Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 58(1); 2024 > Article
-
Original Article
Tumor-infiltrating T lymphocytes evaluated using digital image analysis predict the prognosis of patients with diffuse large B-cell lymphoma -
Yunjoo Cho1
 , Jiyeon Lee1,2
, Jiyeon Lee1,2 , Bogyeong Han3
, Bogyeong Han3 , Sang Eun Yoon4
, Sang Eun Yoon4 , Seok Jin Kim4
, Seok Jin Kim4 , Won Seog Kim4
, Won Seog Kim4 , Junhun Cho1
, Junhun Cho1
-
Journal of Pathology and Translational Medicine 2024;58(1):12-21.
DOI: https://doi.org/10.4132/jptm.2023.11.02
Published online: January 10, 2024
1Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
2Department of Pathology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
3Department of Pathology, Seoul National University, Seoul National College of Medicine, Seoul, Korea
4Division of Hematology and Oncology, Department of Internal Medicine, Sungkyunkwan University School of Medicine, Seoul, Korea
- Corresponding Author: Junhun Cho, MD, Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea Tel: +82-2-3410-1891, Fax: +82-2-3410-2831 E-mail: jununius@naver.com
© 2024 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- The implication of the presence of tumor-infiltrating T lymphocytes (TIL-T) in diffuse large B-cell lymphoma (DLBCL) is yet to be elucidated. We aimed to investigate the effect of TIL-T levels on the prognosis of patients with DLBCL.
-
Methods
- Ninety-six patients with DLBCL were enrolled in the study. The TIL-T ratio was measured using QuPath, a digital pathology software package. The TIL-T ratio was investigated in three foci (highest, intermediate, and lowest) for each case, resulting in TIL-T–Max, TIL-T–Intermediate, and TIL-T–Min. The relationship between the TIL-T ratios and prognosis was investigated.
-
Results
- When 19% was used as the cutoff value for TIL-T–Max, 72 (75.0%) and 24 (25.0%) patients had high and low TIL-T–Max, respectively. A high TIL-T–Max was significantly associated with lower serum lactate dehydrogenase levels (p < .001), with patient group who achieved complete remission after RCHOP therapy (p < .001), and a low-risk revised International Prognostic Index score (p < .001). Univariate analysis showed that patients with a low TIL-T–Max had a significantly worse prognosis in overall survival compared to those with a high TIL-T–Max (p < .001); this difference remained significant in a multivariate analysis with Cox proportional hazards (hazard ratio, 7.55; 95% confidence interval, 2.54 to 22.42; p < .001).
-
Conclusions
- Patients with DLBCL with a high TIL-T–Max showed significantly better prognosis than those with a low TIL-T–Max, and the TIL-T–Max was an independent indicator of overall survival. These results suggest that evaluating TIL-T ratios using a digital pathology system is useful in predicting the prognosis of patients with DLBCL.
- Patient selection
- Ninety-six consecutive patients diagnosed with DLBCL NOS at Samsung Medical Center, Seoul, Korea, from January to December 2018, were enrolled in this study. Small biopsy and diagnostic resection were performed in 66 and 30 patients, respectively. The patients’ clinical and pathological information was evaluated by reviewing their electronic medical records. There were 48 males and 48 females, with a male-to-female ratio of 1:1. The median age of the patients was 61 years (range, 5 to 86 years). According to the Ann Arbor stage, 52 patients (54.2%) were stage I–II and 44 (45.8%) were stage III–IV. The number of cases subtyped by cells of origin (COO) [19] was 32 (33.3%) for the germinal center B-cell (GCB) type and 64 (66.7%) for the non-GCB type. At the time of diagnosis, serum lactate dehydrogenase (LDH) levels were elevated above the normal range in 39 patients (40.6%). All patients initially received R-CHOP chemotherapy, except for five patients who either died or were lost to follow-up before they could be treated. Fifty of the 91 treated patients achieved complete remission (CR) after the standard six course of R-CHOP. Among the 41 patients who did not, nine were only treated with R-CHOP, 17 went on to receive second and/or thirdline chemotherapy, and 15 had autologous stem cell transplantation. Based on the revised International Prognostic Index (R-IPI) [20], which takes into consideration the number of negative prognostic factors present at the time of diagnosis (age >60 years, stage III/IV disease, elevated LDH level, Eastern Cooperative Oncology Group performance status ≥ 2, more than one extranodal site of disease), cases were stratified into low-risk group (0–2 negative factors) and high-risk group (3–5 negative factors). Sixty-three (65.6%) cases were low risk and 33 (34.4%) were high risk. The clinicopathological characteristics of the patients are summarized in Table 1. The median follow-up period was 1,181 days (range, 2 to 1,894 days).
- Computational image analysis
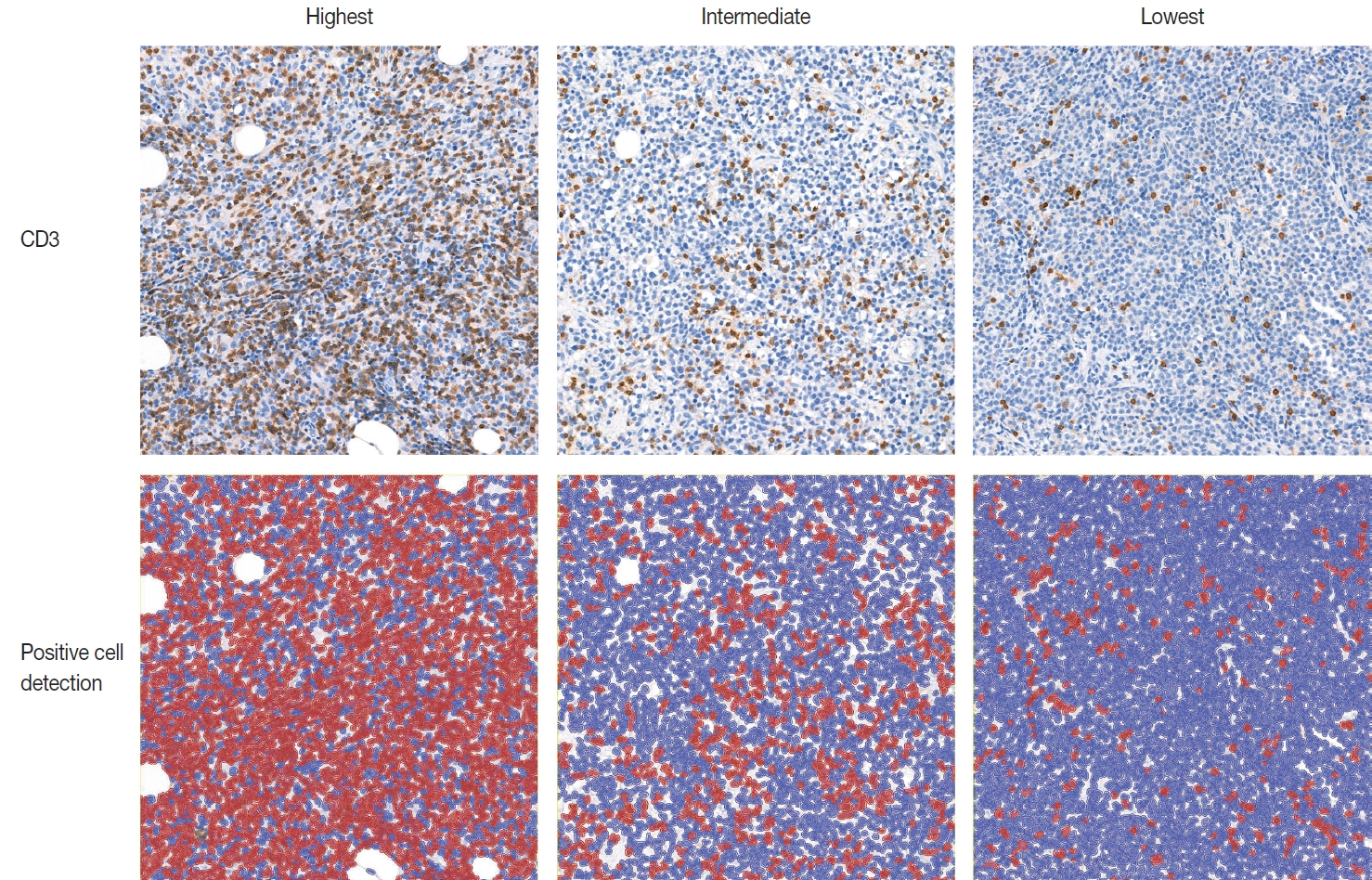
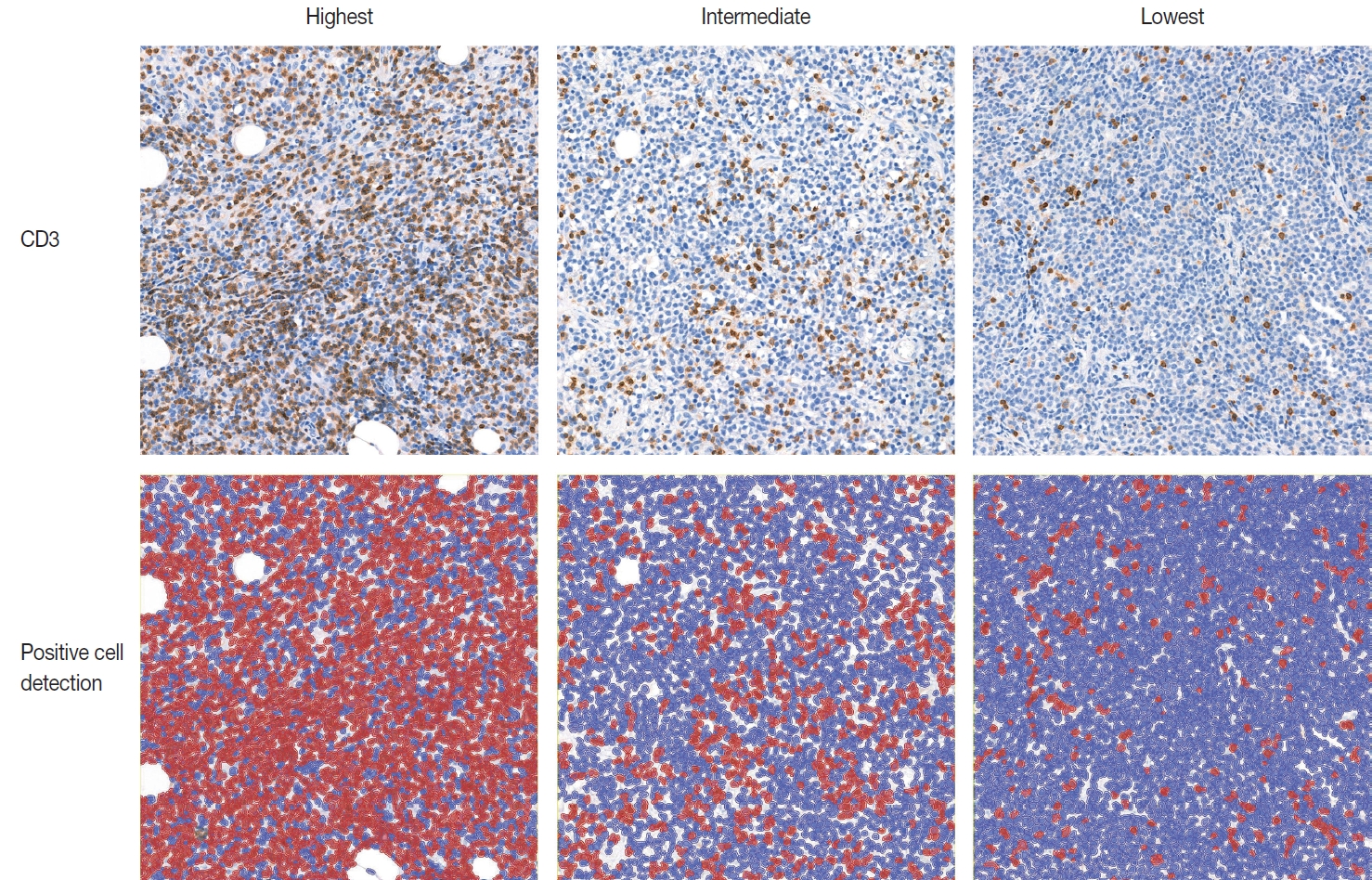
- IHC for CD3 (Polyconal, Dako, Glostrup, Denmark) and CD20 (clone L26, Dako), performed at the time of diagnosis, was used for TIL-T ratio evaluation. All slides were scanned using a Pannoramic 1000 scanner (3DHistech, Budapest, Hungary). The INFINITT DPS (INFINITT Healthcare, Seoul, Korea) was used as the image-viewing system. Two independent pathologists (Y.C. and J.C.), without prior knowledge of the patients’ clinical outcomes, thoroughly evaluated tumor areas with dense B-cell populations, as detected by CD20 IHC. Among the differing densities of CD3-positive lymphocytes, the areas with the highest, lowest, and intermediate densities of CD3-positive cells in each case were manually selected. Areas with the highest and the lowest densities were first identified, then those areas were excluded from determining the intermediate density area. Intermediate density was taken from an area occupying the largest proportion of the tumor with similar level of CD3-positive lymphocyte density. Disagreements were resolved by consensus, and the consensus foci were adopted as final. All the selected areas were then photographed in a 400 × 400 μm square image. The image files were processed using QuPath software [21]. Cell-detection was conducted using QuPath’s built-in “Positive cell detection” to determine the ratio of TIL-T to total cell of each focus (Fig. 1). The TIL-T ratios of the areas with the highest, lowest, and intermediate CD3-positive cell densities were denoted as TIL-T–Max, TIL-T–Min, and TIL-T–Intermediate, respectively.
- Statistical analysis
- Comparisons between clinical features and TIL-T ratios were performed using Pearson’s chi-square test. Overall survival (OS) was defined as the time from the date of diagnosis to death from any cause. Survival distribution was compared using the Kaplan-Meier method and log-rank test. Prognostic variables associated with OS were examined by univariate analysis using the Cox proportional hazards regression model. Only variables significantly associated with survival were included in the multivariate regression analyses. Statistical analyses were performed, and graphics were obtained using R ver. 4.1.3 (https://www.r-project.org). A p-value < .05 was considered statistically significant.
MATERIALS AND METHODS
- Evaluation of TIL-T ratio and comparison with clinicopathological parameters
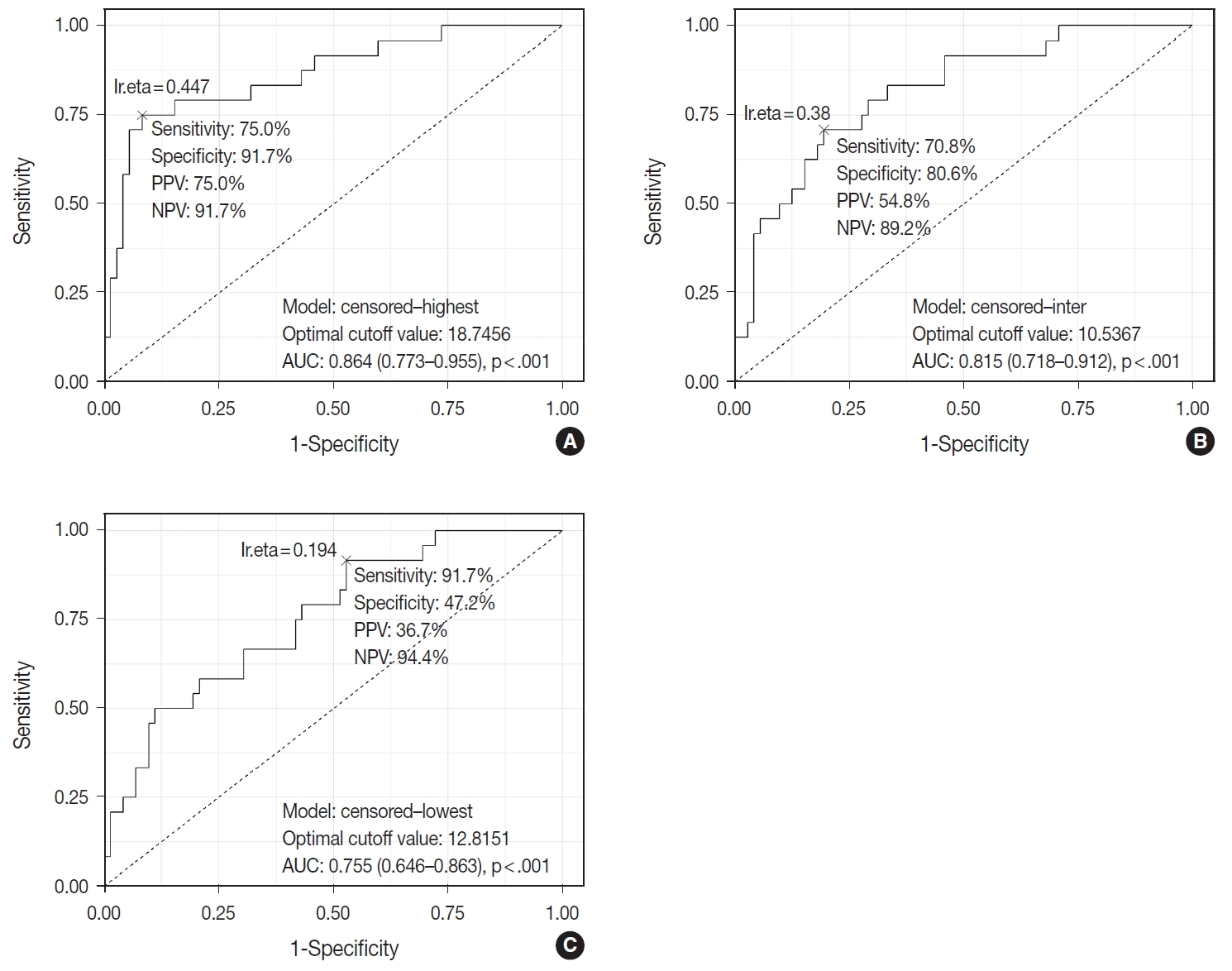
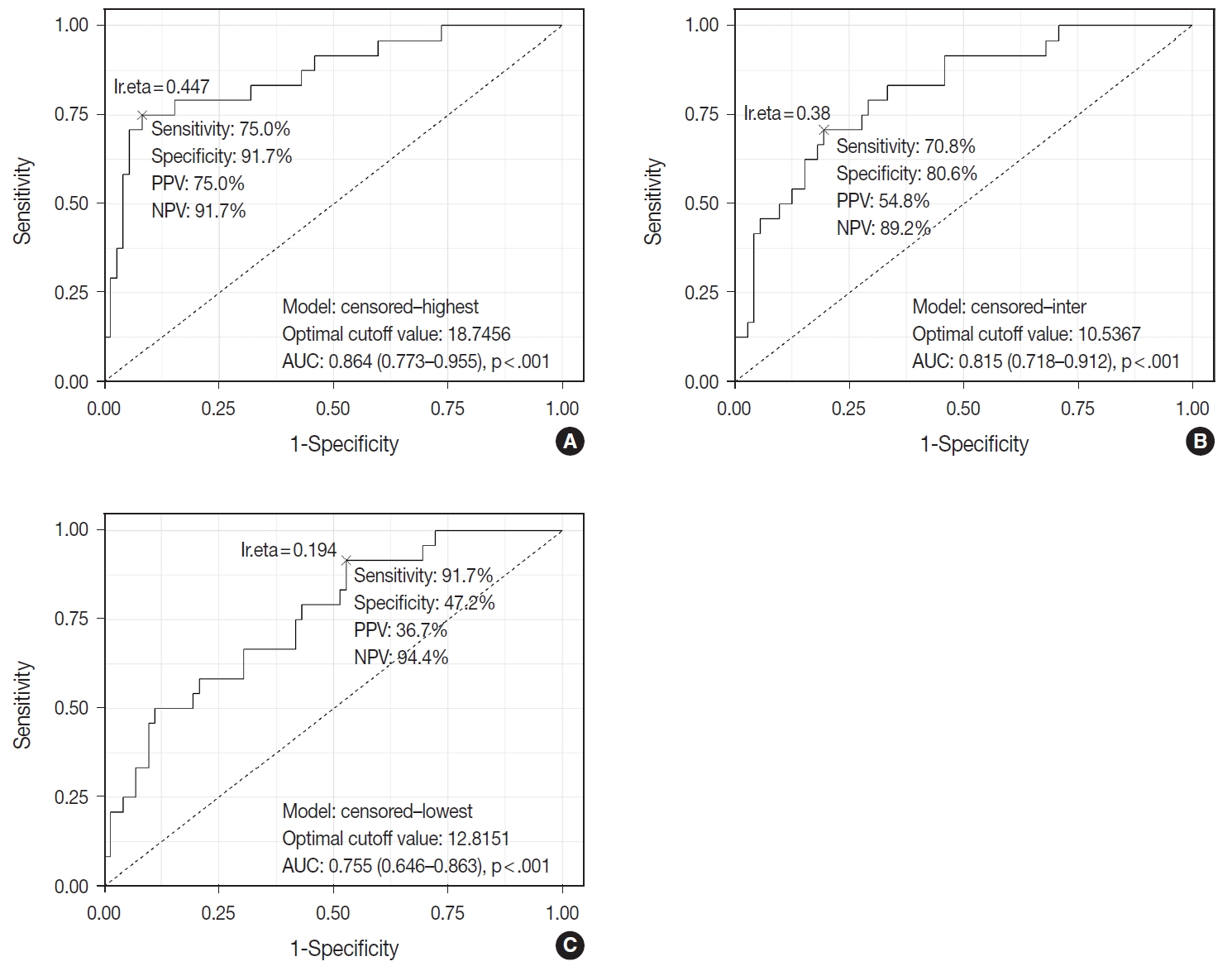
- TIL-T–Max, TIL-T–Min, and TIL-T–Intermediate were calculated as described above. The receiver operating characteristic curve analysis was used to determine the optimal cutoff value with respect to the OS of patients for each TIL-T ratio (Fig. 2). Cutoff points between high and low TIL-T ratios were 19%, 11%, and 13% for TIL-T–Max, TIL-T–Intermediate, and TILT– Min, respectively. With TIL-T–Max as the criterion, 72 (75.0%) had > 19% TIL-T–Max and 24 (25.0%) had < 19% TIL-T–Max; with TIL-T–Intermediate, 64 (66.7%) patients had a high TIL-T-Intermediate and 32 (33.3%) had a low TIL-T–Intermediate; and with TIL-T–Min, 36 (37.5%) had a high TIL-T–Min and 60 (62.5%) had a low TIL-T–Min (Table 1). The mean values for the TIL-T–Max, –Intermediate, and –Min were 41.6 % (range, 0.3% to 98.5%; standard deviation [SD], ± 27.72), 26.3% (range, 0.2% to 97.5%; SD, ± 23.18), and 14.4% (range, 0.1% to 88.8%; SD, ±16.75), respectively. The clinicopathological parameters associated with TIL-T ratios are listed in Table 1. A high TIL-T–Max was significantly associated with serum LDH levels within the normal range (χ2 = 17.634, degrees of freedom [df] = 1, p < .001), with the group of patients that achieved CR after initial R-CHOP therapy (χ2 = 10.908, df = 1, p < .001) and with lowrisk R-IPI (χ2 = 12.945, df = 1, p < .001). A high TIL-T–Intermediate was also significantly associated with low serum LDH levels (χ2 = 8.211, df = 1, p = .004), CR after R-CHOP (χ2 = 9.647, df = 1, p = .002), and a low risk of R-IPI (χ2 = 4.208, df = 1, p = .040). The TIL-T–Min was not significantly associated with any of the clinical variables.
- Survival analysis
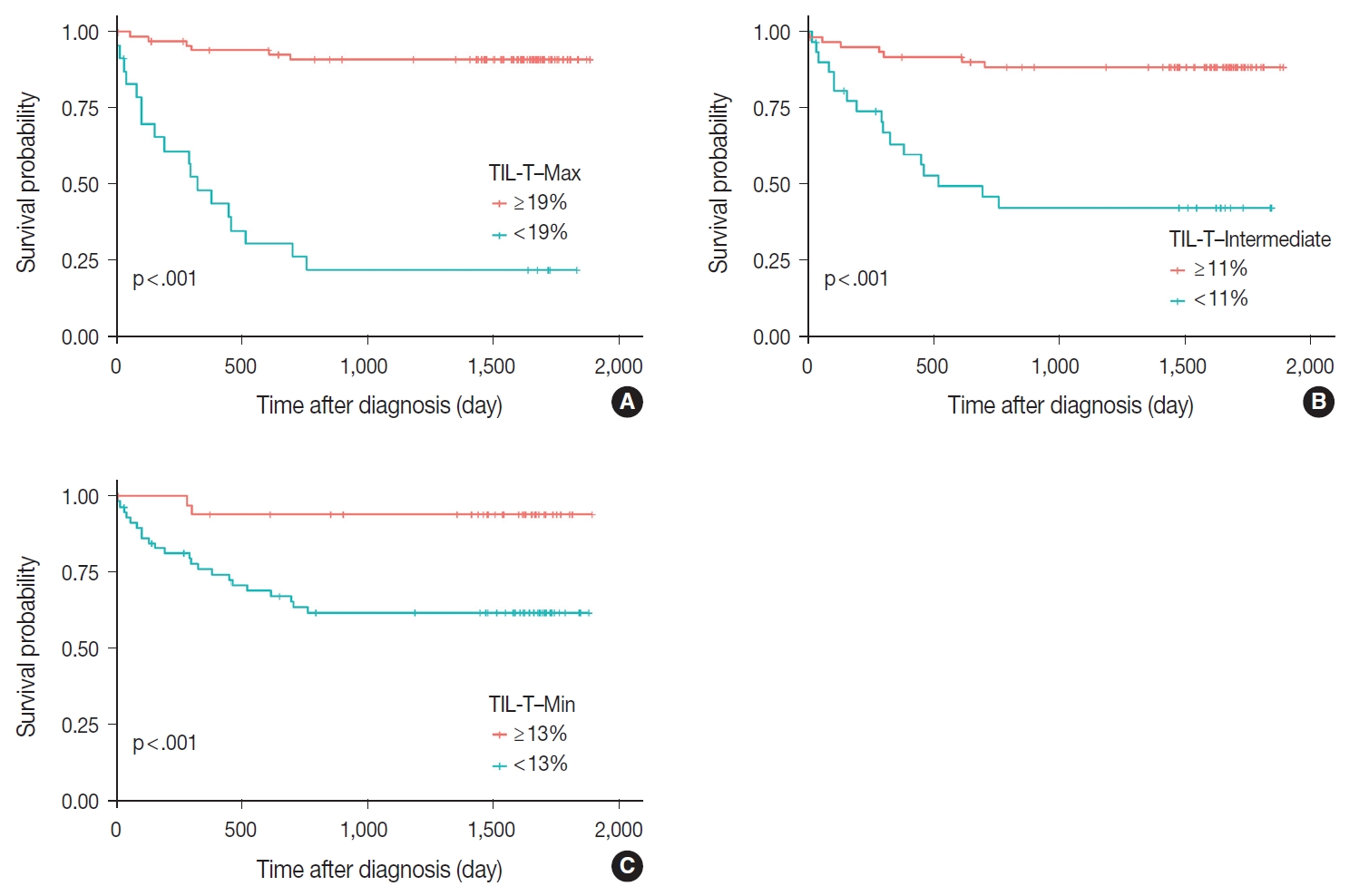
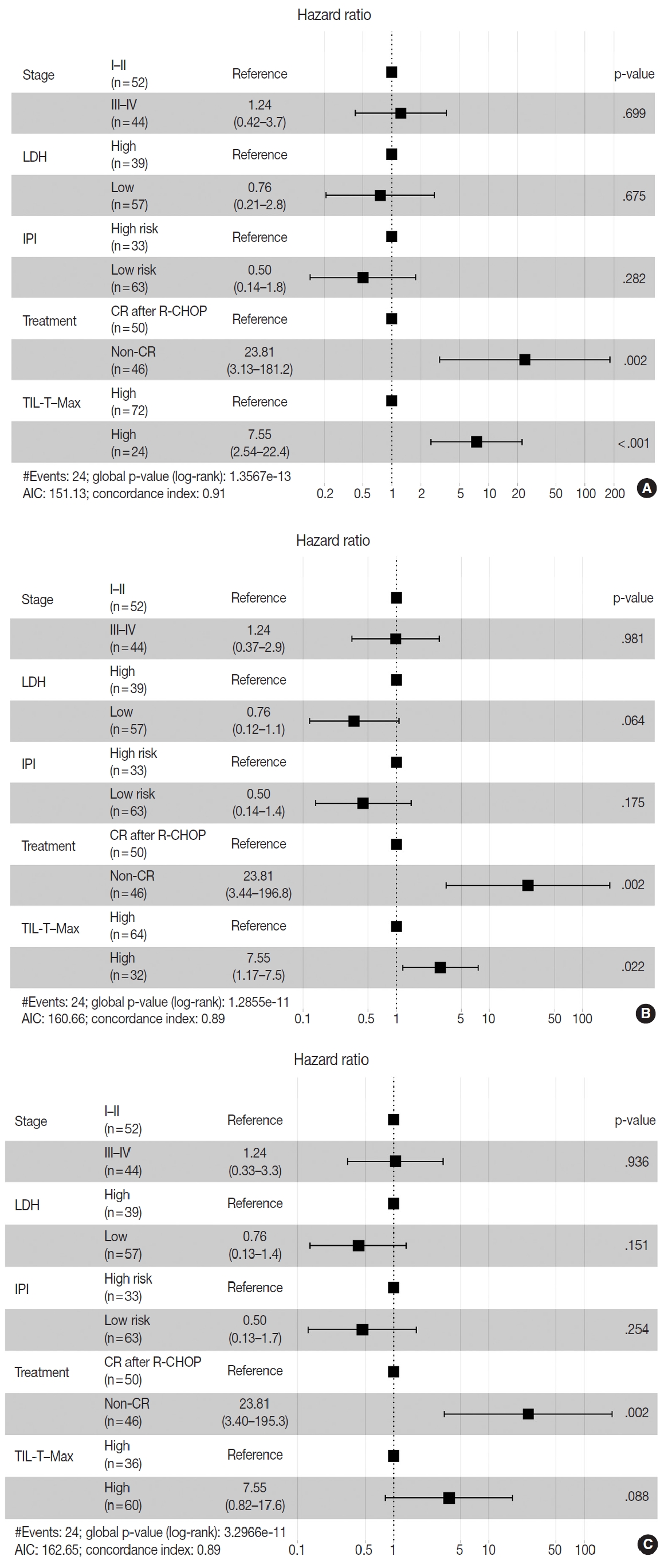
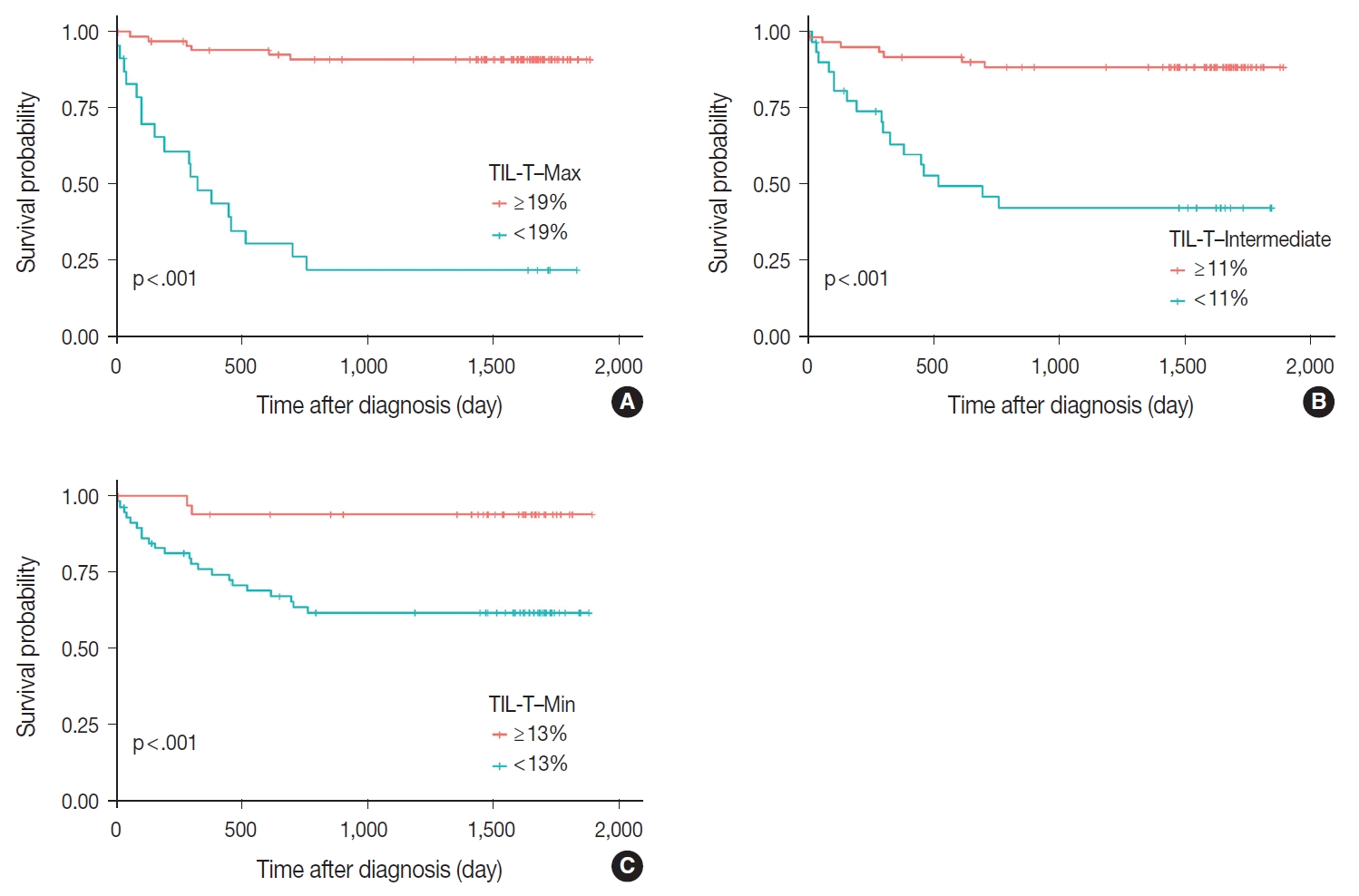
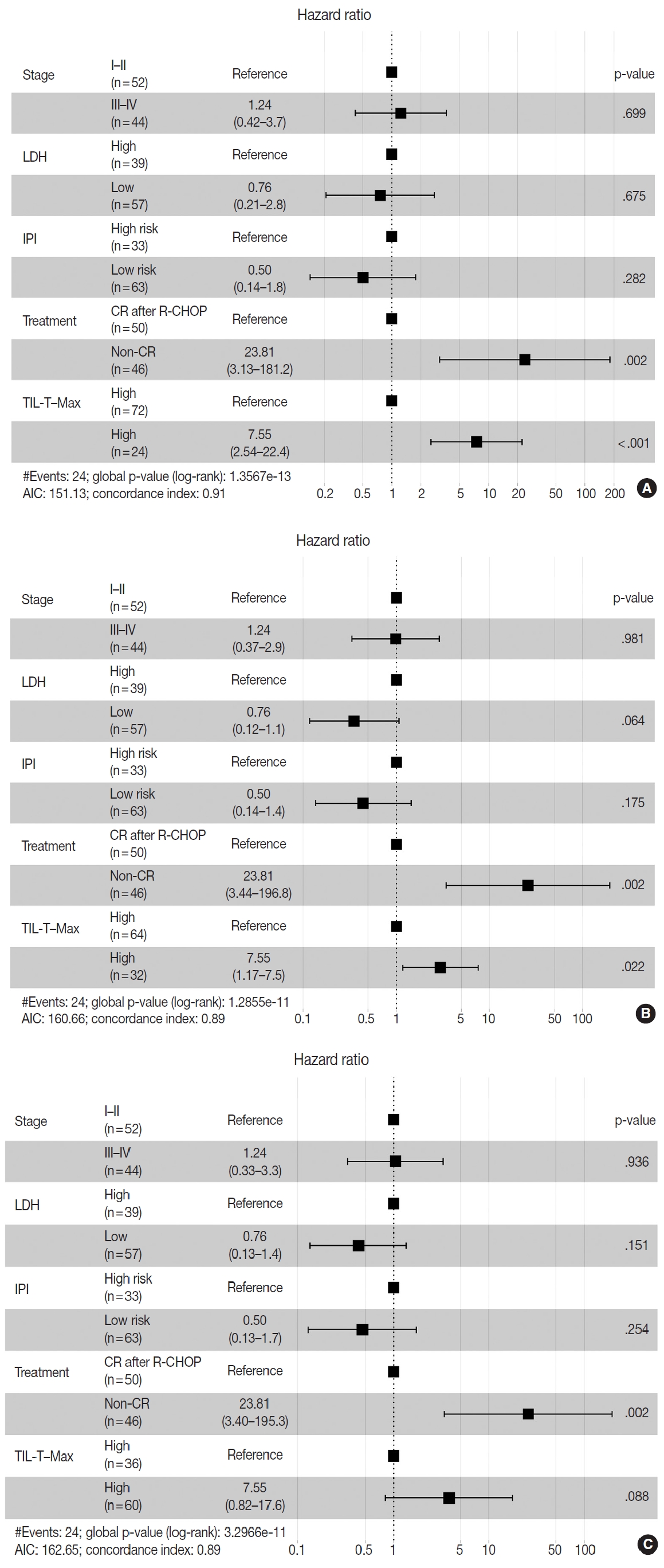
- Patients with a high TIL-T–Max showed a significantly longer OS (p < .001) (Fig. 3A). This trend was also observed in the high TIL-T–Intermediate (p < .001) (Fig. 3B) and high TIL-T– Min (p < 0.001) (Fig. 3C). According to the univariate Cox proportional hazards analysis, a shorter OS was significantly associated with a high Ann Arbor stage, and a longer OS was significantly associated with a serum LDH level within the normal range, patients achieving CR after R-CHOP therapy, and low-risk R-IPI (Table 2). Univariate analysis revealed that patients with low TIL-T–Max had a significantly worse prognosis in OS compared to those with high TIL-T–Max (hazard ratio [HR], 15.8; 95% confidence interval [CI], 6.20 to 40.25; p < .001) (Table 2), and those with low TIL-T–Intermediate compared to high TIL-T– Intermediate (HR, 6.91; 95% CI, 2.85 to 16.74; p < .001) (Table 2). This trend was also observed in the TIL-T–Min; patients with a low TIL-T–Min had worse OS than those with a high TIL-T–Min (HR, 7.98; CI, 1.87 to 33.95; p = .005) (Table 2). In multivariate analysis, a low TIL-T–Max was found to be an independent unfavorable prognostic factor in patients with DLBCL NOS (HR, 7.55; 95% CI, 2.54 to 22.42; p < .001). The same trend was also observed in TIL-T–Intermediate (HR, 2.96; 95% CI, 1.17 to 7.53; p = .022) (Table 2, Fig. 4).
RESULTS
- In this study, we used digital image analysis to evaluate the exact ratio of TIL-T in patients with DLBCL NOS. Low TIL-T ratios in the TIL-T–Max, TIL-T–Intermediate, and TIL-T–Min were associated with shorter OS and were found to be independent prognostic factors in patients with DLBCL.
- Previous studies have focused on the prognostic significance of the total TIL-T ratio in patients with DLBCL. An immunohistochemical study of TIL-T in DLBCL showed no correlation between survival and TIL-T percentage [16]. Another study found that the percentage of CD3+ cells (total T cells) had no impact on DLBCL patient outcomes [18]. However, Xu et al. (2001) [15] found that a TIL-T ratio > 20% showed a favorable prognosis compared to a TIL-T ratio < 20%. Another study reported that patients with high CD3+ TIL-T levels (> 45%) had better eventfree survival and OS rates, although CD4+ TIL-T was statistically more relevant than CD3+ TIL-T in predicting patient outcomes [17]. All the studies mentioned above were conducted using flow cytometry analyses of fresh biopsy tissue, except for one by Lippman et al. (1990) [16]. Flow cytometric analysis of the TIL-T ratio in DLBCL specimens may have contributed to these contradictory results. Flow cytometric analysis, a rapid multi-parametric analysis of single cells in solution, lacks morphological correlation. Sampling errors such as the inclusion of normal lymphoid tissue adjacent to the malignant tumor area can influence the sensitivity of flow cytometry and result in reports that are not representative of the actual TME. Also, flow cytometric analysis cannot take into consideration of the differing areas of densities of TIL-Ts in one tumor specimen. In our study, formalin-fixed paraffin-embedded tissue specimens were used for IHC staining, and morphological evaluation of the whole slide was performed to detect the actual focus of T-lymphocyte infiltration among diffuse neoplastic B-cell populations. Moreover, the correlation between the TIL-T ratio and patient outcome was more statistically significant in the TIL-T–Max, suggesting that the clinical behavior of DLBCL may be more accurately reflected by the TIL-T ratio at the densest T lymphocyte infiltration. This finding underscores the importance of histological assessment in determining the TIL-T ratio in patients with DLBCL. TIL-T levels of tumor are mainly determined by the intrinsic immunogenicity of tumor cells. In partial areas of tumors, it is possible that TIL-T levels are lowered by other factors, such as immune checkpoints. Therefore, among various TIL-T levels in tumors, it could be considered that TIL-T–Max is most likely to accurately reflect the intrinsic immunogenic capacity of tumor cells, which may have contributed to a more precise predictions of a patient prognosis. Further study is required to confirm the prognostic value of the TIL-T–Max and to substantiate the hypothesis behind it.
- In our results, the high TIL-T ratio group according to the TIL-T–Max showed no correlation with patient age, sex, or COO of tumor. The Ann Arbor stage also showed no statistically significant association with the TIL-T–Max. However, serum LDH levels were significantly elevated in patients with a low TIL-T– Max. This suggests that the degree of TIL-T is determined by the immunogenicity of the tumor cells rather than by the patient’s demographic characteristics or the origin of the tumor cells. Tumor immunogenicity is influenced by tumor mutation burden, DNA mismatch repair function, antigen presentation defects, and immune checkpoint genes [22,23]. Therefore, further studies are needed to compare TIL-T levels with the molecular features and expression of immune checkpoint genes in DLBCL. Tumor immunogenicity can affect not only the prognosis of patients, but also their responsiveness to immunotherapy, such as immune checkpoint blockade or CAR-T therapy. Therefore, it is necessary to conduct additional research on the application of the TIL-T ratio criteria presented in our study (a ratio of ≥ 19% measured in the TIL-T–Max area) for immunotherapy in patients with DLBCL through digital image analysis.
- As more pathology departments implement digital reviews, digital image analyses are expected to become more commonplace [24]. It is important to recognize accurate quantification facilitated by artificial intelligence for prognostic and predictive scoring as a diagnostic tool for pathologists. Several studies have shown that digital image analysis is equal to or better than manual scoring by pathologists [25,26]. The application of digital image analysis to assess TIL-T ratios in tissue specimens may help minimize inter- and intra-observer variability, thus contributing to the discovery of accurate cutoff points for TIL-T ratio analysis in the future.
- This study has several limitations. First, this study was a retrospective analysis and lacked validation of the cutoff point used to differentiate between high and low TIL-T levels. Second, molecular analyses of the biological factors that determine the level of TIL-T are lacking. Further studies are required to address these shortcomings, including verification of the results and cutoff values using outside cohorts. Additional subset analyses of different T-cell populations in tissue specimens and the genetic profiles of the different groups of TIL-T ratios may help understand the immunobiology of the TILs in DLBCL.
- Patients with DLBCL with high TIL-T ratios showed a significantly better prognosis than those with low TIL-T ratios, and the TIL-T ratio was an independent indicator of OS. These results suggest that TIL-T may play a critical role in DLBCL disease progression and evaluating the level of TIL-T in DLBCL specimens using digital pathology software may be useful in predicting the clinical behavior and response to immunotherapy in patients with DLBCL.
DISCUSSION
Ethics Statement
All methods were performed in accordance with the Helsinki Declaration, and all protocols of this study were approved by the Institutional Review Board (IRB) of the Samsung Medical Center (IRB file number: SMC 2021-01-093-004). Formal written informed consent was not required with a waiver by the appropriate IRB.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: YC, JC. Data acquisition: SEY, SJK, WSK. Data analysis: YC, JL, BH. Writing—original draft: YC. Writing—review & editing: WSK, JC. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
TIL-T, tumor-infiltrating T lymphocyte; COO, cells of origin; GCB, germinal center B-cell-like; LDH, lactate dehydrogenase; CR, complete remission; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; CTx, chemotherapy; AutoSCT, autologous stem cell transplantation; R-IPI, revised International Prognostic Index.
- 1. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia 2022; 36: 1720-48. PubMedPMC
- 2. Campo E, Jaffe ES, Cook JR, et al. The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. Blood 2022; 140: 1229-53. PubMedPMC
- 3. Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol 2019; 37: 1285-95. ArticlePubMedPMC
- 4. Davies A, Cummin TE, Barrans S, et al. Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large B-cell lymphoma (REMoDL-B): an open-label, randomised, phase 3 trial. Lancet Oncol 2019; 20: 649-62. ArticlePubMedPMC
- 5. Nowakowski GS, Chiappella A, Gascoyne RD, et al. ROBUST: a phase III study of lenalidomide plus R-CHOP versus placebo plus R-CHOP in previously untreated patients with ABC-type diffuse large B-cell lymphoma. J Clin Oncol 2021; 39: 1317-28. ArticlePubMedPMC
- 6. Zhang J, Medeiros LJ, Young KH. Cancer immunotherapy in diffuse large B-cell lymphoma. Front Oncol 2018; 8: 351.ArticlePubMedPMC
- 7. Wang L, Li LR, Young KH. New agents and regimens for diffuse large B cell lymphoma. J Hematol Oncol 2020; 13: 175.ArticlePubMedPMCPDF
- 8. Nicholas NS, Apollonio B, Ramsay AG. Tumor microenvironment (TME)-driven immune suppression in B cell malignancy. Biochim Biophys Acta 2016; 1863: 471-82. ArticlePubMed
- 9. Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer 2014; 14: 517-34. ArticlePubMedPDF
- 10. Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12: 298-306. ArticlePubMedPDF
- 11. Keane C, Vari F, Hertzberg M, et al. Ratios of T-cell immune effectors and checkpoint molecules as prognostic biomarkers in diffuse large B-cell lymphoma: a population-based study. Lancet Haematol 2015; 2: e445-55. ArticlePubMed
- 12. Xu-Monette ZY, Xiao M, Au Q, et al. Immune profiling and quantitative analysis decipher the clinical role of immune-checkpoint expression in the tumor immune microenvironment of DLBCL. Cancer Immunol Res 2019; 7: 644-57. ArticlePubMedPDF
- 13. Sermer D, Batlevi C, Palomba ML, et al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv 2020; 4: 4669-78. ArticlePubMedPMCPDF
- 14. Hopfinger G, Jager U, Worel N. CAR-T cell therapy in diffuse large B cell lymphoma: hype and hope. Hemasphere 2019; 3: e185. ArticlePubMedPMC
- 15. Xu Y, Kroft SH, McKenna RW, Aquino DB. Prognostic significance of tumour-infiltrating T lymphocytes and T-cell subsets in de novo diffuse large B-cell lymphoma: a multiparameter flow cytometry study. Br J Haematol 2001; 112: 945-9. ArticlePubMedPDF
- 16. Lippman SM, Spier CM, Miller TP, Slymen DJ, Rybski JA, Grogan TM. Tumor-infiltrating T-lymphocytes in B-cell diffuse large cell lymphoma related to disease course. Mod Pathol 1990; 3: 361-7. PubMed
- 17. Keane C, Gill D, Vari F, Cross D, Griffiths L, Gandhi M. CD4(+) tumor infiltrating lymphocytes are prognostic and independent of RIPI in patients with DLBCL receiving R-CHOP chemo-immunotherapy. Am J Hematol 2013; 88: 273-6. ArticlePubMed
- 18. Ansell SM, Stenson M, Habermann TM, Jelinek DF, Witzig TE. Cd4+ T-cell immune response to large B-cell non-Hodgkin’s lymphoma predicts patient outcome. J Clin Oncol 2001; 19: 720-6. ArticlePubMed
- 19. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004; 103: 275-82. ArticlePubMed
- 20. Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007; 109: 1857-61. ArticlePubMedPDF
- 21. Bankhead P, Loughrey MB, Fernandez JA, et al. QuPath: Open source software for digital pathology image analysis. Sci Rep 2017; 7: 16878.ArticlePubMedPMCPDF
- 22. Wang S, He Z, Wang X, Li H, Liu XS. Antigen presentation and tumor immunogenicity in cancer immunotherapy response prediction. Elife 2019; 8: e4020. ArticlePDF
- 23. Blankenstein T, Coulie PG, Gilboa E, Jaffee EM. The determinants of tumour immunogenicity. Nat Rev Cancer 2012; 12: 307-13. ArticlePubMedPMCPDF
- 24. Mukhopadhyay S, Feldman MD, Abels E, et al. Whole slide imaging versus microscopy for primary diagnosis in surgical pathology: a multicenter blinded randomized noninferiority study of 1992 cases (pivotal study). Am J Surg Pathol 2018; 42: 39-52. PubMed
- 25. Gudlaugsson E, Skaland I, Janssen EA, et al. Comparison of the effect of different techniques for measurement of Ki67 proliferation on reproducibility and prognosis prediction accuracy in breast cancer. Histopathology 2012; 61: 1134-44. ArticlePubMed
- 26. Stalhammar G, Robertson S, Wedlund L, et al. Digital image analysis of Ki67 in hot spots is superior to both manual Ki67 and mitotic counts in breast cancer. Histopathology 2018; 72: 974-89. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- Do Pre‐Treatment Biopsy Characteristics Predict Early Tumour Progression in Feline Diffuse Large B Cell Nasal Lymphoma Treated With Radiotherapy?
Valerie J. Poirier, Valeria Meier, Michelle Turek, Neil Christensen, Jacqueline Bowal, Matthew D. Ponzini, Stefan M. Keller
Veterinary and Comparative Oncology.2025; 23(1): 82. CrossRef - Comprehensive Analysis of Tumor Microenvironment and PD-L1 Expression Associations with Clinicopathological Features and Prognosis in Diffuse Large B-Cell Lymphoma
Yun-Li Xie, Long-Feng Ke, Wen-Wen Zhang, Fu Kang, Shu-Yi Lu, Chen-Yu Wu, Huan-Huan Zhu, Jian-Chao Wang, Gang Chen, Yan-Ping Chen
Blood and Lymphatic Cancer: Targets and Therapy.2025; Volume 15: 167. CrossRef - Metabolic-immune axis in the tumor microenvironment: a new strategy for prognostic assessment and precision therapy in DLBCL and FL
Chengqian Chen, Wei Guo, Haotian Wang, Luming Cao, Ou Bai
Frontiers in Immunology.2025;[Epub] CrossRef - Integrative analysis of a novel immunogenic PANoptosis‑related gene signature in diffuse large B-cell lymphoma for prognostication and therapeutic decision-making
Ming Xu, Ming Ruan, Wenhua Zhu, Jiayue Xu, Ling Lin, Weili Li, Weirong Zhu
Scientific Reports.2024;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure




Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
| Characteristic | No. of patients (%) | TIL-T–Max |
TIL-T–Intermediate |
TIL-T–Min |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| High (n=72, 75.0%) | Low (n=24, 25.0%) | p-value | High (n=64, 66.7%) | Low (n=32, 33.3%) | p-value | High (n=36, 37.5%) | Low (n=60, 62.5%) | p-value | ||
| Age (yr) | .239 | > .999 | ||||||||
| ≤ 60 | 48 (50.0) | 39 | 9 | 32 | 16 | > .999 | 18 | 30 | ||
| > 60 | 48 (50.0) | 33 | 15 | 32 | 16 | 18 | 30 | |||
| Sex | .630 | > .999 | > .999 | |||||||
| Female | 38 (39.6) | 30 | 8 | 25 | 13 | 14 | 24 | |||
| Male | 58 (60.4) | 42 | 16 | 39 | 19 | 22 | 36 | |||
| Ann Arbor stage | .098 | .096 | > .999 | |||||||
| Low (I–II) | 52 (54.2) | 43 | 9 | 39 | 13 | 20 | 32 | |||
| High (III–IV) | 44 (45.8) | 29 | 15 | 25 | 19 | 16 | 28 | |||
| COO | .803 | .592 | .502 | |||||||
| GCB | 32 (33.3) | 25 | 7 | 23 | 9 | 14 | 18 | |||
| Non-GCB | 64 (66.7) | 47 | 17 | 41 | 23 | 22 | 42 | |||
| Serum LDH level | < .001 | .004 | .077 | |||||||
| Low | 57 (59.4) | 52 | 5 | 45 | 12 | 26 | 31 | |||
| High | 39 (40.6) | 20 | 19 | 19 | 20 | 10 | 29 | |||
| Treatment | < .001 | .002 | .246 | |||||||
| CR after R-CHOP | 50 (52.1) | 45 | 5 | 41 | 9 | 22 | 28 | |||
| Non-CR | 46 (47.9) | 27 | 19 | 23 | 23 | 14 | 32 | |||
| R-CHOP only | 9 | |||||||||
| + 2nd and/or 3rd line CTx | 17 | |||||||||
| + AutoSCT | 15 | |||||||||
| Pre-treatment | 5 | |||||||||
| R-IPI | < .001 | .040 | .202 | |||||||
| Low risk (0–2) | 63 (65.6) | 55 | 8 | 47 | 16 | 27 | 36 | |||
| High risk (3–5) | 33 (34.4) | 17 | 16 | 17 | 16 | 9 | 24 | |||
| Variable | Comparison/referent | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| TIL-T–Max | |||||||
| Age (yr) | > 60/≤ 60 | 0.62 | 0.28–1.40 | .251 | |||
| Sex | Male/Female | 1.42 | 0.61–3.33 | .414 | |||
| Ann Arbor stage | III–IV/I–II | 4.14 | 1.64–10.43 | .003 | 1.24 | 0.42–3.66 | .699 |
| Cell-of-origin | Non-GCB/GCB | 1.60 | 0.64–4.03 | .318 | |||
| Serum LDH level | Low/High | 0.18 | 0.07–0.45 | < .001 | 0.76 | 0.20–2.77 | .675 |
| Treatment | Non-CR/CR after R-CHOP | 37.42 | 5.04–277.70 | < .001 | 23.81 | 3.13–181.15 | .002 |
| R-IPI | Low risk/High risk | 0.15 | 0.06–0.37 | < .001 | 0.50 | 0.14–1.77 | .282 |
| TIL-T–Max | Low/High | 15.81 | 6.20–40.25 | < .001 | 7.55 | 2.54–22.42 | < .001 |
| TIL-T–Intermediate | |||||||
| Age (yr) | > 60/≤ 60 | 0.62 | 0.28–1.40 | .251 | |||
| Sex | Male/Female | 1.42 | 0.61–3.33 | .414 | |||
| Ann Arbor stage | III–IV/I–II | 4.14 | 1.64–10.43 | .003 | 0.99 | 0.34–2.89 | .981 |
| Cell-of-origin | Non-GCB/GCB | 1.60 | 0.64–4.03 | .318 | |||
| Serum LDH level | High/Low | 0.18 | 0.07–0.45 | < .001 | 0.35 | 0.12–1.06 | .065 |
| Treatment | Non-CR/CR after R-CHOP | 37.42 | 5.04–277.70 | < .001 | 26.00 | 3.44–-196.81 | .002 |
| R-IPI | Low risk/High risk | 0.15 | 0.06–0.37 | < .001 | 0.44 | 0.14–1.44 | .175 |
| TIL-T–Intermediate | Low/High | 6.91 | 2.85–16.74 | < .001 | 2.96 | 1.17–7.53 | .022 |
| TIL-T–Min | |||||||
| Age (yr) | > 60/≤ 60 | 0.62 | 0.28–1.40 | .251 | |||
| Sex | Male/Female | 1.42 | 0.61–3.33 | .414 | |||
| Ann Arbor stage | III-IV/I-II | 4.14 | 1.64–10.43 | .003 | 1.05 | 0.33–3.32 | .936 |
| Cell-of-origin | Non-GCB/GCB | 1.60 | 0.64–4.03 | .318 | |||
| Serum LDH level | High/Low | 0.18 | 0.07–0.45 | < .001 | 0.43 | 0.13–1.36 | .151 |
| Treatment | Non-CR/CR after R-CHOP | 37.42 | 5.04–277.70 | < .001 | 25.76 | 3.40–195.27 | .002 |
| R-IPI | Low risk/High risk | 0.15 | 0.06–0.37 | < .001 | 0.47 | 0.13–1.72 | .254 |
| TIL-T–Min | Low/High | 7.98 | 1.87–33.95 | .005 | 3.80 | 0.82–17.58 | .088 |
TIL-T, tumor-infiltrating T lymphocyte; COO, cells of origin; GCB, germinal center B-cell-like; LDH, lactate dehydrogenase; CR, complete remission; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; CTx, chemotherapy; AutoSCT, autologous stem cell transplantation; R-IPI, revised International Prognostic Index.
HR, hazard ratio; CI, confidence interval; TIL-T, tumor-infiltrating T lymphocyte; GCB, germinal center B-cell-like; LDH, lactate dehydrogenase; CR, complete remission; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R-IPI, revised International Prognostic Index.

 E-submission
E-submission