Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 55(5); 2021 > Article
-
Original Article
Proto-oncogene Pokemon in thyroid cancer: a potential promoter of tumorigenesis in papillary thyroid carcinoma -
Kyungseek Chang
 , Sung-Im Do
, Sung-Im Do , Kyungeun Kim
, Kyungeun Kim , Seoung Wan Chae
, Seoung Wan Chae , In-gu Do
, In-gu Do , Hyun Joo Lee
, Hyun Joo Lee , Dong Hoon Kim
, Dong Hoon Kim , Jin Hee Sohn
, Jin Hee Sohn
-
Journal of Pathology and Translational Medicine 2021;55(5):317-323.
DOI: https://doi.org/10.4132/jptm.2021.06.28
Published online: August 9, 2021
Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- Corresponding Author: Sung-Im Do, MD, PhD, Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, 29 Saemunan-ro, Jongno-gu, Seoul 03181, Korea, Tel: +82-2-2001-2389, Fax: +82-2-2001-2398, E-mail: sungim.do@samsung.com
© 2021 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Pokemon is an oncogenic transcription regulator that plays a critical role in cellular differentiation. Although it has been found to be overexpressed in several types of cancer involving different organs, its role in thyroid gland has yet to be reported. The objective of this study was to evaluate the expression of Pokemon in papillary thyroid carcinoma (PTC) based on clinicopathological parameters.
-
Methods
- Tissue microarray samples derived from patients with PTC or benign thyroid disease were used to evaluate Pokemon expression based on immunohistochemical analysis. Correlations of its expression with various clinicopathological parameters were then analyzed.
-
Results
- Pokemon expression was observed in 22.0% of thyroid follicular cells from the normal group, 44.0% from the group with benign thyroid diseases, and 92.1% from the group with PTC (p < .001). The intensity of Pokemon expression was markedly higher in the PTC group. Pokemon expression level and PTC tumor size showed an inverse correlation. T1a tumors showed strong expression levels of Pokemon. However, larger tumors showed weak expression (p = .006).
-
Conclusions
- Pokemon expression is associated with tumorigenesis of PTC, with expression showing an inverse correlation with PTC tumor size. This might be related to the negative regulation of aerobic glycolysis by Pokemon.
- Tissue specimens
- We selected 90 cases of PTC and 25 cases of benign thyroid diseases such as nodular hyperplasia, follicular adenoma, Hashimoto’s thyroiditis, and Graves’ disease from archival cases in Kangbuk Samsung Hospital (Seoul, Republic of Korea). Controls included 68 normal thyroid tissues identified from selected cases. Tissues resected by surgeons were examined by pathologists before fixation in 10% neutral-buffered formalin for 12–24 hours, followed by thorough macroscopic evaluation and sectioning. After automatic tissue processing, sections were embedded in paraffin blocks. Four-micrometer-thick sections were cut from each formalin-fixed, paraffin-embedded (FFPE) tissue block using a rotary microtome, stained with hematoxylin and eosin, covered with a glass coverslip, followed by analysis and diagnosis by two board-certified pathologists. Clinical and pathological information were obtained from pathology reports and electrical medical information systems. Information collected included age, sex, multifocality, the greatest dimension of the tumor, presence of lymphovascular invasion, extrathyroidal extension, lymph node metastasis, number of metastatic lymph nodes, BRAF mutational status, and presence of background thyroiditis. One out of these 90 PTC patients was excluded from statistical analysis due to insufficient data (Table 1).
- Tissue microarray construction
- Tissue microarray (TMA) blocks were constructed in following orders. Briefly, all hematoxylin and eosin-stained slides were reviewed and the two most representative tumor areas were marked on the corresponding FFPE tissue blocks. Two 2-mm-diameter tissue cores were obtained from each block and manually arrayed into recipient TMA blocks. Only one 2-mm-diameter tissue cores were obtained from blocks with largest tumor size less than 5 mm. The assembly was held in an X-Y position with a 1-mm increment between individual cores. Modified biopsy needle was used to bore holes in a recipient block with defined array cores and to transfer the cores into the recipient block. The percentage of tumor volume in each core was > 70%. Two TMA blocks were prepared for each case.
- Immunohistochemistry
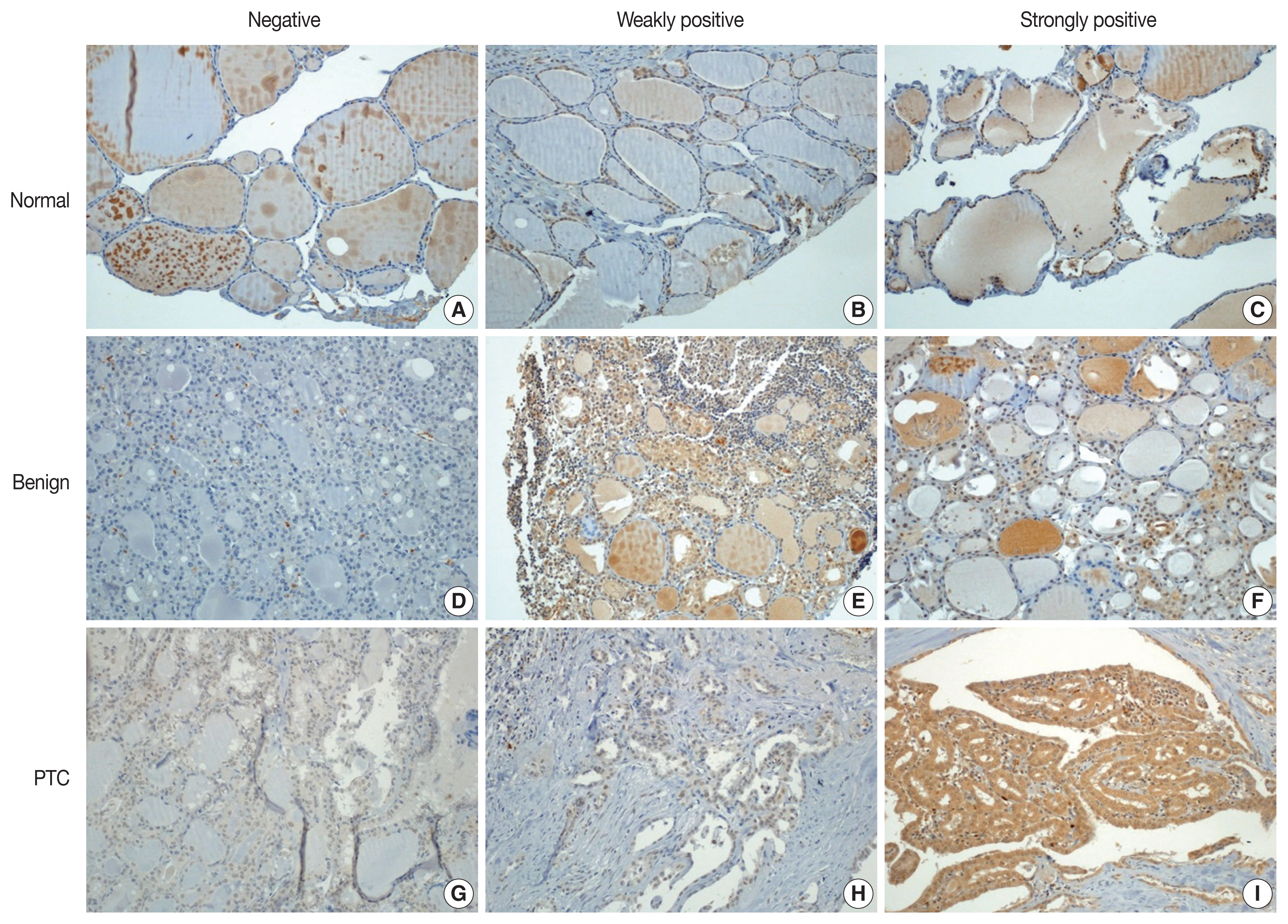
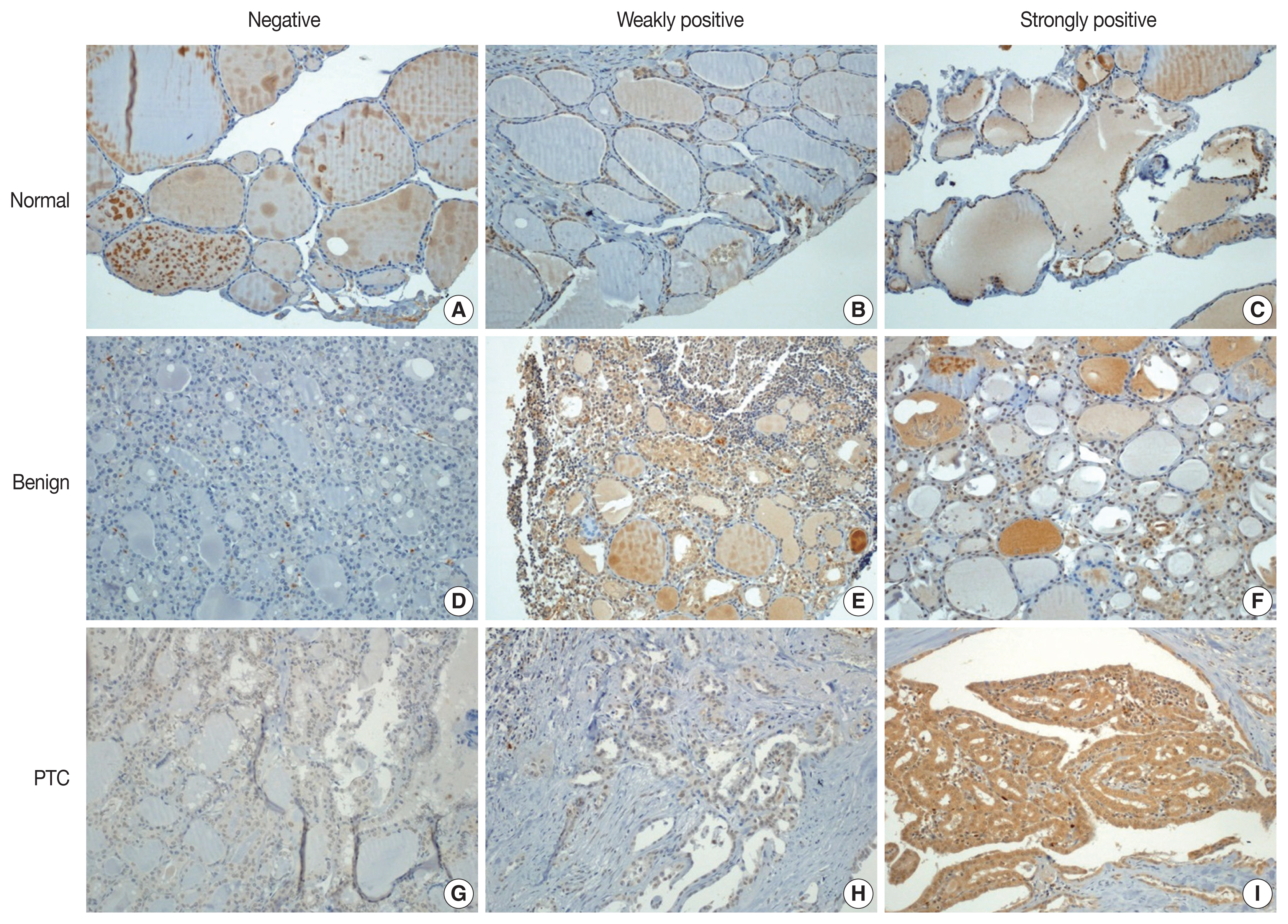
- Immunohistochemical staining was performed using the Bond Polymer Refine Detection kit (Leica Biosystems, Newcastle upon Tyne, UK) in the Bond-Max automatic immunostainer (Leica Biosystems). ZBTB7A/Pokemon antibody (1:200, clone, Novus Biologicals, Littleton, CO, USA) was used as the primary antibody. Immunohistochemical slides were independently evaluated by two pathologists (SID and SWC). Discrepant cases were reviewed. Pokemon expression was interpreted as positive if the cell showed positivity in either the nucleus or the cytoplasm. The intensity of expression was defined as negative, weakly positive, or strongly positive (Fig. 1). Immunohistochemical expression (× 200) of Pokemon in thyroid tissues from normal (Fig. 1A–C), benign thyroid disease (Fig. 1D–F), and PTC (Fig. 1G–I) groups. Pokemon expression intensity is interpreted as negative (Fig. 1A, D, G), weakly positive (Fig. 1B, E, H), or strongly positive (Fig. 1C, F, I). It was determined using TMA cores of those with PTCs (n = 89) or benign thyroid diseases (n = 25). Pokemon expression intensity of normal thyroid tissue was determined in adjacent normal follicular cells where present, if the TMA core carried an identifiable portion of normal thyroid tissue (n = 68).
- Detection of BRAF mutation
- To detect BRAF V600E mutation, nucleic acids were isolated from FFPE tissues using a DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany). Isolated nucleic acids were mixed with a polymerase chain reaction (PCR) master mix using a Seeplex BRAF V600E ACE Detection Kit (Seegene, Seoul, Republic of Korea). These mixtures were then immediately transferred to a preheated thermal cycler for 15 minutes. PCR was carried out in a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA). The cycling amplification program consisted of 35 cycles of denaturation at 94°C for 30 seconds, annealing at 63°C for 30 seconds, and extension at 72°C for 1 minute. Amplified PCR products were loaded onto a 2% agarose gel and visualized with a SafeView Nucleic Acid Stain (Applied Biological Materials, Richmond, BC, Canada). The BRAF V600E mutation was detected using a Gel Documentation System (Bio-Rad Laboratories, Hercules, CA, USA).
- Statistical analysis
- Pearson’s Chi-square test was performed to compare Pokemon expression intensities in thyroid tissues of PTC, benign thyroid disease, and normal groups. It was also used to analyze associations between Pokemon expression intensity and clinicopathological characteristics. Clinical significance was determined using Kruskal-Wallis test (nonparametric method), linear-by-linear association test, and Pearson’s chi-square test. All statistical analyses were performed using SPSS for windows ver. 24.0 (IBM Corp., Armonk, NY, USA). Statistical significance was considered when p-value was less than .05.
- Pathologic TNM staging
- Pathologic TNM staging was referred to American Joint Committee on Cancer 8th edition of the Cancer Staging Manual for PTC. Pathologic T categories included the following: T1a, tumor ≤ 1 cm in the greatest dimension limited to the thyroid; T1b, tumor > 1 cm but ≤ 2 cm in the greatest dimension, limited to the thyroid; T2, tumor > 2 cm, but ≤ 4 cm in the greatest dimension, limited to thyroid; T3a, tumor > 4 cm limited to the thyroid; T3b, gross extrathyroidal extension invading only strap muscles (sternohyoid, sternothyroid, thyrohyoid, or omohyoid muscles) from a tumor of any size; and T4, gross extrathyroidal extension beyond strap muscles. Pathologic N categories included the following: N0, no evidence of locoregional lymph node metastasis; N0a, one or more cytologically or histologically confirmed benign lymph nodes; N1, metastasis to regional nodes; N1a, metastasis to level VI or VII (pretracheal, paratracheal, or prelaryngeal/Delphian, or upper mediastinal) lymph nodes; and N1b, metastasis to unilateral, bilateral, or contralateral lateral neck lymph nodes (levels I, II, III, IV, or V) or retropharyngeal lymph nodes.
MATERIALS AND METHODS
- With collected information shown in Table 1, Pokemon expression intensities in thyroid tissues of normal, benign thyroid disease, and PTC groups were determined (Table 2). Statistically significant (p < .001) differences in Pokemon expression positivity were observed between normal thyroid tissues (22.0%) and thyroid tissues from patients with benign thyroid disease (44.0%) or PTC (92.1%). Results of Pearson’s chi-square test for Pokemon expression intensity in normal, benign, and PTC groups are shown in Supplementary Tables S1–S3. The difference in Pokemon expression intensity between normal and benign thyroid disease groups was statistically significant (p = .035). The difference in Pokemon expression intensity between normal and PTC groups was also statistically significant (p < .001). Similarly, the difference in Pokemon expression intensity between benign thyroid disease and PTC was significant (p < .001).
- Pokemon expression intensity in PTC showed significant correlations with tumor size and corresponding pathologic T category groups (Table 3). Reduced mean tumor size was significantly associated with stronger Pokemon expression intensity (p = .002). Tumor size and TNM stage were also significantly associated with Pokemon expression intensity. When the tumor size was divided into three groups by a separation point at 0.5 cm and 1 cm, a tumor size less than 0.5 cm was associated with Pokemon expression intensity stronger than 66%. However, increased tumor size was associated with less strong or no Pokemon expression intensity (p = .028). Tumor size categorized by conventional TNM staging, excluding T3b and T4 groups for gross extrathyroidal extension to and beyond strap muscles, showed evident negative concordance with Pokemon expression intensity (p = .010). T1a group was associated with expression greater than 66%, whereas groups T1b, T2, and T3a were collectively associated with no or weak expression (p = .006). Other clinicopathological parameters did not show any significant association with Pokemon expression intensity.
RESULTS
- Pokemon can act as a genuine proto-oncogene in vivo [6]. It can repress the expression of tumor suppressor protein ARF [6]. ARF is a negative regulator of the E3 ubiquitin-protein ligase MDM2 which participates in the degradation of p53 via ubiquitin-dependent degradation by the proteasome. Reduced ARF levels can stimulate cell proliferation by decreasing the stability and activity of p53 via MDM2 [6]. Pokemon expression in malignancy is generally related to poor cancer outcome in various organs. It promotes tumorigenesis, acting as a pro-oncogene by repressing or enhancing the expression of genes involved in apoptosis, cell proliferation, and differentiation [18]. For example, Pokemon expression can promote breast cancer progression by up-regulating survivin expression [11]. In the nasopharynx, high Pokemon protein expression is closely associated with non-keratinizing nasopharyngeal carcinoma [19]. In the liver, suppression of Pokemon can impair the invasion of hepatocellular carcinoma (HCC) cells [20]. However, to the best of our knowledge, contribution of Pokemon to thyroid malignancy has yet to be reported.
- Pokemon expression intensity showed a statistically significant (p = .035) difference between normal and benign thyroid disease groups in the present study. Although Pokemon expression was positive in 44% of patients with benign thyroid disease regardless of its expression intensity, it was more significantly (p < .001) associated with PTC than with benign thyroid disease (Table 2). Pokemon overexpression has also been observed in several malignancies, such as T and B cell lymphomas in transgenic mice model via direct binding of Pokemon and the tumor suppressor gene ARF [6], prostate carcinoma via increased Pokemon expression stimulated by epidermal growth factor [16], ovarian carcinomas of all four major histological types (serous, endometrioid, clear cell, and mucinous) via elevated RNA transcription [21], breast carcinoma by up-regulating survivin, a member of the inhibitor of apoptosis proteins [11]. However, the mechanism which Pokemon overexpression leads to tumorigenesis of PTC remains to be investigated.
- When Pokemon expression intensity was compared according to mean PTC tumor size, stronger expression levels were observed in smaller tumors (0.9 ± 0.4 cm), whereas larger tumors (2.1 ± 1.7 cm) showed absent or weak expression. These inverse correlations were evident according to the T category of the tumor when T1a group was compared with the combined group of T1b, T2, and T3a (p = .006) (Table 3). These results were contrary to results of several previous studies of tumors involving various organs, where Pokemon expression was positively correlated with tumor size or T category of breast carcinomas [11], HCC [22], or non-small cell lung cancer [23]. These combined results of particularly strong Pokemon expression in small-sized tumors suggest that Pokemon might play an important role in malignant transformation of thyroid cells and in the early stage of PTC tumor formation, while it might have a diminished role as the tumor grows in size.
- Such inverse correlation between Pokemon expression and tumor size has also been observed in oropharyngeal squamous cell carcinoma (OSCC) which shows a higher relative expression in smaller tumors (< 2 cm) and lower to no expression in larger tumors (≥ 4 cm) [18]. Although the distribution of Pokemon expression is lower in OSCC than in normal oral mucosa, Sartini et al. [18] have suggested that the downregulation of Pokemon might be related to tumor progression. Presumably, our results suggest such downregulation.
- Aerobic glycolysis (the Warburg effect) is a hallmark of human cancer. It plays a crucial role in tumor growth [24]. Receptor tyrosine kinase/PI3K/AKT signaling and MYC act as pro-growth signaling factors that can upregulate aerobic glycolysis. However, Liu et al. [25] have reported that Pokemon, a proto-oncogene, unexpectedly can act as a tumor suppressor by directly binding to the promoter and repressing the transcription of genes responsible for glycolysis metabolism, such as GLUT3, PFKP, and PKM. As a result, a significant decrease in Pokemon copy number variation was observed in several types of human carcinoma in the late stage (N1, N2, N3, M1, stage IV) than in early stage (N0, M0, stage I), including esophageal carcinoma, bladder urothelial carcinoma, colorectal adenocarcinoma, lung squamous cell carcinoma, cutaneous melanoma, and low-grade glioma [25]. We believe that the mechanism underlying the inverse correlation between Pokemon expression and tumor size of PTC might be related to the unexpected negative regulation of aerobic glycolysis by Pokemon.
- Regardless of its contrasting behavior in PTC, Pokemon itself is associated with malignant transformation of tumors in various organs. High levels of Pokemon expression in patients with early stages of PTC may potentially be used for screening and identifying malignancies involving other organs.
- Our study was performed at a single institution with PTC cases retrieved from a two-year period, which invariably resulted in limitations involving the follow-up period of patients with PTC and their survival rates. A multi-institutional study with ethnic and racial diversity is needed to further investigate the effect of Pokemon on PTC. Larger patient groups might be needed to elucidate the effect of Pokemon on parameters showing statistical insignificance in the present study. A long-term survival study may provide further insight into the effect of Pokemon on long-term outcomes of PTC, one of the most common malignancies worldwide.
- In summary, Pokemon overexpression (when compared to normal thyroid tissues) is associated with formation of PTC and benign thyroid diseases. A smaller PTC tumor shows stronger Pokemon overexpression, which supports the hypothesis that Pokemon overexpression can lead to tumorigenesis of PTC. However, a larger PTC tumor may be associated with Pokemon downregulation, which may be related to tumor progression. Targeting Pokemon in early stages could be effective in suppressing PTC formation and potentially facilitate treatment.
DISCUSSION
Supplementary Information
Supplementary Information
The Data Supplement is available with this article at https://doi.org/10.4132/jptm.2021.06.28.
Ethics Statement
All procedures performed in this study were approved by the Institutional Review Board at Kangbuk Samsung Hospital (IRB No. 2016-08-006-001) in accordance with the Helsinki Declaration as revised in 2013. The requirement of informed consent was waived due to its retrospective nature.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: SID, SWC. Data curation: KC, SID, SWC. Formal analysis: KC, SID, SWC. Investigation: KC, SID, SWC. Methodology: SID, SWC. Project administration: SID, KK, SWC. Resources: SID, SWC. Supervision: SID, KK, SWC, IGD, HJL, DHK, JHS. Validation: SID, SWC. Visualization: KC, SID, KK. Writing—original draft: KC, SID, KK. Writing—review & editing: KC, SID, KK. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
Values are presented as mean ± SD or number (%).
Statistical analysis of patient age and mean tumor size was performed using Kruskal-Wallis test. Statistical analysis of other clinicopathological parameters was performed using Pearson’s chi-square test. T category was referred to American Joint Committee on Cancer 8th edition of the Cancer Staging Manual for papillary thyroid carcinoma.
PTC, papillary thyroid carcinoma.
- 1. Al-Brahim N, Asa SL. Papillary thyroid carcinoma: an overview. Arch Pathol Lab Med 2006; 130: 1057-62. ArticlePubMedPDF
- 2. Ito Y, Miyauchi A, Kihara M, Fukushima M, Higashiyama T, Miya A. Overall survival of papillary thyroid carcinoma patients: a single-institution long-term follow-up of 5897 patients. World J Surg 2018; 42: 615-22. ArticlePubMedPMCPDF
- 3. Soares P, Celestino R, Melo M, Fonseca E, Sobrinho-Simoes M. Prognostic biomarkers in thyroid cancer. Virchows Arch 2014; 464: 333-46. ArticlePubMedPDF
- 4. Mercante G, Frasoldati A, Pedroni C, et al. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid 2009; 19: 707-16. ArticlePubMed
- 5. D’Cruz AK, Vaish R, Vaidya A, et al. Molecular markers in well-differentiated thyroid cancer. Eur Arch Otorhinolaryngol 2018; 275: 1375-84. ArticlePubMedPDF
- 6. Maeda T, Hobbs RM, Merghoub T, et al. Role of the proto-oncogene Pokemon in cellular transformation and ARF repression. Nature 2005; 433: 278-85. ArticlePubMedPDF
- 7. Mao A, Chen M, Qin Q, et al. ZBTB7A promotes migration, invasion and metastasis of human breast cancer cells through NF-kappaB-induced epithelial-mesenchymal transition in vitro and in vivo. J Biochem 2019; 166: 485-93. PubMed
- 8. Jeon BN, Yoo JY, Choi WI, Lee CE, Yoon HG, Hur MW. Proto-oncogene FBI-1 (Pokemon/ZBTB7A) represses transcription of the tumor suppressor Rb gene via binding competition with Sp1 and recruitment of co-repressors. J Biol Chem 2008; 283: 33199-210. ArticlePubMedPMC
- 9. Choi WI, Jeon BN, Yun CO, et al. Proto-oncogene FBI-1 represses transcription of p21CIP1 by inhibition of transcription activation by p53 and Sp1. J Biol Chem 2009; 284: 12633-44. ArticlePubMedPMC
- 10. Zhang NN, Sun QS, Chen Z, Liu F, Jiang YY. Homeostatic regulatory role of Pokemon in NF-kappaB signaling: stimulating both p65 and IkappaBalpha expression in human hepatocellular carcinoma cells. Mol Cell Biochem 2013; 372: 57-64. ArticlePubMedPDF
- 11. Zu X, Ma J, Liu H, et al. Pro-oncogene Pokemon promotes breast cancer progression by upregulating survivin expression. Breast Cancer Res 2011; 13: R26.ArticlePubMedPMCPDF
- 12. Yang X, Zu X, Tang J, et al. Zbtb7 suppresses the expression of CDK2 and E2F4 in liver cancer cells: implications for the role of Zbtb7 in cell cycle regulation. Mol Med Rep 2012; 5: 1475-80. PubMed
- 13. Lee DK, Suh D, Edenberg HJ, Hur MW. POZ domain transcription factor, FBI-1, represses transcription of ADH5/FDH by interacting with the zinc finger and interfering with DNA binding activity of Sp1. J Biol Chem 2002; 277: 26761-8. ArticlePubMed
- 14. Choi WI, Jeon BN, Park H, et al. Proto-oncogene FBI-1 (Pokemon) and SREBP-1 synergistically activate transcription of fatty-acid synthase gene (FASN). J Biol Chem 2008; 283: 29341-54. ArticlePubMedPMC
- 15. Aggarwal A, Hunter WJ 3rd, Aggarwal H, et al. Expression of leukemia/lymphoma-related factor (LRF/POKEMON) in human breast carcinoma and other cancers. Exp Mol Pathol 2010; 89: 140-8. ArticlePubMedPMC
- 16. Aggarwal H, Aggarwal A, Agrawal DK. Epidermal growth factor increases LRF/Pokemon expression in human prostate cancer cells. Exp Mol Pathol 2011; 91: 496-501. ArticlePubMedPMC
- 17. Hong X, Hong XY, Li T, He CY. Pokemon and MEF2D co-operationally promote invasion of hepatocellular carcinoma. Tumour Biol 2015; 36: 9885-93. ArticlePubMedPDF
- 18. Sartini D, Lo Muzio L, Morganti S, et al. Pokemon proto-oncogene in oral cancer: potential role in the early phase of tumorigenesis. Oral Dis 2015; 21: 462-9. ArticlePubMed
- 19. Jiao W, Liu F, Tang FZ, et al. Expression of the Pokemon proto-oncogene in nasopharyngeal carcinoma cell lines and tissues. Asian Pac J Cancer Prev 2013; 14: 6315-9. ArticlePubMed
- 20. Kong J, Liu X, Li X, et al. Pokemon promotes the invasiveness of hepatocellular carcinoma by enhancing MEF2D transcription. Hepatol Int 2016; 10: 493-500. ArticlePubMedPDF
- 21. Jiang L, Siu MK, Wong OG, et al. Overexpression of proto-oncogene FBI-1 activates membrane type 1-matrix metalloproteinase in association with adverse outcome in ovarian cancers. Mol Cancer 2010; 9: 318.ArticlePubMedPMCPDF
- 22. Zhang QL, Tian DA, Xu XJ. Depletion of Pokemon gene inhibits hepatocellular carcinoma cell growth through inhibition of H-ras. Onkologie 2011; 34: 526-31. ArticlePubMed
- 23. Apostolopoulou K, Pateras IS, Evangelou K, et al. Gene amplification is a relatively frequent event leading to ZBTB7A (Pokemon) overexpression in non-small cell lung cancer. J Pathol 2007; 213: 294-302. ArticlePubMed
- 24. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029-33. ArticlePubMedPMC
- 25. Liu XS, Haines JE, Mehanna EK, et al. ZBTB7A acts as a tumor suppressor through the transcriptional repression of glycolysis. Genes Dev 2014; 28: 1917-28. ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Systems biology approach delineates critical pathways associated with papillary thyroid cancer: a multi-omics data analysis
Febby Payva, Santhy K. S., Remya James, Amrisa Pavithra E, Venketesh Sivaramakrishnan
Thyroid Research.2025;[Epub] CrossRef - Understanding the dysregulation of PURPL, a novel long intergenic noncoding RNA, in thyroid cancer progression
Mina Kazemzadeh, Reza Safaralizadeh, Amir Ali Mokhtarzadeh, Mohammad Ali Hosseinpour Feizi
Human Gene.2025; 46: 201499. CrossRef - ZBTB7A as a therapeutic target for cancer
Ying Zhou, Xisha Chen, Xuyu Zu
Biochemical and Biophysical Research Communications.2024; 736: 150888. CrossRef - Knockdown of FBI-1 Inhibits the Warburg Effect and Enhances the Sensitivity of Hepatocellular Carcinoma Cells to Molecular Targeted Agents via miR-3692/HIF-1α
Juan Liu, Chao Yang, Xiao-Mei Huang, Pan-Pan Lv, Ya-Kun Yang, Jin-Na Zhao, Si-Yuan Zhao, Wan-Jun Sun
Frontiers in Oncology.2021;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

Fig. 1
| Value (n = 89) | |
|---|---|
| Age (yr) | 43.41 ± 12.14 |
| Sex | |
| Female | 61 (68.5) |
| Male | 28 (31.5) |
| Tumor size (cm) | 1.08 ± 0.94 |
| ≤ 1 | 61 (68.5) |
| > 1 | 28 (31.5) |
| Lymph node metastasis | |
| 0 (absent) | 36 (40.4) |
| ≥ 1 (present) | 53 (59.6) |
| Lymphovascular invasion | |
| Absent | 87 (97.8) |
| Present | 2 (2.2) |
| Extrathyroidal invasion | |
| Absent | 40 (44.9) |
| Present | 49 (55.1) |
| No. of tumors | |
| 1 (single) | 68 (76.4) |
| ≥ 2 (multiple) | 21 (23.6) |
| BRAF V600E mutation | |
| Wild type | 8 (8.9) |
| Mutant | 49 (55.1) |
| Not obtained | 32 (36.0) |
| Background thyroiditis | |
| None | 61 (68.5) |
| Thyroiditis | 28 (31.5) |
| Pokemon expression intensity | Normal (n = 68) | Benign (n = 25) | PTC (n = 89) | p-value |
|---|---|---|---|---|
| Negative | 53 (78.0) | 14 (56.0) | 7 (7.9) | < .001 |
| Weakly positive | 13 (19.1) | 7 (28.0) | 30 (33.7) | |
| Strongly positive | 2 (2.9) | 4 (16.0) | 52 (58.4) |
| Variable | Pokemon expression intensity | p-value | ||
|---|---|---|---|---|
|
| ||||
| Negative (n = 7) | Weak expression (n = 30) | Strong expression (n = 52) | ||
| Age (yr) | 39.0 ± 9.3 | 43.4 ± 12.8 | 43.5 ± 11.7 | .678 |
| Sex | .793 | |||
| Female | 4 (57.1) | 21 (70.0) | 36 (69.2) | |
| Male | 3 (42.9) | 9 (30.0) | 16 (30.8) | |
| Mean tumor size (cm) | 2.1 ± 1.7 | 1.0 ± 0.8 | 0.9 ± 0.4 | .018 |
| Tumor size (cm) | .028 | |||
| ≤ 0.5 | 0 | 8 (26.7) | 11 (21.2) | |
| > 0.5 and ≤ 1 | 1 (14.3) | 14 (46.7) | 27 (51.9) | |
| > 1 | 6 (85.7) | 8 (26.7) | 14 (26.9) | |
| T category by group | .006 | |||
| T1a | 1 (14.3) | 22 (73.3) | 38 (73.1) | |
| T1b, T2, T3a | 6 (85.7) | 8 (26.7) | 14 (26.9) | |
| T category | .010 | |||
| T1a | 1 (14.3) | 22 (73.3) | 38 (73.1) | |
| T1b | 4 (57.1) | 7 (23.3) | 13 (25.0) | |
| T2 | 1 (14.3) | 0 | 1 (1.9) | |
| T3a | 1 (14.3) | 1 (3.3) | 0 | |
| N category | .306 | |||
| N0 | 2 (28.6) | 10 (33.3) | 24 (46.2) | |
| N1a | 4 (57.1) | 15 (50.0) | 26 (50.0) | |
| N1b | 1 (14.3) | 5 (16.7) | 2 (3.8) | |
| Lymphovascular invasion | .134 | |||
| Negative | 7 (100) | 28 (93.3) | 52 (100) | |
| Positive | 0 | 2 (6.7) | 0 | |
| Extrathyroidal invasion | .234 | |||
| Negative | 1 (14.3) | 14 (46.7) | 25 (48.1) | |
| Positive | 6 (85.7) | 16 (53.3) | 27 (51.9) | |
| Number of tumors | .647 | |||
| Single | 6 (85.7) | 24 (80.0) | 38 (73.1) | |
| Multiple | 1 (14.3) | 6 (20.0) | 14 (26.9) | |
| Background disease | .594 | |||
| None | 6 (85.7) | 20 (66.7) | 35 (67.3) | |
| Thyroiditis | 1 (14.3) | 10 (33.3) | 17 (32.7) | |
| BRAF mutation | .296 | |||
| Wild type | 0 | 1 (5.6) | 7 (19.4) | |
| Mutant | 3 (100) | 17 (94.4) | 29 (80.6) | |
Values are presented as mean ± SD or number (%). PTC, papillary thyroid carcinoma.
Values are presented as number (%). Statistical analysis was performed using linear-by-linear association test. PTC, papillary thyroid carcinoma.
Values are presented as mean ± SD or number (%). Statistical analysis of patient age and mean tumor size was performed using Kruskal-Wallis test. Statistical analysis of other clinicopathological parameters was performed using Pearson’s chi-square test. T category was referred to American Joint Committee on Cancer 8th edition of the Cancer Staging Manual for papillary thyroid carcinoma. PTC, papillary thyroid carcinoma.

 E-submission
E-submission