Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 57(5); 2023 > Article
-
Case Study
EWSR1 rearranged primary renal myoepithelial carcinoma: a diagnostic conundrum -
Nilay Nishith
 , Zachariah Chowdhury
, Zachariah Chowdhury
-
Journal of Pathology and Translational Medicine 2023;57(5):284-288.
DOI: https://doi.org/10.4132/jptm.2023.08.08
Published online: September 15, 2023
Department of Onco-Pathology, Mahamana Pandit Madan Mohan Malviya Cancer Centre, Varanasi, India
- Corresponding Author: Nilay Nishith, MD, DNB (Pathology), Department of Onco-Pathology, Mahamana Pandit Madan Mohan Malviya Cancer Centre, BHU Campus, Sundarpur, Varanasi, Uttar Pradesh - 221005, India Tel: +91-9900241232, E-mail: drnilaynishith@gmail.com
© 2023The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Primary renal myoepithelial carcinoma is an exceedingly rare neoplasm with an aggressive phenotype and Ewing sarcoma breakpoint region 1 (EWSR1) rearrangement in a small fraction of cases. In addition to its rarity, the diagnosis can be challenging for the pathologist due to morphologic heterogeneity, particularly on the biopsy specimen. At times, immunohistochemistry may be indecisive; therefore, molecular studies should be undertaken for clinching the diagnosis. We aim to illustrate a case of primary myoepithelial carcinoma of the kidney with EWSR1-rearrangement in a 67-year-old male patient who presented with right supraclavicular mass, which was clinically diagnosed as carcinoma of an unknown primary. An elaborate immunohistochemical work-up aided by fluorescent in-situ hybridization allowed us to reach a conclusive diagnosis. This unusual case report advocates that one should be aware of the histological mimickers and begin with broad differential diagnoses alongside sporadic ones and then narrow them down with appropriate ancillary studies.
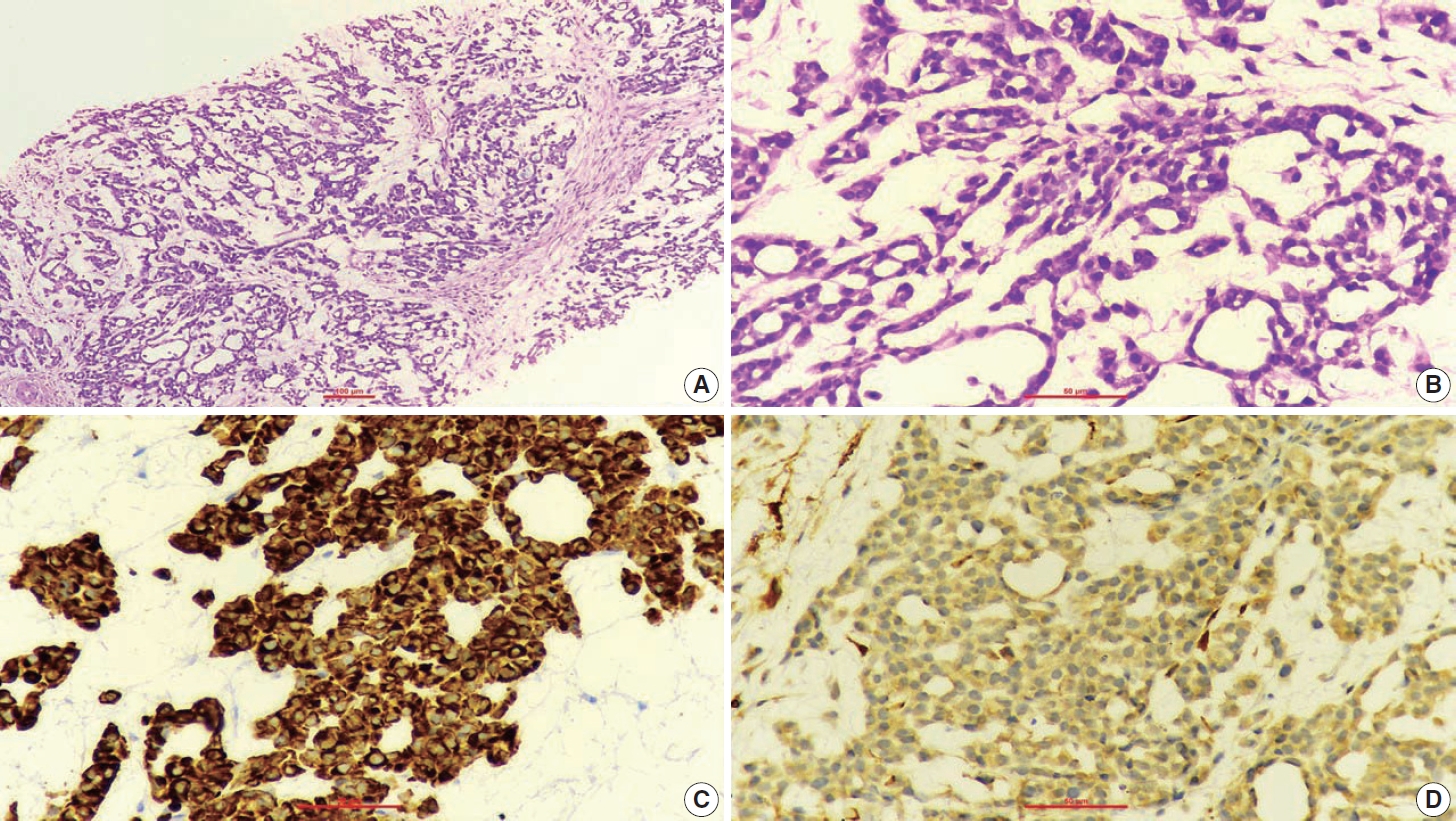
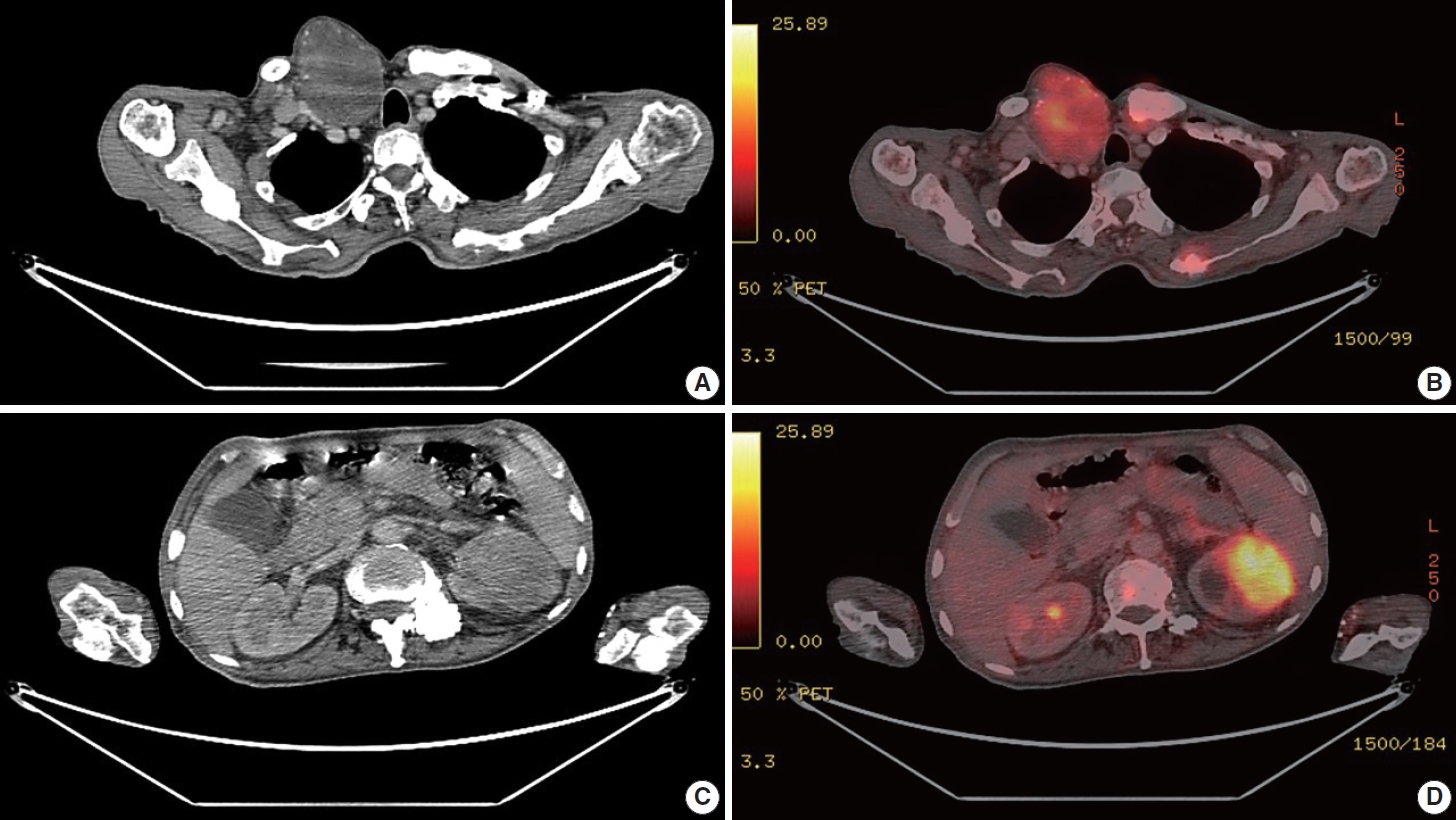
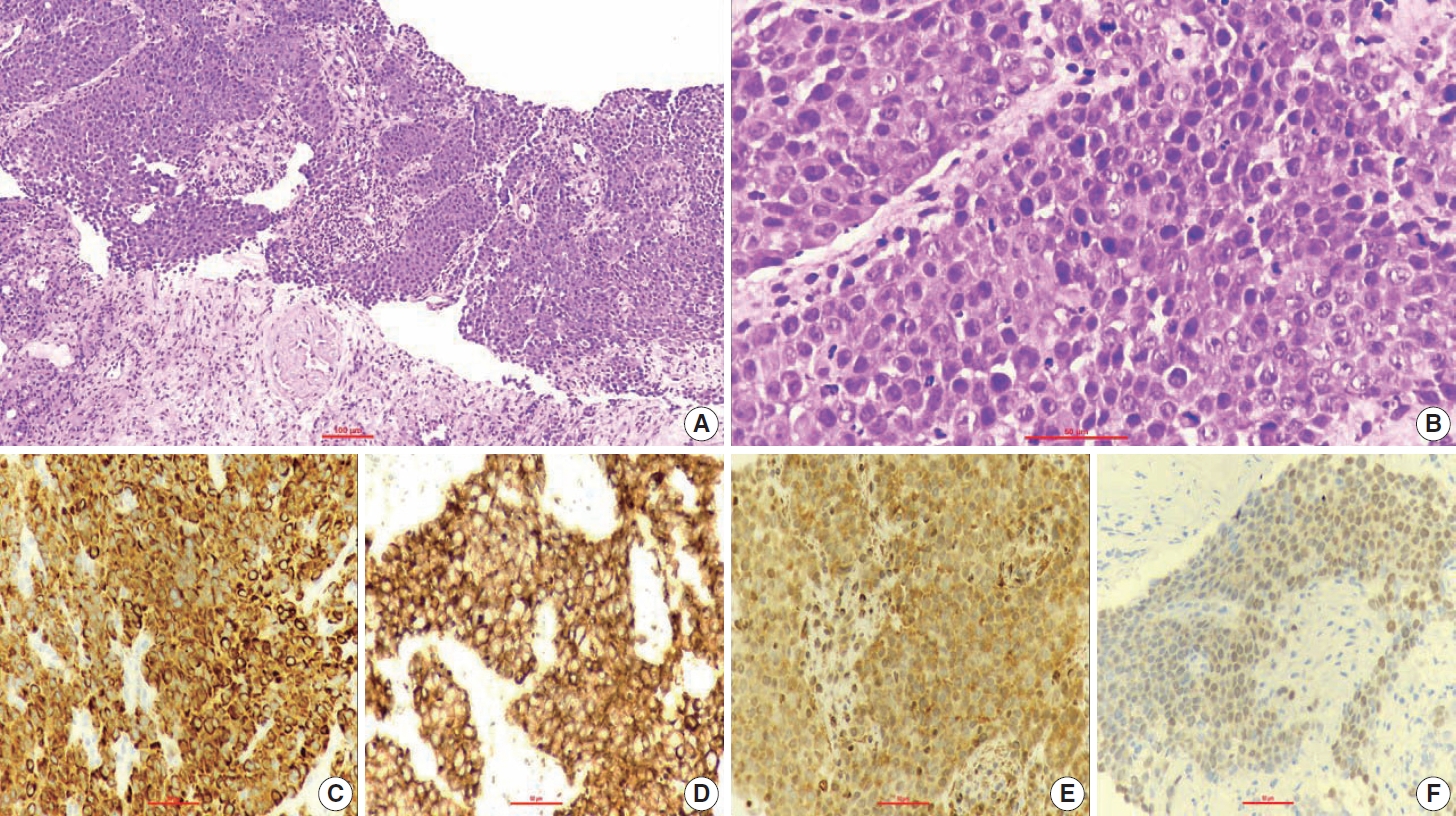
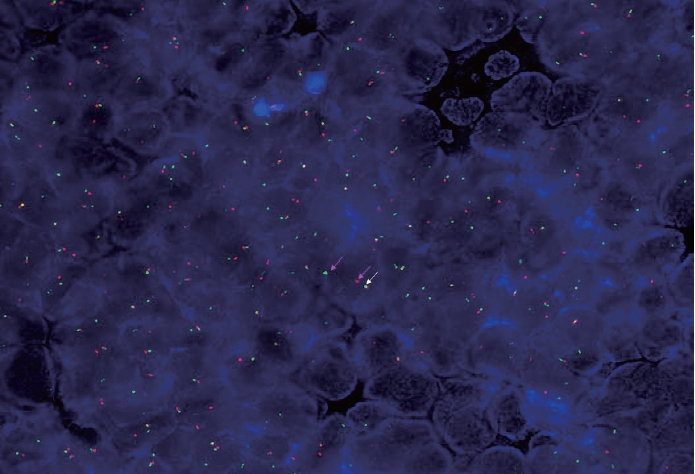
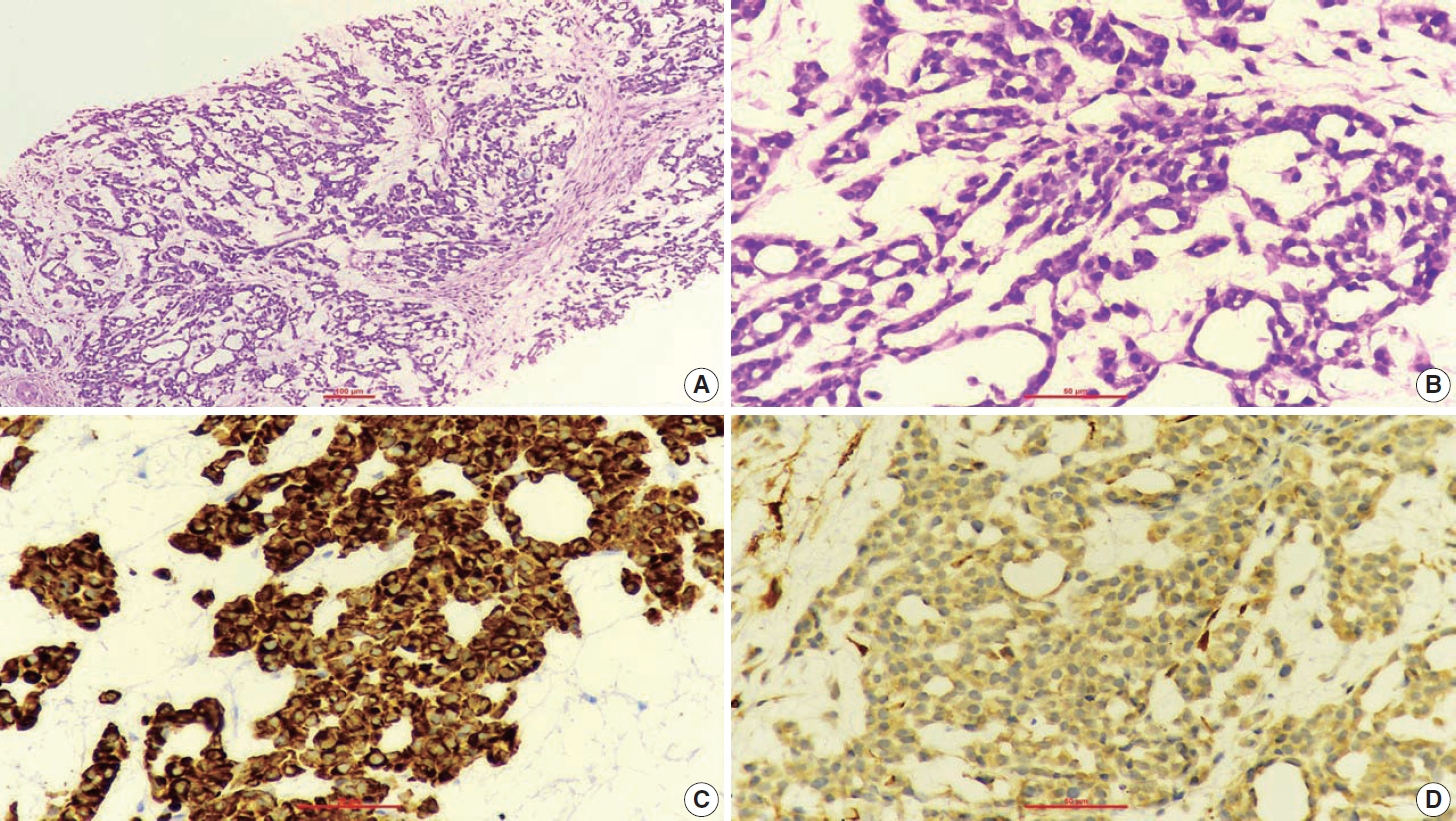
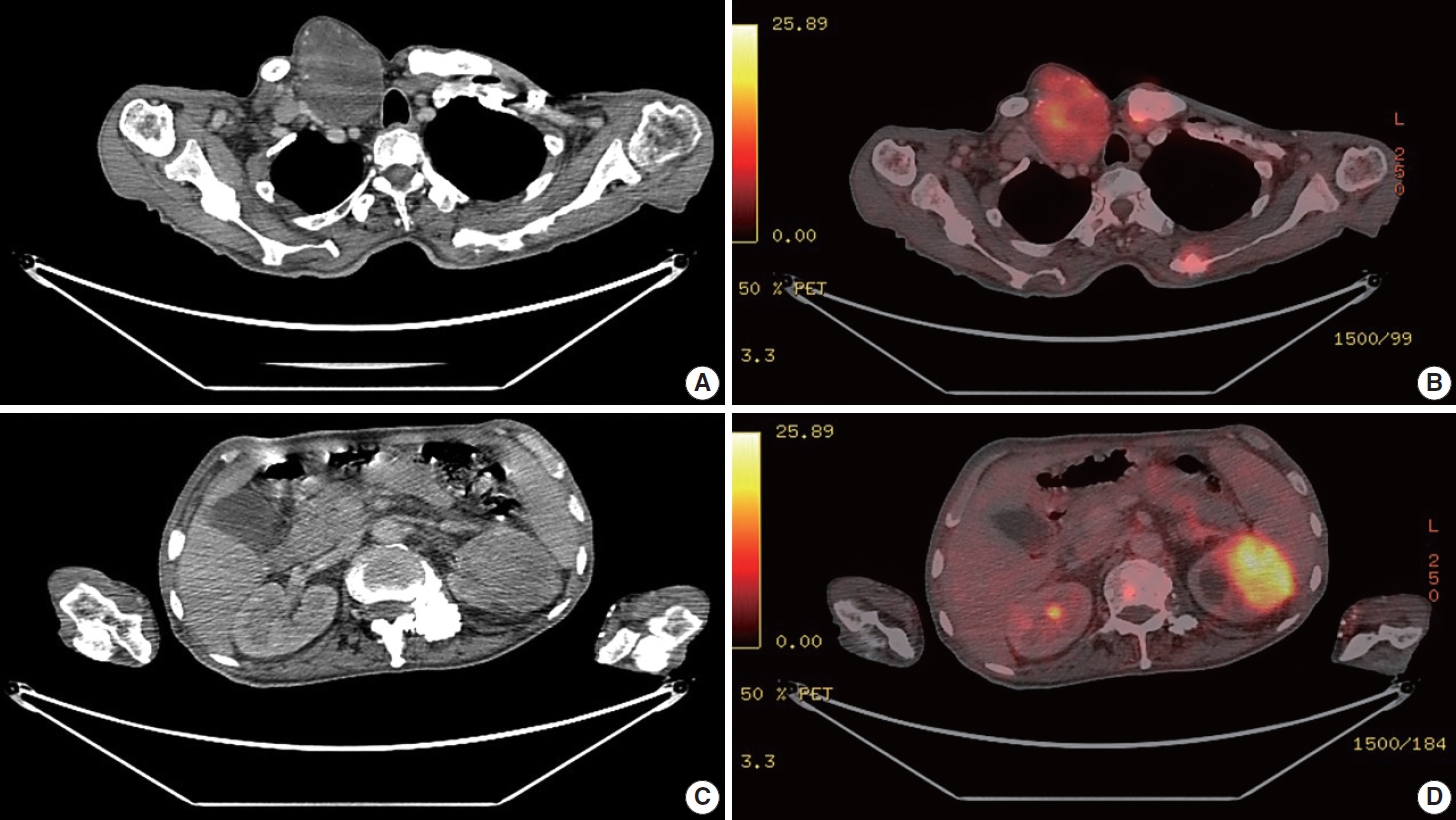
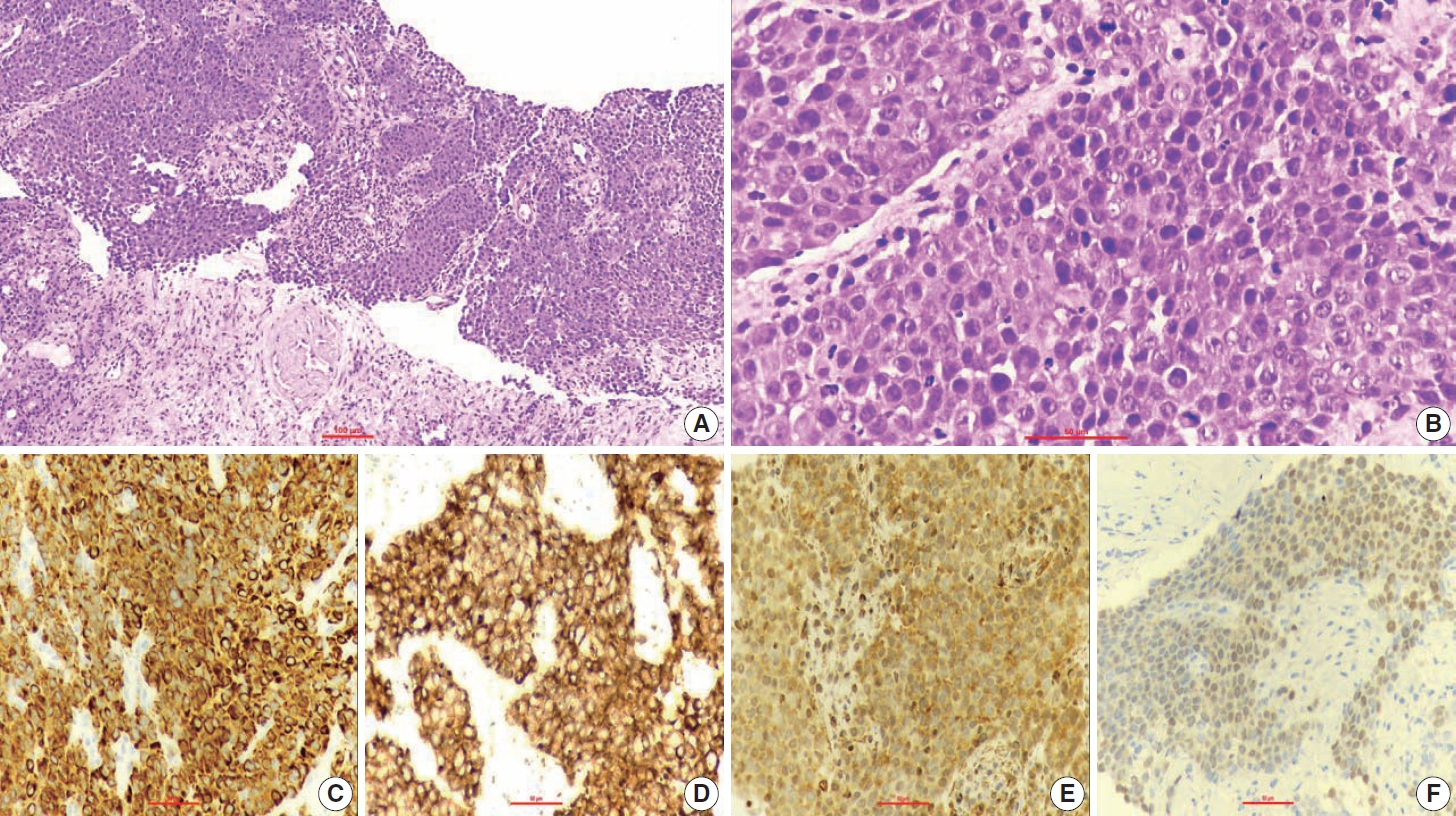
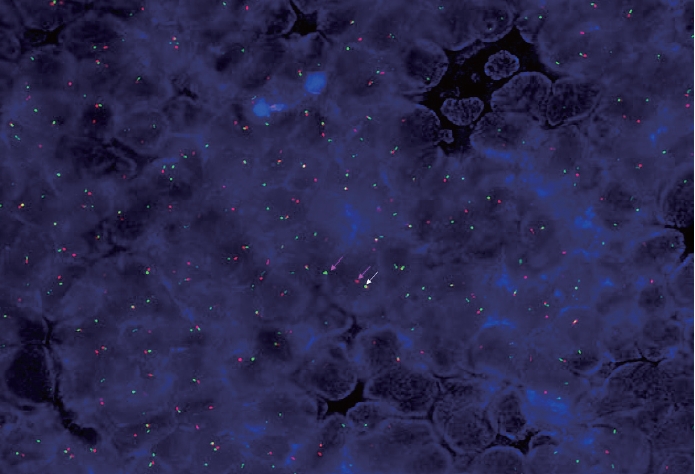
- A 67-year-old male patient presented to the head and neck outpatient department with a right supraclavicular mass. His baseline hematological investigation revealed severe anemia and neutrophilic leukocytosis. Subsequently, the right supraclavicular mass was sampled and sent for histopathological assessment. Microscopic examination favored a benign neoplasm, with the cells arranged in cords, trabeculae, tubules and singly scattered over a loose myxoid stroma. These cells were oval to spindle-shaped and possessed largely monomorphic nuclei, dispersed chromatin, inconspicuous nucleoli, and a scant to moderate amount of eosinophilic cytoplasm (Fig. 1A, B). Nuclear atypia, necrosis, or increased mitotic activity were absent. On immunohistochemistry, the neoplastic cells were positive for AE1/AE3, smooth muscle actin (SMA), and p63 (Fig. 1C, D) while were negative for cytokeratin (CK) 7, CK20, S-100, calponin, paired box 8 (PAX8), NKX3.1, NKX2.2, and TLE-1. Integrase interactor 1 (INI-1) expression was retained in these cells. Thus, a diagnosis of myoepithelioma was rendered. Further, positron emission tomography and computed tomography (PET-CT) was performed, which showed a fluorodeoxyglucose (FDG) avid right supraclavicular mass (eroding the medial end of the clavicle and impinging on the right thyroid lobe); measuring 75×61 mm, SUVmax 5.55 (Fig. 2A, B) along with FDG avid mass noted involving the upper pole and interpolar region of the left kidney; measuring 62×52 mm, SUVmax 10.26 (Fig. 2C, D). Hypermetabolic metastatic retroperitoneal lymph nodes, left adrenal, and lytic skeletal lesions were also present. The renal mass was also sampled to discriminate between a metastasis to the kidney versus a second primary. Microscopically, the cores of renal parenchyma exhibited infiltration by a tumor disposed in nests and sheets. The neoplastic cells are round, with a high nucleo-cytoplasmic ratio, fine to vesicular nuclei, prominent nucleoli, and scant cytoplasm (Fig. 3A, B). Brisk mitotic activity, tumor cell apoptosis, and areas of necrosis were also noted. Immunostaining showed positivity for AE1/AE3, SMA, CD10, p63 (Fig. 3C–F), while they were negative for PAX8, TFE3, S100, ALK1, HMB45, carbonic anhydrase IX, and vimentin. INI1 expression was retained in the tumor cells. Considering the findings of PET-CT, the report was validated as primary renal myoepithelial carcinoma with metastatic right supraclavicular mass. Subsequently, the left renal mass biopsy was subjected to fluorescent in-situ hybridization (FISH), which showed EWSR1 rearrangement (Fig. 4). He is being managed by the Department of Medical Oncology and has been offered the option of supportive care as the best alternative in view of the extensive disease burden, skeletal metastasis, and nodal involvement.
CASE REPORT
- Primary renal myoepithelial carcinoma is a rare tumor, which was first described in 1998, and since then, there have been only a few reported cases in the literature. It typically presents with nonspecific symptoms such as abdominal pain, hematuria, and flank mass [2-4]. Imaging studies, such as computed tomography or magnetic resonance imaging, are often used to diagnose and stage the tumor. However, these imaging modalities are not specific for malignant myoepithelioma and may lead to a misdiagnosis at both usual and uncommon sites such as the kidney [2,3,5,6].
- Renal myoepithelial carcinomas have overlapping histological features and share similarities with their soft tissue counterpart. These are characterized by variable combinations of distinct cellular and stromal components. Different cell types (epithelioid, spindled, plasmacytoid, and clear) can be appreciated in variable proportions and are arranged in cords, trabeculae, solid sheets, and occasionally ducts. The cellular component is embedded in a myxoid/chondromyxoid to hyalinized stroma, with occasional frank chondroid and osseous differentiation. Immunohistochemical studies reveal that the neoplastic cells express myoepithelial markers in variable degrees, including smooth muscle actin, calponin, CD10, and p63. Thus, a diagnosis of malignant myoepithelioma is rendered based on histological and immunohistochemistry findings [1,5,7]. In addition, molecular analysis may help establish the diagnosis, as some cases have been found to harbor EWSR1 rearrangements. Recent studies have identified EWSR1 rearrangements as a potential pathologic mechanism underlying this unusual neoplasm. EWSR1 rearrangement involves the fusion of the EWSR1 gene with a partner gene, such as PBX1, ZNF444, KLF15, or POU5F1 [8-12]. This fusion event results in the formation of an EWSR1 fusion protein, which plays a crucial role in the pathogenesis of primary renal myoepithelial carcinoma. The EWSR1 fusion protein interacts with transcription factors, such as CREB1 and ATF1, and regulates downstream target genes involved in cell proliferation and survival. KLF15 plays a crucial role in podocyte differentiation, mesangial cell proliferation, and renal fibrosis modulation in the kidney. Distal tubular, collecting duct, mesangial epithelial cells, and renal cortical fibroblasts also express KLF15. Based on these observations, it may be speculated that the manifestation of an EWSR1::KLF15 fusion within the kidney as MEC could be organ-specific. However, the clinical significance of EWSR1 rearrangements in malignant myoepithelioma remains unclear [2,3,11,12].
- The rarity of primary renal myoepithelial carcinoma poses a diagnostic challenge for clinicians as well as pathologists. The tumor may be misdiagnosed as other renal malignancies, such as renal cell carcinoma, collecting duct carcinoma, and sarcomatoid renal cell carcinoma. Immunohistochemistry studies are essential to differentiate myoepithelial carcinoma from these histological mimickers. Further, molecular studies, particularly EWSR1 rearrangement analysis, may help in clinching the diagnosis. The identification of these exceptionally rare neoplasms becomes furthermore challenging on biopsy samples. Therefore, it is a good practice to start with broad differential diagnoses alongside sporadic ones and then narrow them down with the assistance of immunohistochemistry.
- In the present case, the initial work-up revealed myoepithelioma of soft tissue origin in the right supraclavicular region. Subsequently, he was thoroughly investigated and found to have a large renal mass with SUVmax greater than the soft tissue lesion, indicating that the former is more metabolically active than the latter and hence, has a higher propensity to metastasize. The histopathological analysis of the renal mass is revealed to be myoepithelial carcinoma exhibiting brisk mitotic activity, tumor cell apoptosis, and areas of necrosis. An extensive panel of immunohistochemical markers was used to exclude all possible differential diagnoses, including primary renal neoplasms and metastatic lesions. Finally, in conjunction with PET-CT findings, he was diagnosed with primary renal myoepithelial carcinoma with metastatic right supraclavicular mass. The diagnosis was confirmed on FISH using EWSR1 break-apart probe. Another pertinent point to note is that the histomorphological characteristics of the primary and metastatic lesions are not the same. This difference could be attributed to the fact that both lesions were large and were diagnosed on biopsy specimens. Therefore, it may be possible that both masses display different histological features owing to the morphological heterogeneity of myoepithelial carcinoma, site of biopsy, and differences in metabolic activity as indicated by SUVmax. An extensive literature search revealed that three of the four prior cases also presented with distant metastasis (mainly lung) at the time of diagnosis [2-4]. However, none have commented upon the histology of the metastatic lesions. On the other hand, we have detailed the histological features of both primary and metastatic lesions and emphasized the differences and the likely reasons for the same.
- The existing literature about primary renal myoepithelial carcinoma is very limited and is available in the form of mere case reports. A thorough appraisal of the published articles indicates that these are highly aggressive tumors with poor prognosis. Surgery remains the mainstay of treatment, and radical nephrectomy is recommended for localized disease. Adjuvant chemotherapy and/or radiotherapy may be considered for high-risk cases or metastatic disease, although their efficacy is uncertain. There is currently no standard systemic therapy for primary renal myoepithelial carcinoma, and clinical trials are urgently needed to develop effective treatment strategies [2-4].
- Primary renal myoepithelial carcinoma is a rare and aggressive tumor, and a subset of these neoplasms show EWSR1 gene rearrangement. The biphasic histology and characteristic immunophenotype help to distinguish MEC from other renal neoplasms. Molecular studies are crucial for accurately diagnosing this malignancy and differentiating it from its histological mimickers. Surgery remains the mainstay of treatment, and adjuvant therapies may be considered for high-risk cases. However, the prognosis remains poor and further research is needed to improve our understanding of the molecular biology of primary renal myoepithelial carcinoma and to develop effective treatment strategies.
DISCUSSION
Ethics Statement
Formal written informed consent was not required with a waiver by the appropriate institutional review board (No. 285/22 MPMMCC, Varanasi, India dated 26th December 2022).
Availability of Data and Material
Data sharing is not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Author contributions
Conceptualization: NN, ZC. Writing—original draft: NN. Writing—review & editing: ZC. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
Acknowledgments
- 1. Jo VY. Soft tissue special issue: myoepithelial neoplasms of soft tissue: an updated review with emphasis on diagnostic considerations in the head and neck. Head Neck Pathol 2020; 14: 121-31. ArticlePubMedPMCPDF
- 2. She YH, Chen YT, Huang YM, et al. Primary renal myoepithelial carcinoma with EWSR-1 gene rearrangement: case report and literature review. Acta Nephrol 2021; 35: 105-11.
- 3. Cajaiba MM, Jennings LJ, Rohan SM, et al. Expanding the spectrum of renal tumors in children: primary renal myoepithelial carcinomas with a novel EWSR1-KLF15 fusion. Am J Surg Pathol 2016; 40: 386-94. PubMed
- 4. Li Q, Mou Z, Yang K, Jiang H. A first case report of primary epithelial myoepithelial carcinoma-like renal tumor showing a perivascular pseudorosette-like pattern: description of morphologic, immunohistochemical, and genetic features. Medicine (Baltimore) 2019; 98: e17245. PubMedPMC
- 5. Yue D, Feng W, Ning C, Han LX, YaHong L. Myoepithelial carcinoma of the salivary gland: pathologic and CT imaging characteristics (report of 10 cases and literature review). Oral Surg Oral Med Oral Pathol Oral Radiol 2017; 123: e182-7. ArticlePubMed
- 6. Hiyama T, Kuno H, Sekiya K, Oda S, Kobayashi T. Imaging of malignant minor salivary gland tumors of the head and neck. Radiographics 2021; 41: 175-91. ArticlePubMed
- 7. Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol 2003; 27: 1183-96. PubMed
- 8. Antonescu CR, Zhang L, Chang NE, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors: a molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer 2010; 49: 1114-24. ArticlePubMedPMC
- 9. Brandal P, Panagopoulos I, Bjerkehagen B, Heim S. t(19;22)(q13;q12) Translocation leading to the novel fusion gene EWSR1-ZNF444 in soft tissue myoepithelial carcinoma. Genes Chromosomes Cancer 2009; 48: 1051-6. ArticlePubMed
- 10. Agaram NP, Chen HW, Zhang L, et al. EWSR1-PBX3: a novel gene fusion in myoepithelial tumors. Genes Chromosomes Cancer 2015; 54: 63-71. ArticlePubMed
- 11. McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiol Rev 2010; 90: 1337-81. PubMed
- 12. Uchida S, Tanaka Y, Ito H, et al. Transcriptional regulation of the CLC-K1 promoter by myc-associated zinc finger protein and kidney-enriched Kruppel-like factor, a novel zinc finger repressor. Mol Cell Biol 2000; 20: 7319-31. PubMedPMC
REFERENCES
Figure & Data
References
Citations

- Primary Ewing Sarcoma of the Kidney
João Lobo, Huiying He, Raheel Ahmed, Bassel Zein-Sabatto, Thomas Winokur, Shi Wei, Shuko Harada, Jesse K. McKenney, Jonathan L. Myles, Jane K. Nguyen, Christopher G. Przybycin, Sean R. Williamson, Cristina Magi-Galluzzi, Reza Alaghehbandan
American Journal of Surgical Pathology.2025; 49(10): 1078. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure





 E-submission
E-submission