Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 56(5); 2022 > Article
-
Case Study
Papillary and medullary thyroid carcinomas coexisting in the same lobe, first suspected based on fine-needle aspiration cytology: a case report -
Hyun Hee Koh1,2
 , Young Lyun Oh1
, Young Lyun Oh1
-
Journal of Pathology and Translational Medicine 2022;56(5):301-308.
DOI: https://doi.org/10.4132/jptm.2022.08.03
Published online: September 13, 2022
1Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
2Department of Pathology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- Corresponding Author: Young Lyun Oh, MD, PhD, Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea, Tel: +82-2-3410-2805, Fax: +82-2-3410-2831, E-mail: yl.oh@samsung.com
© 2022 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Because different types of thyroid malignancies have distinct embryological origins, coexisting tumors are rarely observed. We describe a coexisting papillary thyroid carcinoma (PTC) and medullary thyroid carcinoma (MTC) first suspected by fine-needle aspiration cytology (FNAC). A 57-year-old female presented with an irregular mass in the right thyroid lobe. The cytopathologic findings of fine-needle aspiration showed two components: a papillary-like arrangement consisting of cells with pale enlarged nuclei indicative of PTC and loose clusters comprised of oval cells with granular chromatin indicative of MTC. The diagnosis of a coexisting PTC and MTC was initially confirmed by calcitonin immunocytochemistry and later after total thyroidectomy. Although some surgical case reports of PTC and MTC coexisting in either the same or different lobes have been documented, a case suspected by FNAC before the surgery has rarely been reported. Because appropriate treatment and prognosis of PTC and MTC are different, cytopathologists should be aware of this rare entity.
- Clinical presentation
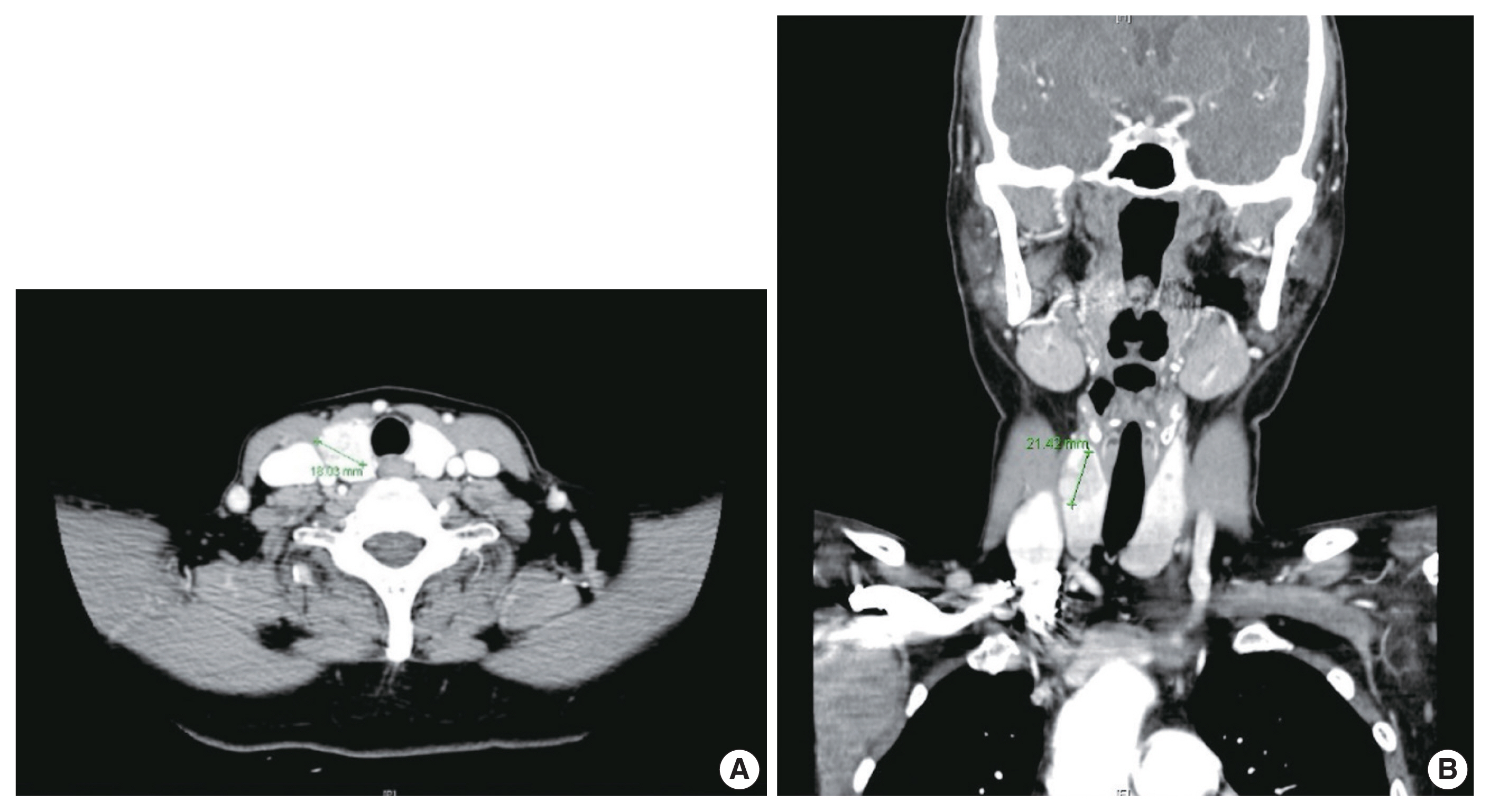
- A 57-year-old female was referred to our institution, Samsung Medical Center. She complained of an incidentally found thyroid abnormality during routine health examination 4 years ago. She had no apparent personal or family history of endocrine disorders. Physical examination revealed a hard fixed mass in the anterior aspect of her neck. Thyroid ultrasonography revealed an irregular mass measuring 2.1 cm which was abutting the anterior capsule in the right lobe and a small nodule measuring 0.4 cm in the left lobe. Thyroid contrast-enhanced CT indicated a calcified mass-like lesion measuring 2.1 × 1.8 cm in the right lobe (Fig. 1). Ultrasonography and CT showed no evidence of lymph node metastasis. Based on radiologic evaluation, the dominant nodule in the right lobe was considered a lesion highly suspicious for malignancy, and the other small nodule in the left lobe an intermediate suspicious lesion.
- Serum levels of TSH (3.07 μIU/mL; normal range, 0.35 to 4.94 μIU/mL), free thyroxine (1.2 ng/dL; normal range, 0.9 to 2.3 ng/dL), and anti-thyroglobulin autoantibody (anti-Tg Ab, 28.4 U/mL; normal range, 0 to 60 U/mL), which reflect thyroid function, were in the normal range. Serum parathyroid hormone level (16.4 pg/mL; normal range, 11 to 62 pg/mL), calcium (9.5 mg/dL; normal range, 8.5 to 10.5 mg/dL), and phosphorous (3.6 mg/dL; normal range, 2.5 to 5.1 mg/dL), which are associated with parathyroid function, were also in the normal range.
- Cytopathologic findings
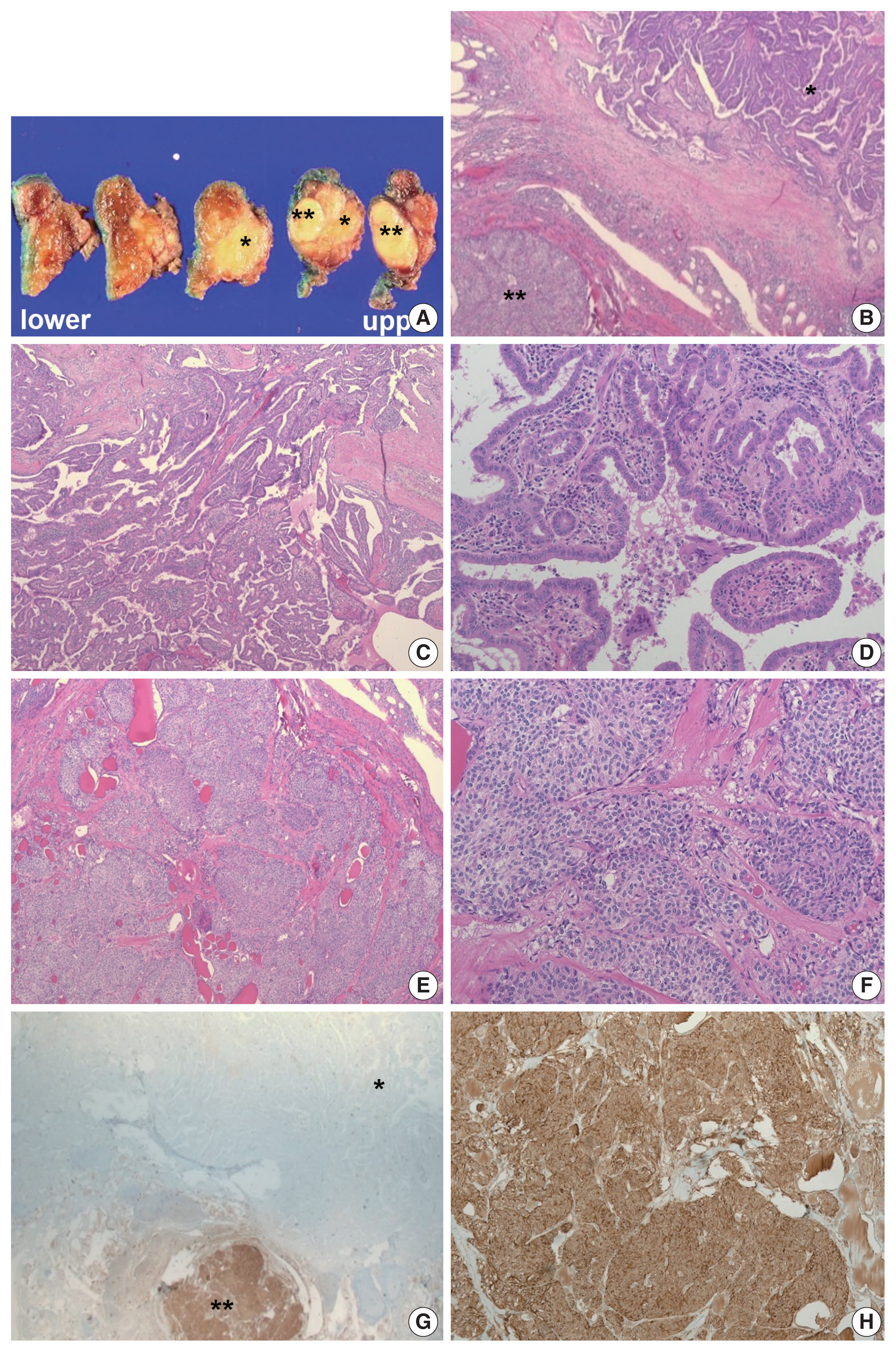
- Under suspicion of thyroid cancer, a single fine-needle aspiration (FNA) of the mass in the right lobe was performed. Cellular smears were placed on slides and stained with hematoxylin and eosin or Papanicolaou staining, and the cytological features of the mass in the right lobe showed admixture of various components, making it difficult to subtype (Fig. 2A, B). Some areas demonstrated cytologic findings, which were unexpectedly divided into two components with a clear distinction (Fig. 2C). First, papillary clusters or syncytium-like arrangements consisting of tumor cells with pale enlarged nuclei, irregular nuclear membranes, and nuclear grooves, often accompanied with psammoma bodies, were observed and supported the diagnosis of PTC (Fig. 2D, E). Some areas having cytologic features admixed with both tumor components often showed intranuclear pseudo-inclusions and multinucleated giant cells (Fig. 2E, F). The other component composed of oval- to spindle-shaped tumor cells with granular chromatin and smooth nuclear membrane forming non-cohesive clusters indicated the possibility of MTC (Fig. 2G). The background was somewhat hemorrhagic with scant colloid. Based on these cytological features, mixed PTC and MTC was suspected; thus, calcitonin immunocytochemistry was performed, and the serum calcitonin level of the patient was measured. The serum calcitonin level was significantly elevated (103.3 pg/mL; normal range, 0 to 12 pg/mL) and calcitonin immunostaining showed strong expression (Fig. 2H), supporting the existence of an MTC component. As a final cytologic diagnosis, suspicious for malignancy (The Bethesda System for Reporting Thyroid Cytopathology, category V) was rendered with an additional note suggestive of mixed PTC and MTC [20].
- Pathologic findings
- Based on these evaluations, the patient underwent total thyroidectomy with anterior compartment neck dissection one month after the first admission. On gross examination, the total thyroidectomy specimen measured 5 × 4.5 cm at the greatest dimension. A well-demarcated multinodular yellowish mass measuring 1.9 × 1.8 cm was observed in the upper-to-mid portion of the right lobe (Fig. 3A), and two small whitish nodules measuring 0.3 cm and 0.2 cm were also present in the mid-portion of the left lobe. The remaining thyroid tissue was unremarkable. Microscopically, a dominant mass in the right lobe consisted of two morphologically distinct components, very closely attached to each other but delineated by normal thyroid tissue and fibrous tissue (Fig. 3B). One component was comprised of well-formed papillary architecture lined by cuboidal cells with nuclear clearing and irregular nuclear membrane (Fig. 3C, D), and the other component showed a cellular mass comprised of a sheet or nest of oval cells with mild atypia and low mitotic activity (Fig. 3E, F). Neither necrosis nor hemorrhage was present in either component. The histologic features of the former were specific for classical type PTC with an extra-thyroidal extension measuring 1.4 cm, and those of the latter were highly suspicious of MTC. Immunostaining was performed for the latter and showed cytoplasmic immunoreactivity for calcitonin; thus, it was diagnosed as an MTC measuring 1.8 cm (Fig. 3G, H). In addition, the histologic findings of the remaining two small nodules in the mid-portion of the left lobe were indicative of classical type PTC, measuring 0.3 cm and 0.2 cm. An anterior compartment neck dissection yielded five regional lymph nodes and one peri-thyroidal lymph node; no lymph node metastasis was observed. This specimen was stage pT1b N0 according to the American Joint Committee on Cancer 8th edition criteria [21].
- Genetic analysis
- Because the patient had a rare coexisting PTC and MTC, genetic analysis associated with thyroid carcinoma was performed, and it was carried out without macrodissection for separating PTC and MTC. Real-time polymerase chain reaction revealed a BRAF V600 mutation and sequencing a BRAF exon 15 V600E mutation. However, sequencing did not reveal the RET gene mutation for MEN2A, MEN2B, or familial MTC.
- Follow-up observation
- After the surgery, the patient underwent radioactive iodine ablation using 80 mCi 131I and has been monitored regularly. Immediately after the surgery, the serum calcitonin level decreased to 3.9 pg/mL, which is in the normal range. One year after thyroidectomy, whole body screening with 123I showed no evidence of recurrence or functioning metastasis. The routinely checked thyroglobulin and anti-Tg Ab serum levels were also in the normal range. The patient was last seen at this institution 33 months following her surgery and is still healthy with no symptoms of discomfort.
CASE REPORT
- The origins of PTC and MTC are embryologically different; thyroglobulin-producing follicular cells derive from a median endodermal anlage, and the C-cells stem from an ultimobranchial body [3,11]. Despite this distinction, several cases regarding the coexistence of PTC and MTC have been reported in the literature, representing less than 1% of all thyroid malignancies [2,14,15]. The coexistence of PTC and MTC may appear either separately as a collision tumor or mixed as a tumor showing dual differentiation. The latter is termed mixed medullary and papillary carcinoma according to the WHO classification and is exceedingly rare. Various hypotheses explaining the coexistence of PTC and MTC have been postulated [9], such as stem cell theory, divergent differentiation theory, field effect theory, hostage theory, and collision theory.
- The Bethesda System for Reporting Thyroid Cytopathology standardizes reporting and cytologic criteria in FNAC of the thyroid [22]. Because the specific histologic features of PTC, including nuclear clearing, nuclear groove, and pseudo-inclusion with papillary architecture, are well known, and cytopathologists often encounter this entity due to its high prevalence, PTC can be easily diagnosed. Conversely, MTC can exhibit a wide range of histologic morphology in terms of both architecture and cytologic features, likening this entity to a chameleon. Some cytologic features, including eccentric nuclei, salt-and-pepper chromatin, inconspicuous nucleoli, binucleation or multinucleation, and ill-defined cytoplasmic border, have been considered pathognomonic findings. However, MTC variably can show nuclear grooves or inclusions, which require distinguishing it from PTC, and the finely granular cytoplasm leads to the possibility of Hürthle cell neoplasm [23]. Due to the various morphologies of MTC, a definitive diagnosis based only on FNAC is difficult, and a large number of differential diagnoses exist. In clinical practice, measuring serum calcitonin level or immunostaining for calcitonin is not routinely performed for all patients with suspected thyroid malignancy. Although serum calcitonin is a useful diagnostic tool for detecting MTC with high sensitivity, it may be increased due to a hypercalcemic state such as renal failure, and a small-sized MTC may fail to increase the level; thus, routine measurement remains debatable. Therefore, cytopathologists can misdiagnose or overlook MTC. Because the surgical plan, management after surgery, and indicators for surveillance of PTC and MTC are entirely different, recognizing the possible coexistence of PTC and MTC and considering it as a differential diagnosis is necessary to not miss concurrent lesions. Similarly, in our case, if the cytopathologist had not been aware of the possibility of coexisting PTC and MTC, the MTC component of the FNA specimen would have been overlooked, and the serum calcitonin level would not have been checked before the surgery. In addition, MTC has more propensity for locoregional invasion than PTC; thus, the extent of surgery should be carefully determined, and the prognosis is usually worse than for PTC. The preoperative cytologic diagnosis determined the surgical plan and an adequate surveillance strategy implemented after surgery that was clinically relevant.
- Due to the aggressive clinical course of MTC, accurate preoperative detection is a prerequisite for improving prognosis; thus, the cytopathologist’s suspicion of MTC based on FNAC, the first step of diagnosis, should be emphasized. Because diagnosing MTC based on cytomorphology alone is very challenging due to its rarity compared with PTC and the wide spectrum of cytologic features that often overlap with other neoplasms, the detection rate of FNAC in patients with MTC ranged from 12.5%–88.2% in a meta-analysis including 15 relevant studies and 641 MTC patients who underwent FNAC [24]. Seven cytomorphologic features were readily recognized in FNAC of MTC: high cellularity, cellular pleomorphism, plasmacytoid cells, round cells, discohesive cells, salt-and-pepper chromatin, and binucleation or multinucleation [25]. In another study, the following important cytologic parameters for MTC were also suggested: a dispersed cell pattern of polygonal cells, azurophilic cytoplasmic granules, and eccentric nuclei with coarse granular chromatin and amyloid [26]. In the current case, discohesive cells arranged in a dispersed pattern with moderate to high cellularity were observed, corroborating with the previous literature, and spindle cell morphology with granular chromatin also supports the diagnosis of MTC. However, the presence of pseudo-inclusion, known as the representative but not specific feature, might have led to a misdiagnosis of PTC; thus, immunostaining for calcitonin was helpful for an accurate diagnosis. Conclusively diagnosing MTC only using FNAC should be approached with caution; thus, cytopathologists are sometimes obliged to categorize difficult smears into atypia of undetermined significance or follicular lesions of undetermined significance, namely AUS/FLUS. When FNAC findings are unmet for the diagnostic criteria of any subtype or show various findings suggestive of two or more components, cytopathologists might suspect the presence of MTC, attempt immunocytochemistry for calcitonin, and recommend that clinicians consider the measurement of serum calcitonin levels.
- Concurrent occurrence of PTC and MTC was initially described by Lamberg et al. in 1981 [17], and Biscolla et al. [27] reported a fairly high rate (27/196, 13.8%) of concurrent PTC among patients with MTC in 2004. Several case reports of concurrent PTC and MTC have been published [2,8,9,14,17,18]. Although our case showed coexisting PTC and MTC separated by intervening normal thyroid tissue in the shape of a collision tumor, they were located very close to each other and appeared as one mass in the right thyroid lobe on ultrasonography and CT. Furthermore, the PTC and MTC components measured 1.4 cm and 1.8 cm, respectively, which are very similar. Consequently, the cytologic specimen based on FNA could involve both components evenly.
- Kim et al. [9] recently reported an original article containing comprehensive information on 10 cases of concurrent PTC and MTC; only four cases showed concurrent PTC and MTC in the same thyroid lobe. The maximal PTC diameter measured less than 1 cm (papillary microcarcinoma) in all but one case, and in only one case, the PTC was 1.7 cm and the MTC 0.5 cm in size. Because papillary microcarcinoma is a very prevalent neoplasm, accounting for up to 30% of all PTCs, the authors [9] suggested that concomitant PTC and MTC might be a simple coincidence. However, the coexisting PTC and MTC in the present study were very closely attached to each other, and their sizes were very similar. The various hypotheses mentioned above for a coexisting PTC and MTC should be reconsidered for explaining this phenomenon.
- Collectively, we presented a case of coexisting PTC and MTC in the same lobe in which the two components were attached but separated by thin intervening stroma, resulting in both components aspirated using FNA before surgical removal. Because the surgical plan and clinical course for PTC and MTC are different, cytopathologists should be aware of this entity. Although the pathogenesis of the coexistence of PTC and MTC is still poorly understood and diverse hypotheses have been suggested, this case may aid in determining the pathogenesis.
DISCUSSION
Ethics Statement
This study was approved by the Institutional Review Board of Samsung Medical Center (approval number: 2021-09-047). In addition, the patient had previously provided her written informed consent for publication of this case report and any accompanying images; her anonymity was fully respected throughout the publication process.
Availability of Data and Material
All data generated during this study are included in this case report. Further enquiries can be directed to the corresponding author.
Code Availability
Not applicable.
Author Contributions
Conceptualization: YLO. Data curation: HHK, YLO. Investigation: HHK. Visualization: HHK. Writing—original draft: HHK. Writing—review & editing: YLO. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
No funding to declare.



- 1. Kazaure HS, Roman SA, Sosa JA. Aggressive variants of papillary thyroid cancer: incidence, characteristics and predictors of survival among 43,738 patients. Ann Surg Oncol 2012; 19: 1874-80. ArticlePubMedPDF
- 2. Sadat Alavi M, Azarpira N. Medullary and papillary carcinoma of the thyroid gland occurring as a collision tumor with lymph node metastasis: a case report. J Med Case Rep 2011; 5: 590.PubMedPMC
- 3. De Felice M, Di Lauro R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev 2004; 25: 722-46. ArticlePubMed
- 4. Baloch ZW, LiVolsi VA. Special types of thyroid carcinoma. Histopathology 2018; 72: 40-52. ArticlePubMedPDF
- 5. Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 2003; 63: 1454-7. PubMed
- 6. Marsh DJ, Learoyd DL, Andrew SD, et al. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinoma. Clin Endocrinol (Oxf) 1996; 44: 249-57. ArticlePubMedPDF
- 7. Alberti L, Carniti C, Miranda C, Roccato E, Pierotti MA. RET and NTRK1 proto-oncogenes in human diseases. J Cell Physiol 2003; 195: 168-86. ArticlePubMed
- 8. Greco C, Brigante G, Taliani E, Corrado S, Simoni M, Madeo B. Concomitant medullary thyroid carcinoma with paraganglioma-like pattern and papillary thyroid carcinoma. Endocrinol Diabetes Metab Case Rep 2019; 2019: 19-0094. ArticlePubMedPMC
- 9. Kim WG, Gong G, Kim EY, et al. Concurrent occurrence of medullary thyroid carcinoma and papillary thyroid carcinoma in the same thyroid should be considered as coincidental. Clin Endocrinol (Oxf) 2010; 72: 256-63. ArticlePubMed
- 10. Maitra A. The endocrine system. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran pathologic basis of disease. 9th ed. Philadelphia: Elsevier publications, 2015; 1073-140. Article
- 11. Braverman LE. Werner and Ingbar’s the thyroid: a fundamental and clinical text. Philadelphia: Lippincott Williams and Wilkins, 2005.
- 12. Wells SA Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015; 25: 567-610. ArticlePubMedPMC
- 13. Jimenez C, Hu MI, Gagel RF. Management of medullary thyroid carcinoma. Endocrinol Metab Clin North Am 2008; 37: 481-96. ArticlePubMed
- 14. Rossi S, Fugazzola L, De Pasquale L, et al. Medullary and papillary carcinoma of the thyroid gland occurring as a collision tumour: report of three cases with molecular analysis and review of the literature. Endocr Relat Cancer 2005; 12: 281-9. ArticlePubMed
- 15. Sizemore GW. Medullary carcinoma of the thyroid gland. Semin Oncol 1987; 14: 306-14. PubMed
- 16. Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs . 4th ed. Lyon: IARC Press, 2017.
- 17. Lamberg BA, Reissel P, Stenman S, et al. Concurrent medullary and papillary thyroid carcinoma in the same thyroid lobe and in siblings. Acta Med Scand 1981; 209: 421-4. ArticlePubMed
- 18. Younes N, Shomaf M, Al Hassan L. Simultaneous medullary and papillary thyroid carcinoma with lymph node metastasis in the same patient: case report and review of the literature. Asian J Surg 2005; 28: 223-6. ArticlePubMed
- 19. Ahn D, Sohn JH, Park JY. A case of concurrent papillary and medullary thyroid carcinomas detected as recurrent medullary carcinoma after initial surgery for papillary carcinoma. J Korean Thyroid Assoc 2013; 6: 80-4. Article
- 20. Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology: definitions, criteria and explanatory notes. 2nd ed. Cham: Springer, 2010.
- 21. Tuttle M, Morris LF, Haugen B, et al. Thyroid-differentiated and anaplastic carcinoma. In: Amin MB, Edge SB, Greene F, eds. AJCC cancer staging manual. 8th ed. New York: Springer International Publishing, 2017; 881-98.
- 22. Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. J Am Soc Cytopathol 2017; 6: 217-22. ArticlePubMed
- 23. Green I, Ali SZ, Allen EA, Zakowski MF. A spectrum of cytomorphologic variations in medullary thyroid carcinoma: fine-needle aspiration findings in 19 cases. Cancer 1997; 81: 40-4. ArticlePubMed
- 24. Trimboli P, Treglia G, Guidobaldi L, et al. Detection rate of FNA cytology in medullary thyroid carcinoma: a meta-analysis. Clin Endocrinol (Oxf) 2015; 82: 280-5. PubMed
- 25. Liu CY, Chen CC, Bychkov A, et al. Constitutive cytomorphologic features of medullary thyroid carcinoma using different staining methods. Diagnostics (Basel) 2021; 11: 1396.ArticlePubMedPMC
- 26. Papaparaskeva K, Nagel H, Droese M. Cytologic diagnosis of medullary carcinoma of the thyroid gland. Diagn Cytopathol 2000; 22: 351-8. ArticlePubMed
- 27. Biscolla RP, Ugolini C, Sculli M, et al. Medullary and papillary tumors are frequently associated in the same thyroid gland without evidence of reciprocal influence in their biologic behavior. Thyroid 2004; 14: 946-52. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Evaluation of Diagnostic Accuracy of Medullary Thyroid Carcinoma Using Fine‐Needle Aspiration Cytology—Based on a Single Tertiary Centre Experience
Si‐Yi Chen, Dong‐Mei Gu
Cytopathology.2026;[Epub] CrossRef - Synchronous papillary and medullary thyroid carcinoma with distinct genetic mutations: A case report
Huanyu Jiang, Lijuan Zhou, Gang Zou, Haidong Zhang, Zhenkun Yu
Oral Oncology.2025; 161: 107191. CrossRef - Coexisting papillary and medullary thyroid carcinomas in a 60 year old male: a case report
Allahdad Khan, Anam Malik, Abdul Ahad Riaz, Muhammad Hussnain Sadiq, Muhammad Shahzaib Arshad, Alka Rani, Ibrahim Nagmeldin Hassan
Annals of Medicine & Surgery.2025; 87(10): 6740. CrossRef - Dedifferentiated Leiomyosarcoma of the Uterine Corpus with Heterologous Component: Clinicopathological Analysis of Five Consecutive Cases from a Single Institution and Comprehensive Literature Review
Suyeon Kim, Hyunsik Bae, Hyun-Soo Kim
Diagnostics.2024; 14(2): 160. CrossRef - Coexisting Medullary and Papillary Thyroid Carcinomas: A Case of Dual Neoplasia With a High Risk of Misdiagnosis
Santiago Sierra Castillo, Maria A Henao Rincón, David Aristizabal Colorado, David Alexander Vernaza Trujillo, Alin Abreu Lomba
Cureus.2024;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure




 E-submission
E-submission