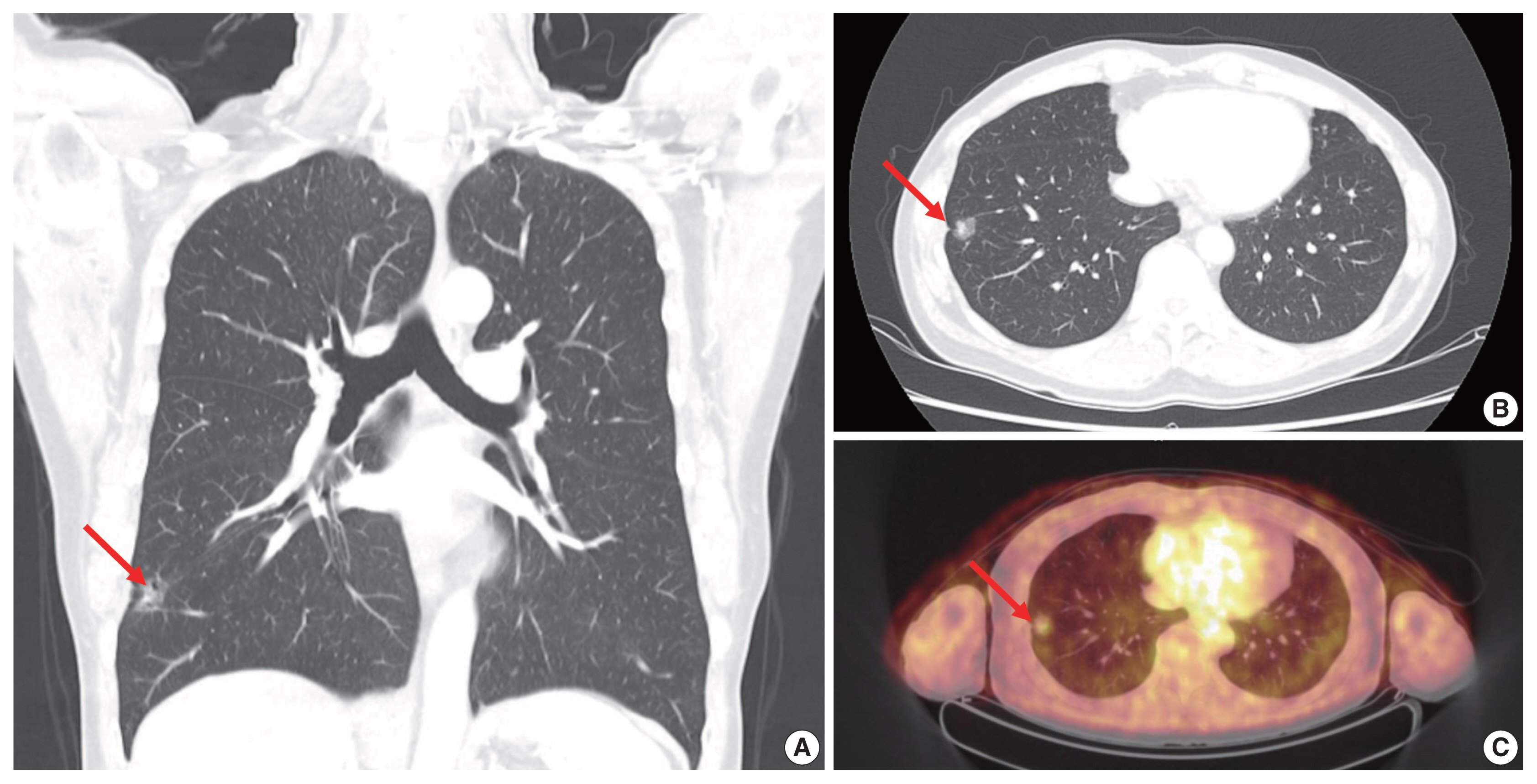
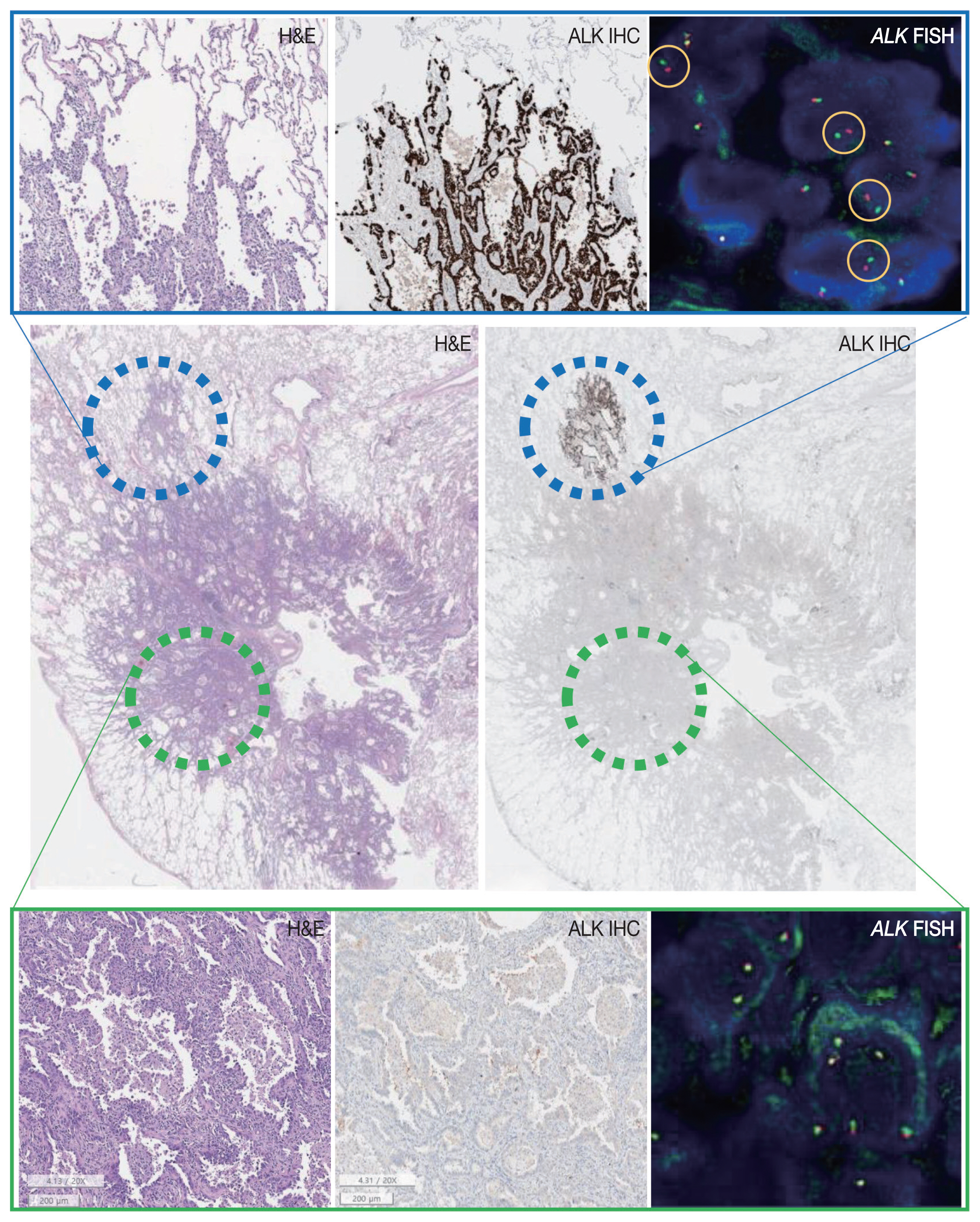
- A 63-year-old male patient who was a never-smoker presented with an incidentally found solitary pulmonary nodule. A pure ground-glass nodule (GGN) at the right lower lobe (RLL) had been detected 3 years previously, and a recent follow-up chest computed tomography (CT) exam had shown a GGN ~1.7 cm in diameter with a 0.5-cm solid portion (Fig. 1A, B). A positron emission tomography–CT fusion scan showed mild uptake by the GGN but no other hypermetabolic lesion (Fig. 1C). A lobectomy of the RLL was performed, and pathologic evaluation of the lesion confirmed a primary LUAD, 1.9×1.4×1.3 cm in size, with a predominantly acinar pattern but also associated lepidic and papillary patterns. There was no evidence of pleural invasion or nodal metastasis (pT1bN0, IA) (Fig. 2).
- Tests for EGFR mutation and ALK translocation were carried out as part of the routine molecular work-ups for non–small cell lung cancer (NSCLC). A missense mutation in EGFR exon 21 (L858R) was found by EGFR PANAMutyper (PANAGENE, Daejeon, Korea). ALK IHC (clone D5F3, Cell Signaling Technology, Danvers, MA, USA) showed focal positivity within the part of the tumor characterized by lepidic pattern (Fig. 2). Fluorescence in-situ hybridization (FISH) using a break-apart probe (Abbott Molecular Inc., Des Plaines, IL, USA) confirmed an ALK translocation in the ALK-positive area and its absence in the ALK-negative area of the tumor.
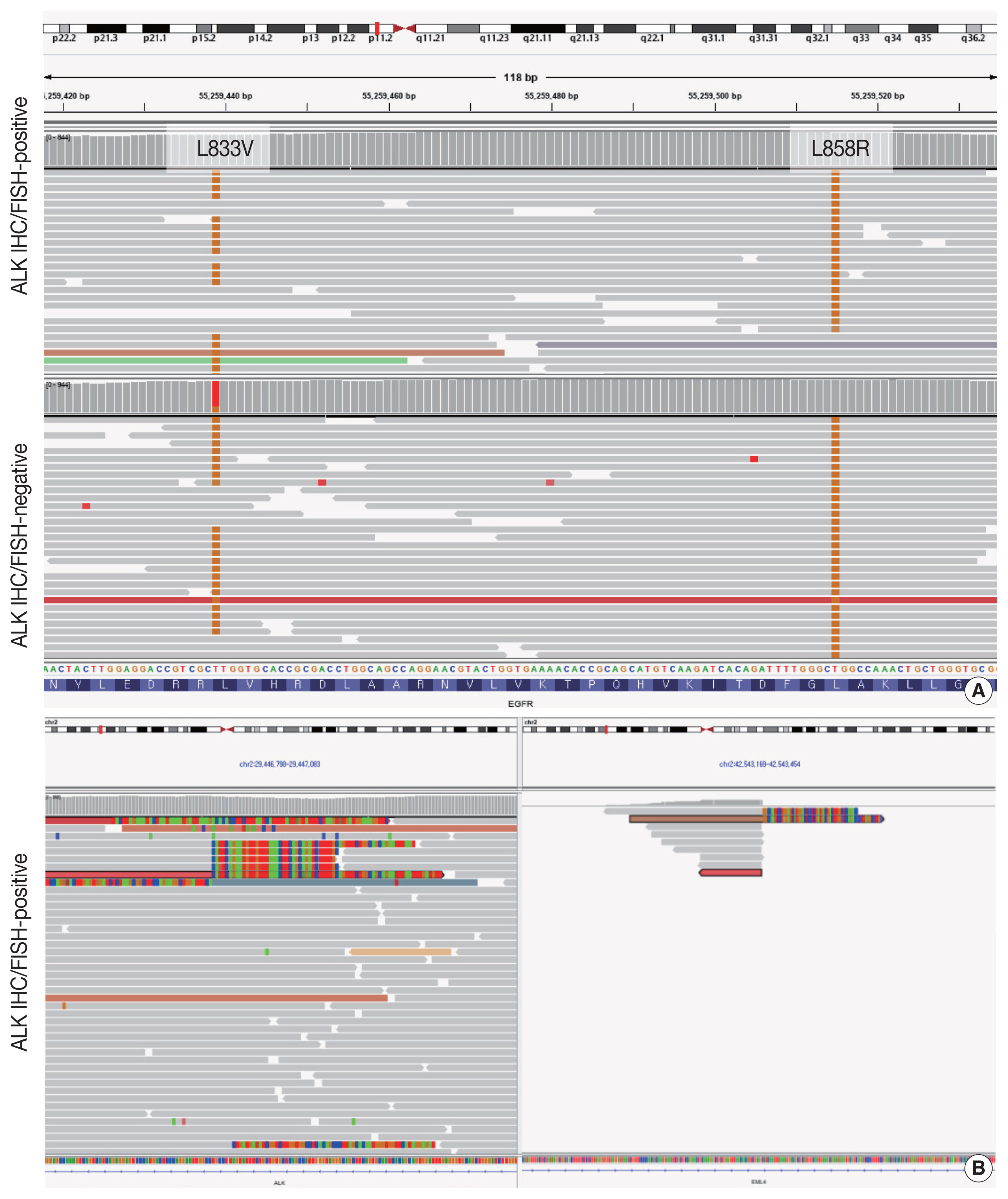
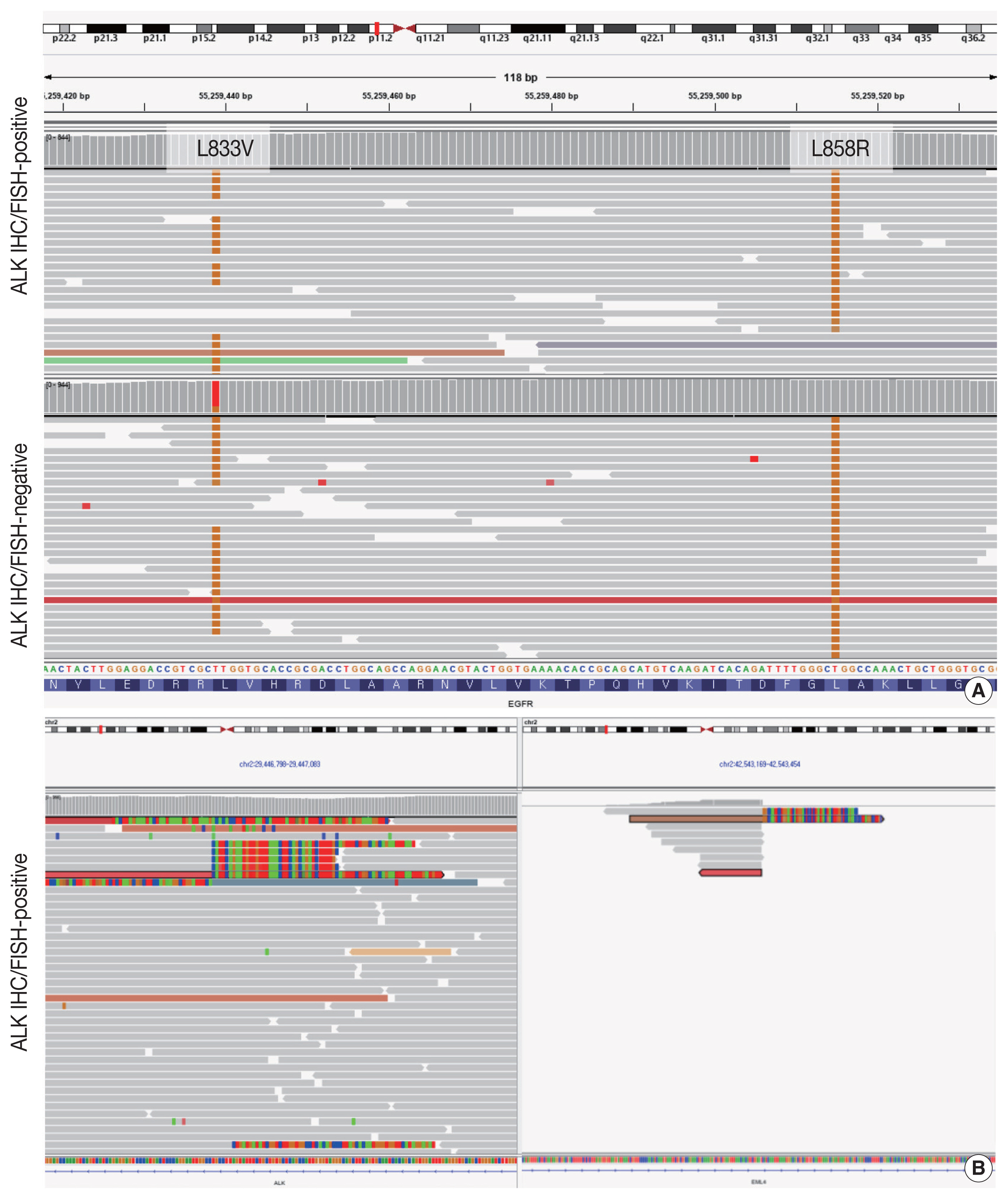
- Targeted next-generation sequencing (NGS) using Axen Cancer Panel 1 (Macrogen Inc., Seoul, Korea), comprising 88 cancer-related genes, was performed separately on the ALK IHC/FISH-positive and ALK IHC/FISH-negative tumor tissue. ALK IHC/ FISH-positive area was carefully microdissected from formalin-fixed, paraffin-embedded (FFPE) slide as shown in Fig 2, and ALK IHC/FISH-negative tumor tissue was obtained from a separate FFPE block with no ALK IHC-positive portions. The tissue from ALK IHC/FISH-negative area harbored EGFR L858R (variant allele frequency [VAF], 20.0%) and EGFR L833V (VAF, 19.5%) mutations (Fig. 3A). The two EGFR mutations were found in the same read, suggesting their presence in the same allele. Targeted sequencing of the ALK IHC/FISH-positive area revealed an EML4 (echinoderm microtubule-associated protein-like 4)-ALK fusion with breakpoints occurring at intron 18 of EML4 and intron 19 of ALK (Fig. 3B), as well as the same EGFR L858R and L833V mutations found in the ALK IHC/FISH-negative area, albeit at lower VAFs (5.9% and 3.4%, respectively) (Fig. 3A).
CASE REPORT
- Here we report a case of early-stage LUAD with concomitant EGFR and ALK alterations. In the mutated EGFR background of the tumor, a focal area with an additional ALK translocation was identified by IHC and FISH. NGS confirmed that the ALK-positive lesion harbored dual EGFR and ALK alterations and that identical EGFR mutations were shared by both ALK-positive and -negative portions of the tumor, suggesting the divergence of an EGFR/ALK co-altered subclone from the original EGFR-mutant clone.
- Both EGFR and ALK alterations are major driver mutations in LUAD, and was considered to be mutually exclusive [3]. However, rare cases with concomitant EGFR and ALK alterations have been reported [4–9]. The prevalence of concomitant EGFR mutation and ALK translocation among NSCLC patients was estimated to be 0.9% [6]. Although our case is not the first report of a LUAD carrying both EGFR and ALK alterations, its clinicopathological features were unique.
- In previous reports of LUAD with concomitant EGFR/ALK alterations, the tumors were often clinically advanced [4,5,7–10], whereas in our patient the tumor was stage IA LUAD. These findings suggest that co-alterations of EGFR and ALK can occur in the very early phase of tumorigenesis. Pathologic evaluation of the tumor revealed the lepidic growth pattern of the ALK-positive portion whereas the pattern in the ALK-negative portion was predominantly acinar. LUADs with concomitant EGFR/ ALK alterations frequently exhibited a solid, cribriform, or micropapillary patterns [7], while our case suggests that LUAD with co-altered EGFR/ALK can also include low-grade histologic patterns. These findings demonstrate that LUAD with concomitant EGFR/ALK alterations is not limited to a single morphology but may, instead, be characterized by a wide histologic spectrum.
- Despite previous reports of concomitant EGFR/ALK co-altered LUADs, the spatial and sequential contexts of the two alterations are poorly understood. Yang et al. [5] identified seven cases of LUAD with co-localized expression of mutant EGFR and ALK, determined in serial sections of the FFPE tumor samples. Their observations evidenced the intra-tumor and potential intracellular coexistence of EGFR and ALK alterations in certain LUADs. Our case also shows that EGFR and ALK can be co-altered in LUAD within the same tumor population, as verified by targeted sequencing. Moreover, the sequencing results provided additional insights, by revealing combined L858R and L833V mutations in the EGFR gene. This is an extremely rare finding, with only a single case identified among 783 NSCLCs analyzed [11]. Thus, it is highly unlikely that the EGFR L858R and L833V mutations in the ALK-positive and ALK-negative areas occurred independently, de novo; rather, a more plausible scenario is that the two-point mutations arose as a single first hit, followed by ALK translocation in a subclone stemming from the main tumor that subsequently followed a divergent evolution.
- The spatial distribution of the mutant EGFR and ALK fusion proteins in two advanced LUAD cases was previously analyzed using IHC, and the homogeneous co-localization of the EGFR/ALK alterations was determined in both [8]. This finding contrasts with the present case, in which the ALK translocation was likely to have been a sub-clonal event that gives rise to LUAD with co-altered EGFR/ALK. Further studies are needed to verify that, in tumorigenesis of LUAD with concomitant EGFR/ALK alterations, the two events were indeed consecutive.
- Nevertheless, the possibility of collision tumor cannot be completely ruled out; current extraction-based, bulk sequencing cannot verify that two EGFR mutations and ALK translocation exist in the very same tumor cell. However, we performed NGS after careful microdissection of the tumor area showing lepidic pattern, where virtually every tumor cells stained positive for ALK protein. This implies that the likelihood of the presence of EGFR-only-mutated tumor cell population within the micro-dissected area would be extremely low. Recent advances in single-cell sequencing technology would help deciphering the clonal events in single-cell resolution.
- There is currently no consensus regarding the treatment of LUAD with concomitant EGFR/ALK alterations, and conflicting results on the efficacy of EGFR tyrosine kinase inhibitors (TKI) versus ALK inhibitors as first-line treatment have been reported [4–9]. Won et al. [4] found that the clinical outcome of patients treated with ALK inhibitors was substantially better than that of patients treated with EGFR TKIs. However, based on their own multi-center retrospective analysis and review of published data, Schmid et al. [9] concluded that EGFR/ALK co-altered LUADs were more likely to be resistant to ALK inhibitors than to EGFR TKIs. Yang et al. [5] attempted to explain these conflicting results by considering the phosphorylation status of EGFR and ALK as potential predictive biomarkers of treatment response, but the number of patients analyzed was too small to draw definitive conclusions.
- In our patient, although kinase inhibitor treatment was not needed, as the tumor was small enough to be locally excised, it is reasonable to assume that the treatment response would have been better with an EGFR TKI than with an ALK inhibitor, because the ALK translocation was present only in a subclone of the tumor. Identification of the initial genetic alteration may therefore aid in determining the primary driver mutation in EGFR/ALK co-altered LUAD, even in advance-stage tumors, as it may, in turn, lead to optimal treatment selection.
- In summary, we report a case of early-stage, EGFR-mutated LUAD with a focal EGFR/ALK co-alteration. Our study suggests that concurrent mutations of EGFR and ALK can arise via divergent tumor evolution, even in the relatively early phases of tumorigenesis.
DISCUSSION
Ethics Statement
This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (No. H-1911-035-1077) and written informed consent from the patient was waived by IRB decision.
Author Contributions
Conceptualization: YKJ, DHC. Data curation: KCL, JK. Formal analysis: KCL, JK, YKJ. Investigation: KCL, JK. Visualization: KCL, JK. Writing—original draft: KCL, JK, YKJ. Writing—review & editing: YKJ, DHC. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
This study was funded by Basic Science Research Program (grant No.: NRF-2016R1D1A1B01015964) through the National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology (MEST).
- 1. Zheng M. Classification and pathology of lung cancer. Surg Oncol Clin N Am 2016; 25: 447-68. ArticlePubMed
- 2. Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med 2018; 142: 321-46. PubMed
- 3. Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res 2013; 19: 4273-81. PubMed
- 4. Won JK, Keam B, Koh J, et al. Concomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann Oncol 2015; 26: 348-54. ArticlePubMed
- 5. Yang JJ, Zhang XC, Su J, et al. Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res 2014; 20: 1383-92. ArticlePubMedPDF
- 6. Lee JK, Kim TM, Koh Y, et al. Differential sensitivities to tyrosine kinase inhibitors in NSCLC harboring EGFR mutation and ALK translocation. Lung Cancer 2012; 77: 460-3. ArticlePubMed
- 7. Lee T, Lee B, Choi YL, Han J, Ahn MJ, Um SW. Non-small cell lung cancer with concomitant EGFR, KRAS, and ALK mutation: clinicopathologic features of 12 cases. J Pathol Transl Med 2016; 50: 197-203. ArticlePubMedPMCPDF
- 8. Baldi L, Mengoli MC, Bisagni A, Banzi MC, Boni C, Rossi G. Concomitant EGFR mutation and ALK rearrangement in lung adeno-carcinoma is more frequent than expected: report of a case and review of the literature with demonstration of genes alteration into the same tumor cells. Lung Cancer 2014; 86: 291-5. ArticlePubMed
- 9. Schmid S, Gautschi O, Rothschild S, et al. Clinical outcome of ALK-positive non-small cell lung cancer (NSCLC) patients with de novo EGFR or KRAS co-mutations receiving tyrosine kinase inhibitors (TKIs). J Thorac Oncol 2017; 12: 681-8. ArticlePubMed
- 10. Fan J, Dai X, Wang Z, et al. Concomitant EGFR mutation and EML4-ALK rearrangement in lung adenocarcinoma is more frequent in multifocal lesions. Clin Lung Cancer 2019; 20: e517-30. ArticlePubMed
- 11. Hata A, Yoshioka H, Fujita S, et al. Complex mutations in the epidermal growth factor receptor gene in non-small cell lung cancer. J Thorac Oncol 2010; 5: 1524-8. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Machine learning-based characterization of a PANoptosis-associated model for enhancing prognosis and immunotherapy response in lung adenocarcinoma patients
Ziqiao Fu, Jia Zeng, Xiaomin Xiong, Weimin Zhong
Discover Oncology.2025;[Epub] CrossRef - Identification and validation of molecular subtype and prognostic signature for lung adenocarcinoma based on neutrophil extracellular traps
Yanhua Zuo, Guangyi Leng, Ping Leng
Pathology and Oncology Research.2023;[Epub] CrossRef - Machine Learning-Based Integration Develops a Macrophage-Related Index for Predicting Prognosis and Immunotherapy Response in Lung Adenocarcinoma
Zuwei Li, Minzhang Guo, Wanli Lin, Peiyuan Huang
Archives of Medical Research.2023; 54(7): 102897. CrossRef - Big data analysis identified a telomere-related signature predicting the prognosis and drug sensitivity in lung adenocarcinoma
Weiyi Zhang
Medicine.2023; 102(46): e35526. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure




 E-submission
E-submission