Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 56(5); 2022 > Article
-
Original Article
Inflammation and tissue remodeling contribute to fibrogenesis in stricturing Crohn’s disease: image processing and analysis study -
Mustafa Erdem Arslan1
 , Rupinder Brar1
, Rupinder Brar1 , Lianna Goetz2
, Lianna Goetz2 , Dipti Karamchandani2,3
, Dipti Karamchandani2,3 , Michael W. Mikula4
, Michael W. Mikula4 , Kyle Hodge5
, Kyle Hodge5 , Hua Li1
, Hua Li1 , Sangtae Ahn6
, Sangtae Ahn6 , Hwajeong Lee1
, Hwajeong Lee1
-
Journal of Pathology and Translational Medicine 2022;56(5):239-248.
DOI: https://doi.org/10.4132/jptm.2022.05.18
Published online: July 4, 2022
1Department of Pathology, Albany Medical Center, Albany, NY, USA
2Division of Anatomic Pathology, Department of Pathology, Penn State Health Milton S. Hershey Medical Center, Hershey, PA, USA
3Division of Pathology, University of Texas Southwestern Medical Center, Dallas, TX, USA
4Department of Pathology, Johns Hopkins Hospital, Baltimore, MD, USA
5Albany Medical College, Albany, NY, USA
6GE Global Research, Niskayuna, NY, USA
- Corresponding Author: Hwajeong Lee, MD, Department of Pathology, Albany Medical Center, 47 New Scotland Ave., MC81, Albany, NY 12208, USA Tel: +1-518-262-6254, Fax: +1-518-262-3663, E-mail: LeeH5@amc.edu
© 2022 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Inflammation and structural remodeling may contribute to fibrogenesis in Crohn’s disease (CD). We quantified the immunoexpression of calretinin, CD34, and calprotectin as a surrogate for mucosal innervation, telocytes (interstitial cells playing a role in networking), and inflammation, respectively, and correlated them with bowel alterations in stricturing CD.
-
Methods
- Primary resection specimens for ileal CD (n = 44, 31 stricturing CD, 13 inflammatory CD) were identified. Left-sided ulcerative colitis and trauma cases were used as controls. Proximal and distal margin and middle (diseased) sections were stained for calretinin, CD34, and calprotectin. Microscopic images were captured from the mucosa (calretinin), submucosa (calprotectin), and myenteric plexus (CD34), and the immunostaining was quantified using image processing and analysis. Bowel thickness at the corresponding sections were measured and correlated with the amount of immunoexpression.
-
Results
- A total of 2,037 images were analyzed. In stricturing CD, submucosal alteration/thickening at the stricture site correlated with calprotectin staining and inversely correlated with calretinin staining at the proximal margin. Muscularis propria alteration/thickening at the stricture site correlated with mucosal calretinin staining at the proximal margin. Submucosal alteration/thickening at the proximal margin correlated with calretinin and CD34 staining at the proximal margin and inversely correlated with CD34 staining at the stricture site. Calretinin immunostaining at the distal margin was significantly higher in stricturing CD than the controls.
-
Conclusions
- Inflammation and tissue remodeling appear to contribute to fibrogenesis in stricturing CD. Increased mucosal calretinin immunostaining distal to the diseased segment could be helpful in diagnosing CD in the right clinical context.
- Study and control groups
- The pathology database was searched for primary ileocecal resection specimens for ileal CD. Clinical data including indication for surgery, age, gender as well as clinical, endoscopic or histologic evidence of recurrence within 1 year following resection (early recurrence) and by the end of the follow-up were obtained from the electronic medical records. Colonic CD, re-excision of anastomosis for recurrent CD, and pure fistulating CD without stricture were excluded. Cases were further divided into stricturing CD and inflammatory CD based on clinical history, imaging findings, and indication for surgery.
- Archived H&E slides of cases were retrieved and resection margins were reviewed. As mucosal calretinin immunostaining can be obscured by inflammation, all cases with inflamed margins were further excluded from this study. Sections of uninflamed proximal (ileum) and distal (cecum or proximal right colon) margins and the mid-sections (i.e., one with the most severe fibrosis) were selected for immunohistochemistry. Total colectomies for left-sided ulcerative colitis (n=16), ileocecal resections for trauma (n=3) and a completion right hemicolectomy following previous polypectomy (n=1) were used as controls wherein uninflamed terminal ileal sections and uninflamed proximal right colon sections were used as surrogates for proximal and distal margins, respectively.
- Bowel thickness measurement and immunohistochemistry in CD
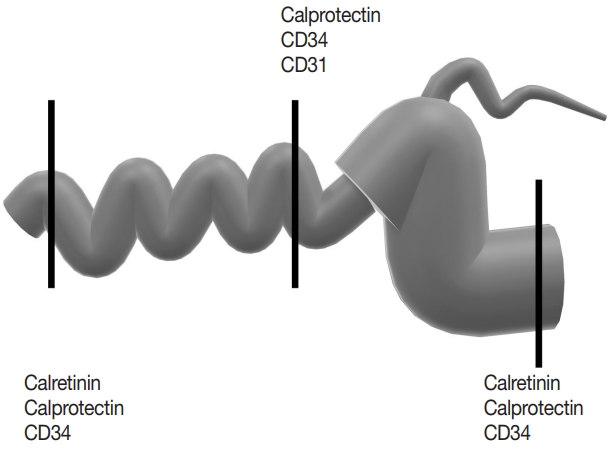
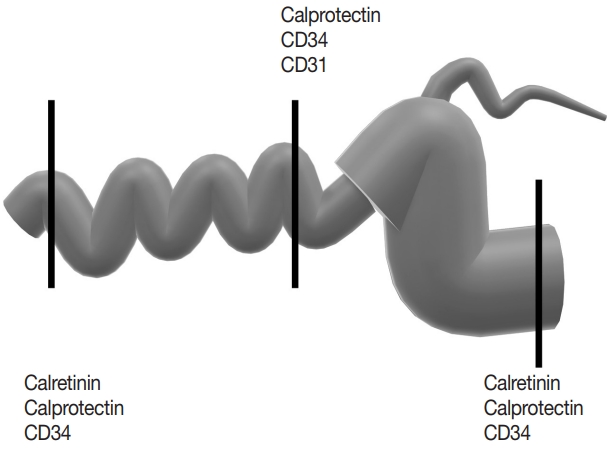
- For CD cases, the bowel thickness excluding the mucosa was measured microscopically. The submucosal (SM) and muscularis propria (MP) thickness was measured from the H&E slides of the sections that were chosen for immunohistochemistry. SM, MP, the ratio (SM divided by MP; SM/MP), and the proportion of SM and MP relative to combined thickness (SM and MP divided by SM+MP; SM/[SM+MP] and MP/[SM+MP]) were recorded for analysis. Immunohistochemistry for calretinin, CD34, calprotectin and CD31 was performed on 5-μm-thick tissue sections taken from representative FFPE tissue blocks on a Ventana Benchmark Ultra using the OptiView DAB detection kit (Ventana Medical Systems, Inc., Tucson, AZ, USA). Mucosal calretinin immunostaining is oftentimes obscured by inflammation and tissue damage; therefore, calretinin stain was not performed on the middle section of CD (Fig. 1, Supplementary Table S1).
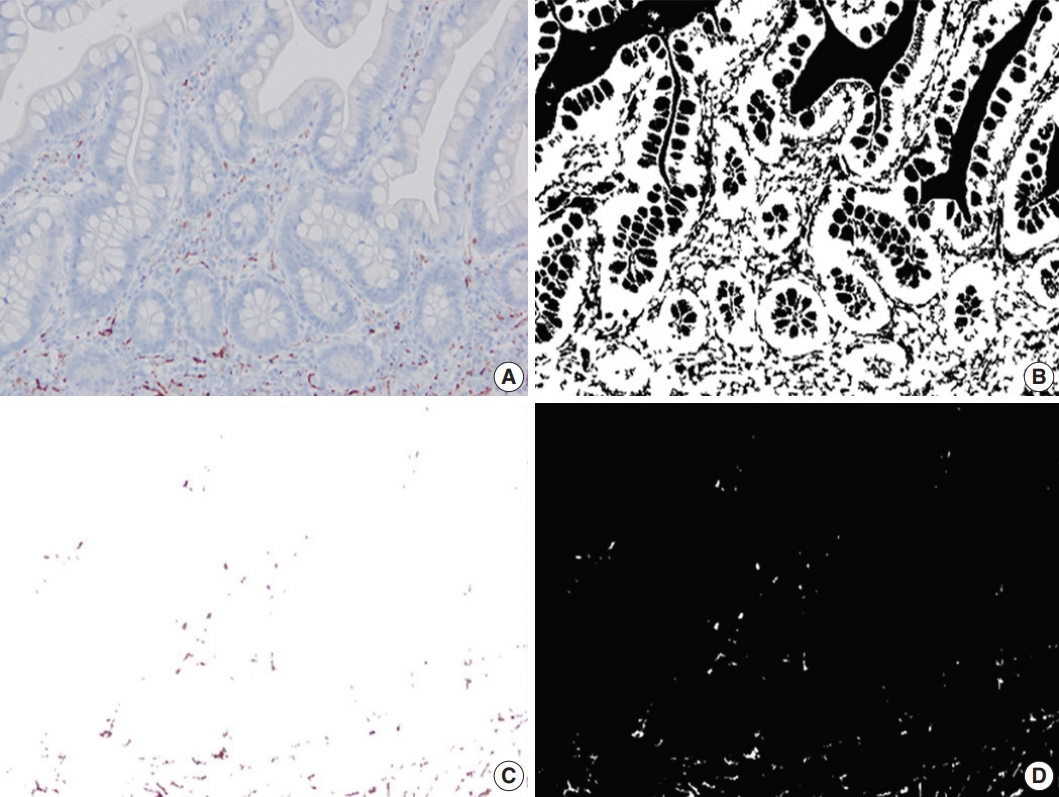
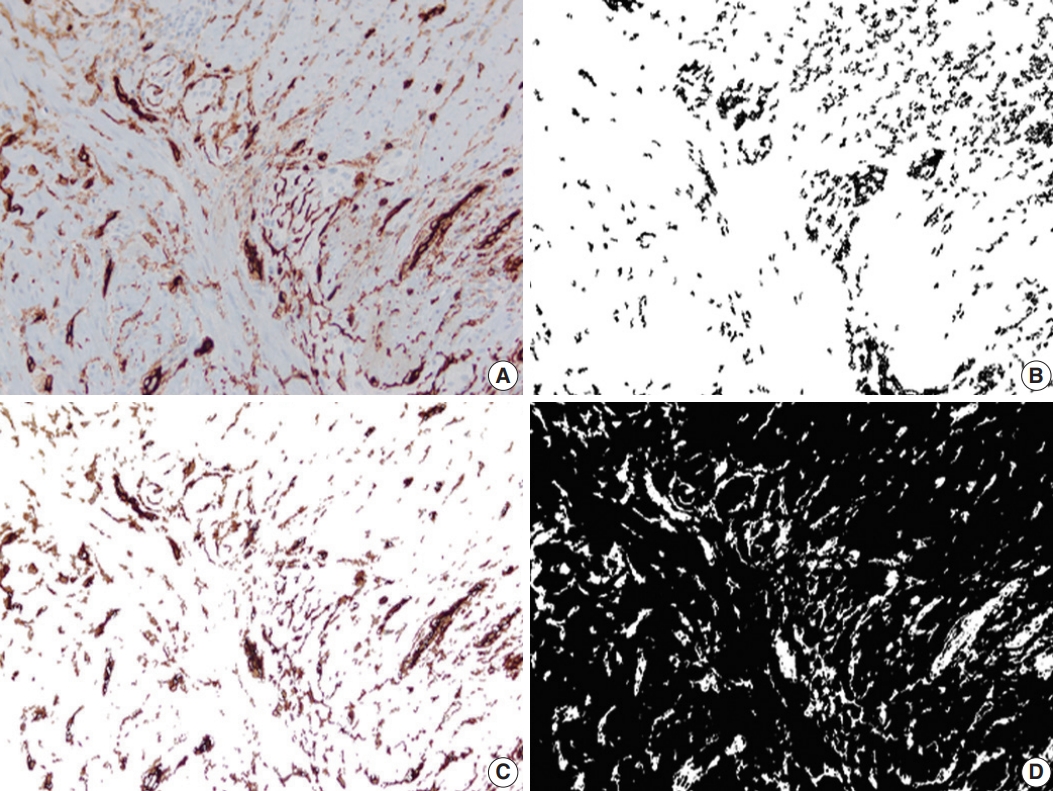
- Image capture, processing, and analysis
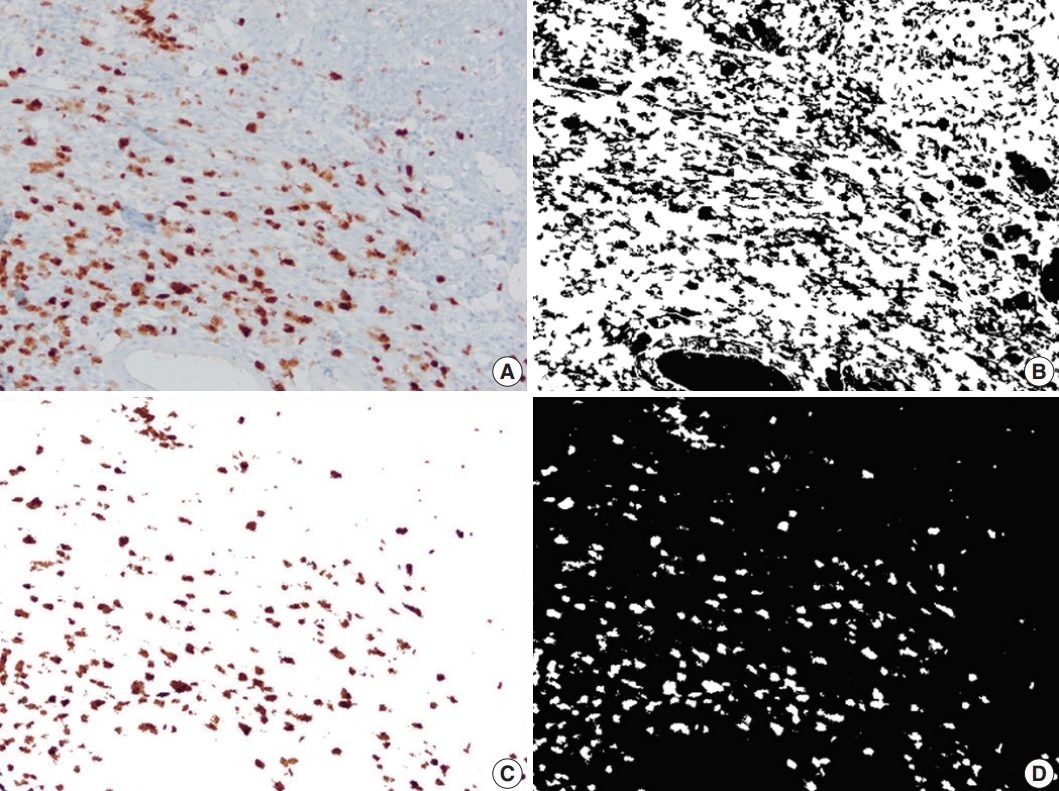
- Up to six static microscopic images were captured from immunostained slides at ×200 magnification per slide in JPEG format (Olympus cellSens Entry, Life Science Solutions, Waltham, MA, USA). The images were captured from the mucosa for calretinin, submucosa for calprotectin and from the myenteric plexus for CD34. No images were captured from CD31-immunostained slides. Pixel count (PC) was defined as the percentage of immunostained pixels out of the total number of pixels in an image. PC for calretinin (PC-calr), CD34 (PC-CD34), and calprotectin (PC-calp) were calculated using Image Processing and Analysis (MATLAB) as previously described [19-21].
- Data analysis
- Pearson’s correlation tests were performed for the SM and MP thickness and the ratios to test whether these parameters can be interchangeably used to represent submucosal and muscularis propria alteration/thickness. The mean PC for captured images was calculated for each slide and designated as location (proximal/mid/distal)-PC (pixel count)–stain (calr/CD34/calp). For example, “proximal-PC-calr” indicates PC of calretinin immunostaining at the proximal margin section. In CD, proximal-PC-calr and the ratio between proximal and distal-PC-calr (proximal/distal-PCcalr) were correlated using Pearson’s correlation test to determine whether these two parameters can be interchangeably used as a measure of mucosal calretinin immunostaining at the proximal margin. Similarly, proximal-PC-CD34 and the ratio between proximal and mid-PC-CD34 (proximal/mid-PC-CD34) were correlated. For mid-PC-CD34 and mid-PC-calprotectin, the highest PC (mid-PC-CD34-hotspot and mid-PC-calp-hotspot) of the PCs of a given slide was determined and was correlated with the mid-PC-CD34 and mid-PC-calp using Pearson’s correlation test. These PC parameters were correlated with the parameters of bowel alteration/thickness (SM, MP, SM/MP, SM/[SM+ MP], and MP/[SM+MP]) at the proximal margin, mid-section, and distal margin. The last analysis was repeated for stricturing and inflammatory CD subgroups. It was again repeated to compare CD with and without early recurrence, and CD with and without recurrence by the end of the follow-up period. In the controls, proximal-PC-calr/CD34/calp (ileal section) and distal-PC-calr/CD34/calp (proximal right colon section) were compared with those in CD and the CD subgroups. A p-value<.05 was considered statistically significant.
MATERIALS AND METHODS
- Study and control groups
- A total of 44 ileal CD with uninflamed margin sections were identified. Thirty-one were stricturing and 13 were inflammatory CD. The mean age of the patients was 34 years (range, 12 to 65 years). The male-to-female ratio was 17:27.
- The indication for surgery was stricture/obstruction for the stricturing CD. Five patients had concurrent fistula. For inflammatory CD, the indication for surgery was medical refractoriness or abscess formation. No patient had concurrent fistula in the inflammatory CD subgroup.
- The control group consisted of 16 total colectomies for left-sided ulcerative colitis, three ileocecal resections for trauma and one case of completion right hemicolectomy following polypectomy. The mean age of the control group patients was 36 years (range, 9 to 60 years). The male-to-female ratio was 13:7.
- Postoperative recurrence in CD
- A postoperative follow-up of more than 1 year was available in 33 patients (24 stricturing, 9 inflammatory CD). The mean follow-up duration was 64 months (range, 14 to 173 months). By the end of the follow-up, 21 patients (15 stricturing, 6 inflammatory CD) had recurrent CD and 12 (9 stricturing, 3 inflammatory CD) did not have any signs of recurrence. Ten of these recurrences (8 stricturing CD, 2 inflammatory CD) were early recurrence (recurrence within 1 year following resection). There was no difference in the frequencies of recurrence in stricturing CD versus inflammatory CD (Fisher exact test, p>.05).
- Bowel thickness measurement in CD
- The bowel thickness in stricturing CD did not differ from that of inflammatory CD (student’s t-test, p>.05). The three parameters of SM—SM, SM/MP, SM/(SM+MP)—showed strong correlations between one another (r ranging from 0.63 to 0.98, p< .05) in the proximal and distal margin and the mid-section. Therefore, all of these three parameters were considered to represent the measure of submucosal alteration/thickening. Similarly, the two parameters of MP—MP, MP/(SM+MP)—showed a correlation between one another (r ranging from 0.34 to 0.57, p< .05) in all sections, thus were considered to represent the measure of altered muscularis propria/thickening (Supplementary Table S2).
- In all CD, the bowel thickness was greater in the mid-section compared to the proximal margin. In the mid-section, the SM/(SM+MP) was greater and MP/(SM+MP) was smaller compared to the proximal margin (p<.05). In other words, while the mid-section was thicker in all CD, SM alteration/thickening was a major contributor of the bowel thickening in the mid-section compared to MP thickening. There were no differences in the bowel thickness parameters between stricturing and inflammatory CD.
- Image capture, processing, and analysis
- A total of 2037 images (1551 images from CD, 486 images from the control group) were analyzed. In CD, the proximal-PC-calr showed a strong correlation with the ratio between proximal-PC-calr and distal-PC-calr (proximal/distal-PC-calr; r=0.92, p< .05). Therefore, both proximal-PC-calr and proximal/distal-PC-calr were considered to represent the measure of mucosal calretinin staining at the proximal margin. Also, the proximal-PC-CD34 showed a statistically significant correlation with the ratio between proximal-PC-CD34 and mid-PC-CD34 (proximal/mid-PCCD34; r=0.60, p<.05). Thus, both were considered to represent the density of CD34 immunostaining at the proximal margin. Similarly, the PC-hotspots of CD34 and calprotectin at the mid-section were strongly correlated with the average PCs for CD34 and calprotectin, respectively (r=0.96 and r=0.97, p<.05). Thus, for CD34 and calprotectin at the mid-section, both average PC and PC-hotspot were considered to represent the magnitude of immunostaining. In the CD group, the mean PC of CD34 and calprotectin immunostaining in the mid-section was significantly lower (CD34; p<.001) and higher (calprotectin; p<.001) compared to both margins. In the control group, the mean of distal-PC-calr was significantly lower than that in CD, especially stricturing CD (p<.001). There was no difference in the means of proximal-PC-calr, proximal-PC-CD34, proximal-PC-calp, distal-PC-CD34 or distal-PC-calp between the CD and the control group (Table 1, Figs. 2–4). On microscopic assessment, the number of endothelial cells staining with CD31 was negligible compared to the amount of CD34 staining in the same section. In other words, increased CD34 staining in a given field was not attributable to increased vascularity. Therefore, no images were captured or analyzed by image processing and analysis from CD31 stained slides.
- Bowel wall alteration/thickening vs. PC in CD vs. recurrence
- Submucosal alteration/thickening at the proximal margin correlated with the mucosal calretinin staining and myenteric CD34 staining at the proximal margin and inversely correlated with CD34 staining in the mid-section. Submucosal alteration/thickening in the mid-section inversely correlated with proximal calretinin immunostaining. At the distal margin, the submucosal thickness inversely correlated with CD34 staining in the mid-section. Furthermore, in stricturing CD, submucosal thickness/ alteration at the mid-section correlated with calprotectin immunostaining in the mid-section and CD34 immunostaining at the distal margin. Compared to the submucosal changes, MP alteration/thickening at the proximal margin and mid-section showed an opposite pattern of associations with PC parameters (Supplementary Fig. S1, S2).
- In inflammatory CD, muscularis alteration/thickening at the proximal margin correlated with proximal CD34 staining whereas submucosal alteration/thickening at the distal margin correlated with CD34 staining at the distal margin. Muscularis alteration/thickening at the distal margin showed an inverse correlation with CD34 staining at the distal margin (Table 2). There were no differences in the parameters of bowel wall thickness or calretinin/CD34 pixel counts at the proximal margin between CD with recurrence vs. without recurrence by the end of the follow-up, and CD with early recurrence vs. without early recurrence.
RESULTS
- Inflammation, fibrosis, bowel wall thickening, and neural hypertrophy/hyperplasia are well-known common histologic findings in CD [9,23]. Few studies have quantitatively addressed the alterations of these histologic components using rather limited panels of immunostains and/or special stains [11]. Belai et al. performed calretinin (a calcium-binding protein) immunohistochemistry on eight ileal samples from CD and compared them to samples from adults (mean age, 50 years) and older (mean age, 80 years) adults. Compared to adult control tissue, sections from CD and older adults showed increased immunolabeling for calretinin in the myenteric ganglia. The authors postulated that an altered calcium homeostasis in myenteric neurons may be involved in the pathologic changes of CD [24]. Recently, Chen et al. [25] performed smooth muscle actin immunohistochemistry and Masson’s trichrome special stain on stenotic bowel segments of CD. Based on staining pattern, the authors concluded that smooth muscular hypertrophy, rather than collagen deposition (fibrosis), is a major contributor of stenosis in CD [25]. Ueno et al. observed that ileal mucosa from stricturing CD harbors an increased number of CD34-positive fibrocytes compared to non-stricturing CD [5].
- In this study, we quantified calretinin, CD34, and calprotectin immunostaining in lieu of mucosal innervation, interstitial telocytes, and inflammation, respectively, on full-thickness samples from CD and correlated them with microscopically measured bowel thickness. Calretinin immunohistochemistry is widely used in the diagnostic work-up of Hirschsprung disease in FFPE tissue. The presence of mucosal calretinin-positive nerve fibers indicates intact innervation of the examined bowel segment, thus virtually excluding Hirschsprung disease [26]. Telocytes are interstitial cells that are thought to be involved in intercellular signaling and bowel motility [27]. They have a small ovoid cell body surrounded by peripheral projections (telopodes), which vary in length, shape, number, and thickness. These distinctive features seperate telocytes from fibroblasts, myofibroblasts, interstitial cells of Cajal and other interstitial cells [27]. CD34 immunohistochemistry has been used as the most reliable marker of telocytes in the myenteric plexus in CD [16-18]. Calprotectin immunohistochemistry has been used as a marker of inflammation in IBD studies. Fukunaga et al. [14] have shown an increased amount of calprotectin immunohistochemical staining in FFPE colon samples from patients with IBD compared to normal controls. Likewise, calprotectin immunostaining showed a positive correlation with the degree of appendiceal inflammation in ulcerative colitis and indeterminate colitis [15]. Our study confirms that the amount of calprotectin immunostaining reflects the degree of inflammation in tissue sections.
- In our study, in the proximal margin of CD without apparent disease, submucosal alteration/thickness positively correlated with calretinin (mucosal innervation) and CD34 staining (telocytes) and inversely correlated with CD34 staining in the mid-section. In the mid-stenotic/fibrotic section, submucosal alteration/thickness positively correlated with calprotectin staining (inflammation) but inversely correlated with calretinin staining at the proximal margin. Submucosal thickness at the distal margin without apparent disease again inversely correlated with CD34 staining in the mid-section. Notably, MP at the proximal margin and mid-section tended to have an opposite pattern of correlation with the PC parameters. This trend was not reproduced when the inflammatory CD subgroup was analyzed separately. There was no difference in the amount of calretinin or CD34 staining at the proximal margin between the subgroups with and without recurrence, and with and without early recurrence.
- Milia et al. [16] have shown that in CD, stricture sites harbor fewer telocytes compared to unaffected segments using CD34 immunohistochemistry. Our findings were in line with Milia et al.’s observation [16]. Thus, we hypothesize that mucosal innervation at the stricture site would be proportionally reduced, although calretinin immunostain was not feasible in the mid-sections of CD. Additionally, we observed a positive correlation between the calretinin and CD34 immunostaining vs. submucosal alteration/thickness at the proximal margin. Therefore, submucosal fibrosis appears to be associated with tissue remodeling represented by an attenuated telocytes and innervation, at least at an advanced stage of fibrosis.
- One may argue that because submucosal neural hyperplasia is common at the stricture site of CD, mucosal innervation would be increased rather than decreased in the mid-section of CD. We postulate that the submucosal neural hypertrophy seen in CD is a reactive process to compensate for impeded bowel movement, similar to Hirschsprung disease. In conventional Hirschsprung disease, submucosal nerve hypertrophy is predominantly seen in the transition zone and aganglionic bowel segment, but usually not in the proximal ganglionated bowel [21].
- In contrast, given the positive correlation between the submucosal alteration/thickness and calprotectin immunostaining in the mid-section in stricturing CD, it appears that inflammation contributes to the progression of submucosal fibrosis and thickening. It is interesting to note that there was an inverse correlation between the mucosal innervation at the proximal margin and the submucosal alteration/thickness at the mid-section. We postulate that at some point, the main driver leading to alteration of the submucosa at the mid-section also attenuates proximal alterations and further augments localized alterations and stricturing in the middle.
- The opposite patterns of association between altered MP and PC parameters at the proximal margin and stricture site appear to indicate that when fibrosis progresses in the submucosa, the muscularis layer undergoes compensatory alterations to offset submucosal changes. This hypothesis is further supported by the fact that the MP was thicker than submucosa at the proximal margin whereas in the mid-section, the submucosa was thicker than the MP.
- In our study, the main difference between stricturing CD and inflammatory CD was that no correlations were found between the proximal submucosal alteration/thickness vs. PC parameters in the inflammatory CD subgroup. Also, the submucosal alteration/thickness in the mid-section did not correlate with the degree of inflammation in the inflammatory CD subgroup. This may be because we had relatively small number of inflammatory CD cases. Alternatively, a subset of inflammatory CD may represent a subclinical phase of stricturing CD [28], wherein submucosa at the proximal margin may not have been sufficiently altered. It is also possible that the inflammation and telocyte attenuation leading to fibrosis may occur at a relatively later stage in stricturing CD.
- Previous studies have shown that the presence of myenteric and or submucosal plexitis at the proximal margin of ileocolonic resection may predict postoperative CD recurrence, especially an early recurrence [29-31]. We compared mucosal calretinin staining at the proximal margin of our cases as a surrogate for neural function. Our negative results in regard to neural function at the proximal margin may be due to our selection criteria. We selected resections with no inflammation at the margins, which was confirmed by very low calprotectin pixel counts at the margins; therefore, none of our CD cases had plexitis at the proximal margin.
- As we have used new methodology to study novel markers, we cannot compare our results with other studies with different selection criteria and methods. However, some notable differences appear worthy of comment. For example, we have shown that the submucosal alteration/thickening in the mid-section correlated with the amount of inflammation at the submucosa level in stricturing CD. Previous studies have shown that fibrogenesis in CD cannot be solely explained by inflammation; i.e., some patients still develop strictures even after inflammation is medically controlled [32,33]. Similarly, a recent mouse model study has shown that altered mammalian target of rapamycin/autophagy pathway via the interleukin (IL)-23/IL-22 axis may play a role in fibrosis progression in the absence of inflammation in CD [13]. As Chen et al. [25] have hypothesized, the main driver of fibrosis progression may be inflammation in an early phase, whereas it may be some other process, such as smooth muscle hyperplasia, in a later stage. This awaits further study.
- Also, Ueno et al. [5] used CD34 immunohistochemistry as a marker of fibrocytes and reported that CD34 immunofluorescence was increased in the ileal mucosal biopsies of stricturing CD compared to non-stricturing CD patients. In contrast, we used CD34 immunohistochemistry as a marker of telocytes and targeted myenteric plexus as previously reported [16,34]. Therefore, the difference in the CD34 staining distribution between our and Ueno et al’s study appears to be due to the difference in the bowel layer and the cell types we targeted.
- A novel finding of our study is that the mean of distal-PC-calr in non-CD controls was significantly less than that in all CD, stricturing CD and inflammatory CD. In contrast, there was no difference in the means of proximal-PC-calr and proximal- and distal-PC-D34 between CD and controls. Therefore, distal-calretinin-PC may be potentially useful in diagnosing CD in an appropriate clinical context.
- One of the limitations of our study is the small number of inflammatory CD in our cohort. This appears to be an inherent limitation of a study using resected CD specimens, as the most common indication for surgical resection for CD is stricture [4]. However, there were certain differences when the stricturing CD subgroup was compared with all CD to include inflammatory CD. These suggest that our inflammatory CD subgroup is likely distinct from stricturing CD. Also, as this is a cross-sectional study, we cannot demonstrate temporal alterations of each marker we have studied. A longitudinal study using serial mucosal biopsies may be helpful in this regard. In addition, the distance between the margin and the diseased lesion and the exact length of diseased segment were not available in many cases. These parameters may have influenced some of our results. The main strength of our study is that for the first time, to the best of our knowledge, we applied a digital pathology tool to evaluate potential markers of fibrogenesis in CD. Although we acknowledge that it would be difficult to apply this tool in daily practice, it allowed us to generate relatively objective continuous variables that correlated with various parameters of bowel wall alteration.
- In summary, inflammation and tissue remodeling represented by alterations in the density of telocytes and mucosal innervation appear to contribute to fibrogenesis in stricturing CD. Whether the tissue remodeling we demonstrate herein is specific to stricturing CD or similar pattern can be seen any wound healing process including other types of strictures is to be studied.
DISCUSSION
Supplementary Information
Ethics Statement
The study was approved by the Institutional Review Board (IRB) at Albany Medical College (protocol #5718 06/18/2020) and Penn State Medical Center. All procedures performed in the current study were approved by IRB in accordance with the 1964 Helsinki declaration and its later amendments. Formal written informed consent was not required with a waiver by the appropriate IRB.
Availability of Data and Material
All data generated or analyzed during the study are included in this published article (and its supplementary information files).
Code Availability
Not applicable.
Author contributions
Conceptualization: Hwajeong Lee. Data curation: MEA, RB, LG, DK, KH, Hua Li, SA. Formal analysis: MEA, SA, Hwajeong Lee. Investigation: MEA, Hwajeong Lee. Methodology: MEA, SA, Hwajeong Lee. Project administration: Hwajeong Lee. Supervision: Hwajeong Lee. Visualization: MEA, SA. Writing—original draft: MEA, Hwajeong Lee. Writing—review & editing: RB, LG, DK, MWM, KH, Hua Li, SA. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest. SA contributed to this article in his personal capacity. The views expressed are his own and do not necessarily represent the views of General Electric.
Funding Statement
No funding to declare.
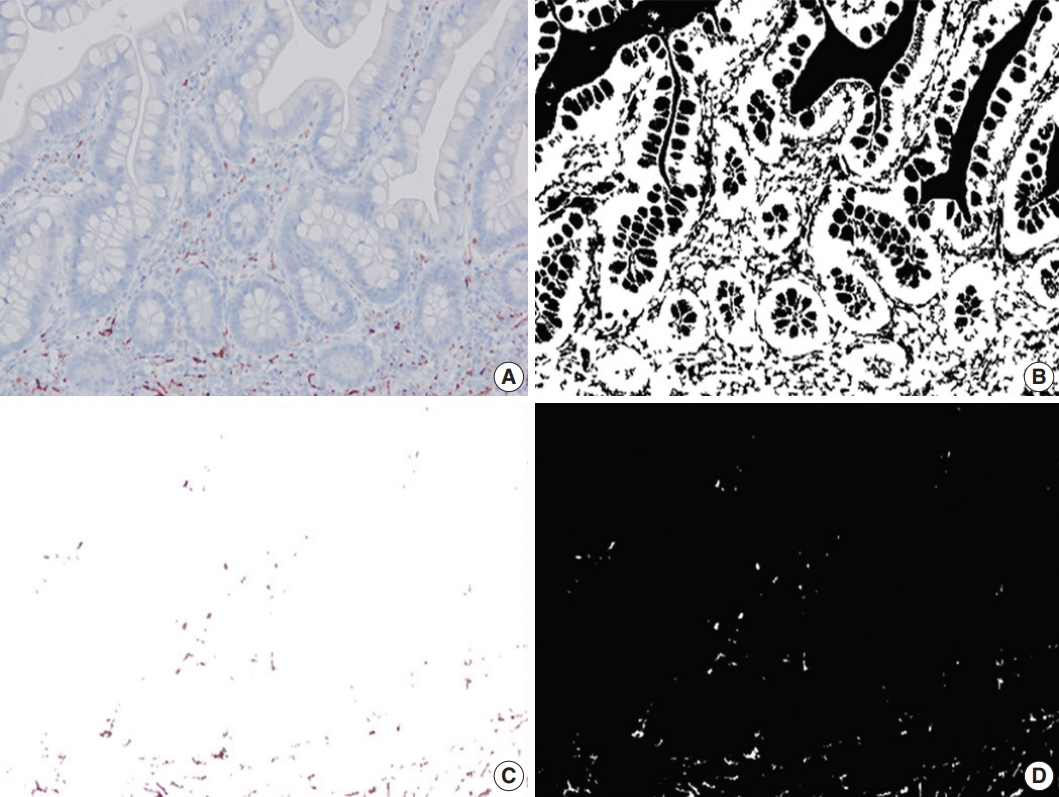
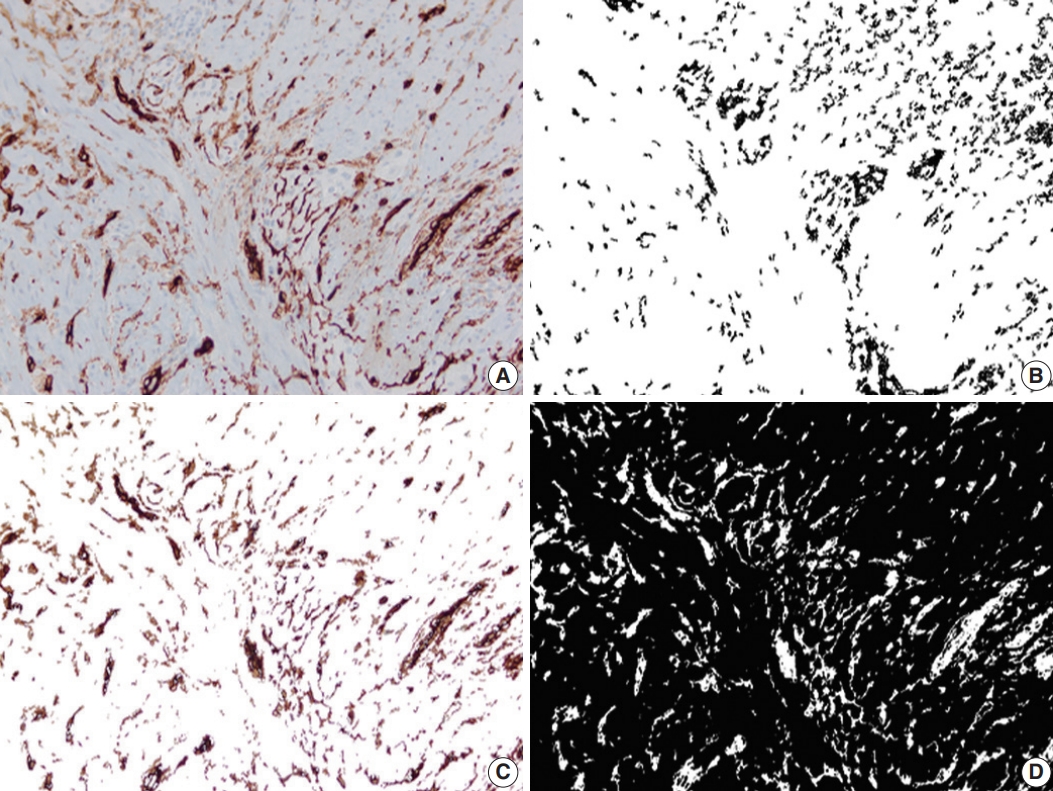
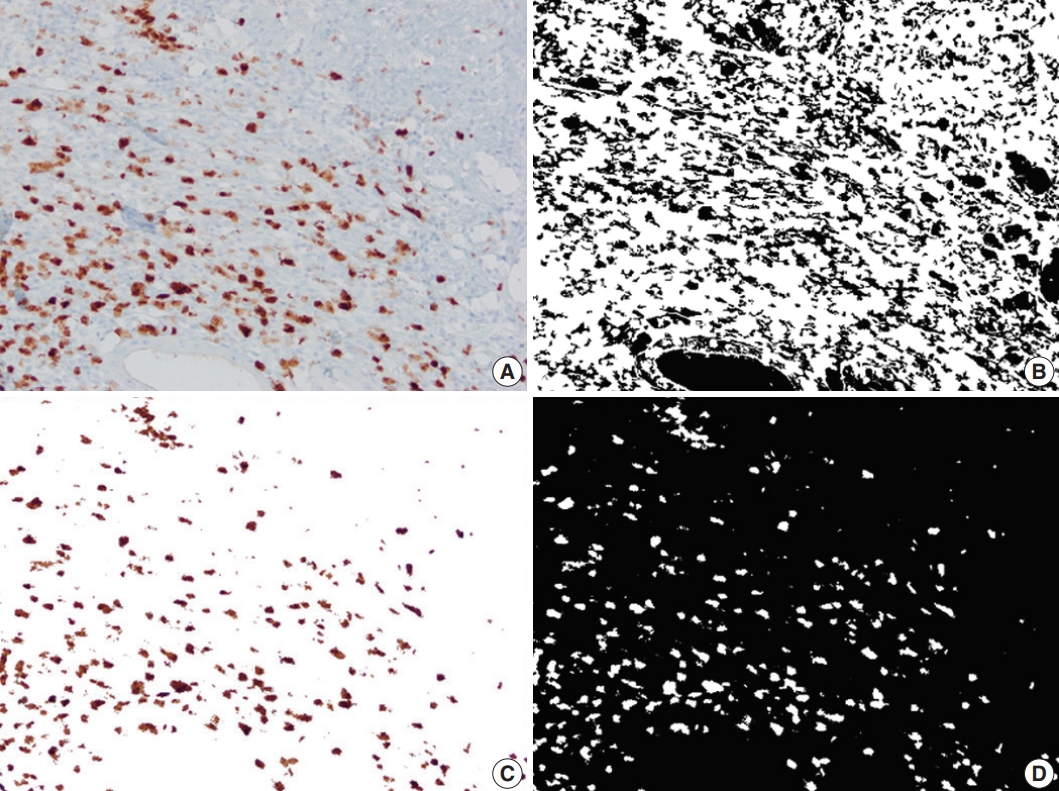
| Parameter | All CD | Stricturing CD | Inflammatory CD | Controls |
|---|---|---|---|---|
| No. of cases | 44 | 31 | 13 | 20 |
| No. of images | 1,791 | 1,321 | 470 | 486 |
| Proximal margin | ||||
| Proximal-PC-calr, mean (%) | 1.352 | 1.415 | 1.202 | 1.113 |
| Proximal-PC-CD34, mean (%) | 17.718 | 17.245 | 19.284 | 17.800 |
| Proximal/mid-CD34 | 1.479 | 1.481 | 1.439 | - |
| Proximal-PC-calp, mean (%) | 0.362 | 0.397 | 0.218 | 0.338 |
| Distal margin | ||||
| Distal-PC-calr, mean (%) | 2.407 | 2.480 | 2.234 | 1.434a |
| Distal-PC-CD34, mean (%) | 18.972 | 19.287 | 16.297 | 15.156 |
| Proximal/distal-PC-calr | 0.588 | 0.594 | 0.574 | 1.062 |
| Distal-PC-calp, mean (%) | 0.396 | 0.436 | 0.274 | 1.120 |
| Mid-section | ||||
| Mid-PC-CD34, mean (%) | 13.473 | 13.087 | 14.394 | - |
| Mid-PC-CD34-hotspot, mean (%) | 17.330 | 16.795 | 18.606 | - |
| Mid-PC-calp, mean (%) | 10.975 | 9.929 | 13.468 | - |
| Mid-PC-calp-hotspot, mean (%) | 16.524 | 15.139 | 19.826 | - |
The numbers in round bracket indicate r in stricturing Crohn’s disease; The numbers in square bracket indicate r in inflammatory Crohn’s disease; all p < .05.
p, proximal; PC, pixel count; calr, calretinin; p/d, proximal/distal; p/m, proximal/mid; m, mid; calp, calprotectin; SM, submucosal thickness; MP, muscularis propria thickness.
- 1. Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn’s disease. Dis Mon 2018; 64: 20-57. ArticlePubMed
- 2. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46-54. ArticlePubMed
- 3. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55: 749-53. ArticlePubMedPMC
- 4. Lee H, Westerhoff M, Shen B, Liu X. Clinical aspects of idiopathic inflammatory Bowel disease: a review for pathologists. Arch Pathol Lab Med 2016; 140: 413-28. ArticlePubMedPDF
- 5. Ueno A, Jijon HB, Peng R, et al. Association of circulating fibrocytes with fibrostenotic small Bowel Crohn’s disease. Inflamm Bowel Dis 2022; 28: 246-58. ArticlePubMedPDF
- 6. Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc 2017; 92: 1088-103. ArticlePubMed
- 7. Zeitz J, Fournier N, Labenz C, et al. Risk factors for the development of fistulae and stenoses in Crohn disease patients in the Swiss Inflammatory Bowel Disease Cohort. Inflamm Intest Dis 2017; 1: 172-81. ArticlePubMedPMCPDF
- 8. Chan WPW, Mourad F, Leong RW. Crohn’s disease associated strictures. J Gastroenterol Hepatol 2018; 33: 998-1008. ArticlePubMedPDF
- 9. Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory Bowel diseases. Gastroenterology 2017; 152: 340-50. ArticlePubMedPMC
- 10. Latella G, Sferra R, Speca S, Vetuschi A, Gaudio E. Can we prevent, reduce or reverse intestinal fibrosis in IBD? Eur Rev Med Pharmacol Sci 2013; 17: 1283-304. PubMed
- 11. Gordon IO, Bettenworth D, Bokemeyer A, et al. Histopathology scoring systems of stenosis associated with small Bowel Crohn’s disease: a systematic review. Gastroenterology 2020; 158: 137-50. ArticlePubMedPMC
- 12. Wu F, Chakravarti S. Differential expression of inflammatory and fibrogenic genes and their regulation by NF-kappaB inhibition in a mouse model of chronic colitis. J Immunol 2007; 179: 6988-7000. PubMed
- 13. Mathur R, Alam MM, Zhao XF, et al. Induction of autophagy in Cx3cr1(+) mononuclear cells limits IL-23/IL-22 axis-mediated intestinal fibrosis. Mucosal Immunol 2019; 12: 612-23. ArticlePubMedPMCPDF
- 14. Fukunaga S, Kuwaki K, Mitsuyama K, et al. Detection of calprotectin in inflammatory bowel disease: fecal and serum levels and immunohistochemical localization. Int J Mol Med 2018; 41: 107-18. ArticlePubMedPMC
- 15. Kmeid M, Arker SH, Petchers A, et al. Appendiceal inflammation in colectomy is independently correlated with early pouchitis following ileal pouch anal anastomosis in ulcerative colitis and indeterminate colitis. Ann Diagn Pathol 2021; 55: 151838.ArticlePubMed
- 16. Milia AF, Ruffo M, Manetti M, et al. Telocytes in Crohn’s disease. J Cell Mol Med 2013; 17: 1525-36. ArticlePubMedPMCPDF
- 17. Wollheim FA. Telocytes, communicators in healthy stroma and relation to inflammation and fibrosis. Joint Bone Spine 2016; 83: 615-8. ArticlePubMed
- 18. Faussone-Pellegrini MS, Gherghiceanu M. Telocyte’s contacts. Semin Cell Dev Biol 2016; 55: 3-8. ArticlePubMed
- 19. Najjar S, Ahn S, Kasago I, et al. Image processing and analysis of mucosal calretinin staining to define the transition zone in Hirschsprung disease: a pilot study. Eur J Pediatr Surg 2019; 29: 179-87. ArticlePubMed
- 20. Cordeiro-Rudnisky F, Ahn S, Sheuka N, et al. Transition zone in total colonic aganglionosis and colorectal Hirschsprung’s disease shows a similar trend of mucosal innervation: image processing and analysis study. Pediatr Dev Pathol 2020; 23: 127-31. ArticlePubMedPDF
- 21. Najjar S, Ahn S, Umrau K, et al. Increasing trend of calretinin-positive mucosal innervation from aganglionic zone toward transition zone in Hirschsprung's disease. Eur J Pediatr Surg 2022; 32: 191-7. ArticlePubMed
- 22. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012; 380: 1590-605. ArticlePubMed
- 23. Strobach RS, Ross AH, Markin RS, Zetterman RK, Linder J. Neural patterns in inflammatory bowel disease: an immunohistochemical survey. Mod Pathol 1990; 3: 488-93. PubMed
- 24. Belai A, Burnstock G. Distribution and colocalization of nitric oxide synthase and calretinin in myenteric neurons of developing, aging, and Crohn’s disease human small intestine. Dig Dis Sci 1999; 44: 1579-87. ArticlePubMedPDF
- 25. Chen W, Lu C, Hirota C, Iacucci M, Ghosh S, Gui X. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn’s fibrostenosing bowel strictures: a semiquantitative analysis by using a novel histological grading scheme. J Crohns Colitis 2017; 11: 92-104. ArticlePubMed
- 26. Guinard-Samuel V, Bonnard A, De Lagausie P, et al. Calretinin immunohistochemistry: a simple and efficient tool to diagnose Hirschsprung disease. Mod Pathol 2009; 22: 1379-84. ArticlePubMedPDF
- 27. Popescu LM, Faussone-Pellegrini MS. Telocytes: a case of serendipity: the winding way from interstitial cells of cajal (ICC), via interstitial cajal-like cells (ICLC) to telocytes. J Cell Mol Med 2010; 14: 729-40. ArticlePubMedPMC
- 28. Irwin J, Ferguson E, Simms LA, Hanigan K, Carbonnel F, RadfordSmith G. A rolling phenotype in Crohn’s disease. PLoS One 2017; 12: e0174954.ArticlePubMedPMC
- 29. Ferrante M, de Hertogh G, Hlavaty T, et al. The value of myenteric plexitis to predict early postoperative Crohn’s disease recurrence. Gastroenterology 2006; 130: 1595-606. ArticlePubMed
- 30. Bressenot A, Chevaux JB, Williet N, et al. Submucosal plexitis as a predictor of postoperative surgical recurrence in Crohn’s disease. Inflamm Bowel Dis 2013; 19: 1654-61. ArticlePubMed
- 31. Tandon P, Malhi G, Abdali D, et al. Active margins, plexitis, and granulomas increase postoperative Crohn’s recurrence: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021; 19: 451-62. ArticlePubMed
- 32. Crespi M, Dulbecco P, De Ceglie A, Conio M. Strictures in Crohn’s disease: from pathophysiology to treatment. Dig Dis Sci 2020; 65: 1904-16. ArticlePubMedPDF
- 33. Alfredsson J, Wick MJ. Mechanism of fibrosis and stricture formation in Crohn's disease. Scand J Immunol 2020; 92: e12990.ArticlePubMedPMCPDF
- 34. Manetti M, Rosa I, Messerini L, Ibba-Manneschi L. Telocytes are reduced during fibrotic remodelling of the colonic wall in ulcerative colitis. J Cell Mol Med 2015; 19: 62-73. ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

- Pathophysiologic implications and therapeutic potentials of telocytes in multiorgan fibrosis
Irene Rosa, Eloisa Romano, Bianca Saveria Fioretto, Mirko Manetti
Current Opinion in Rheumatology.2026; 38(1): 26. CrossRef - Serum S100A8/A9 Correlates to Surgery‐Free Interval in Idiopathic Subglottic Stenosis
Laura M. Mafla, Raymond J. So, Ibrahim Abd‐Elazem, Samuel L. Collins, Yee Chan‐Li, Gabriela Lilly, Ioan A. Lina, Alexander H. Gelbard, Alexander T. Hillel, Kevin M. Motz
The Laryngoscope.2025; 135(5): 1724. CrossRef - Telocytes in inflammatory bowel diseases: contributions to pathology and therapeutic potentials
Ronaldo Paolo Panganiban, Christina McAninch, Marina Chulkina, Irina V. Pinchuk
Frontiers in Cell and Developmental Biology.2025;[Epub] CrossRef - Synergistic effects of vedolizumab and JAK 1,2,3 inhibitors in Crohn’s disease: insights from a systems biology and artificial intelligence-based approach
Ignacio Marín-Jiménez, Mónica Sierra-Ausín, Teresa Letosa-Abián, Jesús Aparicio, Carmen Montoto-Otero, Silvia Sánchez-Ramón
Frontiers in Immunology.2025;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure




Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
| Parameter | All CD | Stricturing CD | Inflammatory CD | Controls |
|---|---|---|---|---|
| No. of cases | 44 | 31 | 13 | 20 |
| No. of images | 1,791 | 1,321 | 470 | 486 |
| Proximal margin | ||||
| Proximal-PC-calr, mean (%) | 1.352 | 1.415 | 1.202 | 1.113 |
| Proximal-PC-CD34, mean (%) | 17.718 | 17.245 | 19.284 | 17.800 |
| Proximal/mid-CD34 | 1.479 | 1.481 | 1.439 | - |
| Proximal-PC-calp, mean (%) | 0.362 | 0.397 | 0.218 | 0.338 |
| Distal margin | ||||
| Distal-PC-calr, mean (%) | 2.407 | 2.480 | 2.234 | 1.434 |
| Distal-PC-CD34, mean (%) | 18.972 | 19.287 | 16.297 | 15.156 |
| Proximal/distal-PC-calr | 0.588 | 0.594 | 0.574 | 1.062 |
| Distal-PC-calp, mean (%) | 0.396 | 0.436 | 0.274 | 1.120 |
| Mid-section | ||||
| Mid-PC-CD34, mean (%) | 13.473 | 13.087 | 14.394 | - |
| Mid-PC-CD34-hotspot, mean (%) | 17.330 | 16.795 | 18.606 | - |
| Mid-PC-calp, mean (%) | 10.975 | 9.929 | 13.468 | - |
| Mid-PC-calp-hotspot, mean (%) | 16.524 | 15.139 | 19.826 | - |
| p-PC-calr | p/d-PC-calr | p-PC-CD34 | p/m-PC-CD34 | m-PC-CD34 | m-PC-CD34-hotspot | m-PC-calphotspot | d-PC-CD34 | ||
|---|---|---|---|---|---|---|---|---|---|
| Proximal margin | |||||||||
| SM | - | - | - | 0.41 (0.46) | –0.46 (–0.45) | –0.42 (–0.42) | - | - | |
| SM/MP | 0.39 (0.40) | 0.37 (0.42) | - | 0.31 (0.42) | –0.32 | –0.32 | - | - | |
| SM/(SM + MP) | 0.34 | 0.33 (0.37) | - | (0.40) | –0.31 | –0.31 | - | - | |
| MP | - | - | (–0.37) [0.60] | [0.62] | - | - | - | - | |
| MP/(SM + MP) | –0.34 | - | - | - | 0.50 | 0.48 | - | - | |
| Mid-section | |||||||||
| SM | - | - | - | - | - | - | - | (0.39) | |
| SM/MP | - | - | - | - | - | - | (0.39) | - | |
| SM/(SM + MP) | –0.39 [–0.56] | –0.35 (–0.38) | - | - | - | - | - | - | |
| MP | [0.82] | - | - | - | - | - | - | - | |
| MP/(SM + MP) | 0.39 [0.56] | 0.35 (0.40) | - | - | - | - | - | - | |
| Distal margin | |||||||||
| SM | - | - | - | - | - | –0.32 | - | [0.57] | |
| SM/MP | - | - | - | - | - | - | - | [0.56] | |
| SM/(SM + MP) | - | - | - | - | - | - | - | [0.64] | |
| MP | 0.31 | - | - | - | - | - | - | - | |
| MP/(SM + MP) | - | - | - | - | - | - | - | [–0.64] | |
CD, Crohn’s disease; PC, pixel count; calr, calretinin; calp, calprotectin. p < .05 by student’s t-test.
The numbers in round bracket indicate r in stricturing Crohn’s disease; The numbers in square bracket indicate r in inflammatory Crohn’s disease; all p < .05. p, proximal; PC, pixel count; calr, calretinin; p/d, proximal/distal; p/m, proximal/mid; m, mid; calp, calprotectin; SM, submucosal thickness; MP, muscularis propria thickness.

 E-submission
E-submission